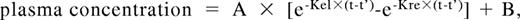Abstract
ABO blood groups greatly influence circulating von Willebrand factor (VWF) levels, and O group subjects have lower VWF values. In this study, we investigated whether ABO groups affect VWF survival by monitoring the post-DDAVP (1-desamino-8-d arginine vasopressin) time courses of VWF antigen (VWF:Ag), VWF collagen binding (VWF:CB), and factor VIII (FVIII) in 47 healthy subjects (28 O and 19 non-O blood groups). The elimination half-life (T1/2el) of VWF was found significantly shorter in O than in non-O subjects (10.0 ± 0.8 hours vs 25.5 ± 5.3 hours, respectively; P < .01), as was the T1/2el of VWF:CB (7.9 ± 0.5 hours vs 20.9 ± 4.5 hours; P < .01). A direct linear correlation was found between basal VWF:Ag and T1/2el, subjects with higher VWF levels having longer-surviving VWF. ABO blood groups appeared to strongly influence VWF clearance, but not its synthesis or release from endothelial cells. The VWF propeptide to VWF:Ag ratio, useful for predicting an increased VWF clearance, was found significantly higher in O than in non-O individuals (1.6 ± 0.1 vs 1.2 ± 0.5, P < .001), with values that correlated inversely with T1/2el (P < .001). Based on these findings, we conclude that the lower VWF values in O group individuals is attributable to a shorter VWF survival and circulating VWF values are strongly influenced by its half-life.
Introduction
Platelet plug formation at the site of vascular injury is initiated by von Willebrand factor (VWF) interacting with the subendothelial matrix, followed by its binding to platelet glycoprotein (GP) Ib1,2 and subsequent platelet activation and aggregation. VWF is synthesized by endothelial cells and megakaryocytes,3,4 and one of its main particular features is a polymer structure ranging in size from 500 000 to more than 20 million Dalton,5 the largest forms being hemostatically the most efficient.6
A broad range of values characterizes plasma VWF levels, which average around 10 μg/mL. Acquired and inherited factors both modulate plasma VWF levels, and twin studies have demonstrated that 66% of all variations in plasma VWF are genetically determined, while 30% of them depend on ABO blood group,7 O blood group individuals having plasma VWF levels 25% lower than non-O subjects.8 ABO group genotyping shows that O1O1 subjects have the lowest VWF levels, and non-O group individuals heterozygous for the O1 allele have significantly lower VWF levels than AA, AB, or BB subjects.9,10 Glycosylation accounts for 19% of VWF by weight, and ABO determinants identified on the N-linked oligosaccharide chains are part of this glycosylation process.11,12 ABO groups are added to the N-linked glycan chains of VWF in the post-Golgi compartment of endothelial cells before VWF secretion, albeit with the variable contribution of the endothelial cells from different vascular beds.13 The carbohydrate moiety plays an important part in VWF polymerization and function,14 and also affects the liver-mediated clearance of VWF. In animal models, the removal of sialic acid has been shown to induce an increase in VWF clearance,15 and the half-life of VWF is halved in mice characterized by an aberrantly glycosylated VWF (due to the absence of the enzyme ST3Gal-IV).16 Moreover, recombinant VWF, lacking in carbohydrate, is cleared from the circulation faster than its glycosylated counterpart,16 and posttranslational changes in VWF induced by Galgt2, aberrantly expressed in endothelial cells, lead to a 20-fold increase in VWF clearance.17
ABO group determinants may also regulate the susceptibility of VWF to the proteolytic action of ADAMTS13, proteolysis being faster in the case of the O blood group.18 A different susceptibility to cleavage by ADAMTS13 may thus be one of the ways in which ABO group affects VWF removal from the circulation and consequent VWF levels.
Although the mechanisms behind ABO blood group and VWF levels have yet to be fully clarified, it has been clearly demonstrated that the effects are mediated by the ABO antigen structures on the N-linked oligosaccharide chains of circulating VWF, and particularly by H antigen expression.19 Understanding these mechanisms is of clinical relevance: non-O individuals have been shown to carry a significantly greater risk of venous thromboembolism, ischemic heart disease, and peripheral vascular disease,21-23 while the O blood group is much more common in von Willebrand disease (VWD) patients than in the general population, suggesting that the O group may favor the phenotypic expression of the disorder.24,25
The aim of this study was to shed light on the mechanisms involved in the VWF modulating effect of ABO blood groups. We investigated the pharmacokinetic parameters associated with post-DDAVP (1-desamino-8-d arginine vasopressin) variations in plasma VWF and FVIII concentrations in healthy individuals divided into O and non-O groups.
Methods
Healthy volunteers were studied in accordance with the Declaration of Helsinki, after we had obtained their written informed consent and the University of Padua institutional review board's approval of the study.
Blood was drawn from the antecubital vein and anticoagulated using sodium citrate (1:10, vol/vol). VWF:Ag was measured with a home-made ELISA (enzyme-linked immunosorbent assay) method,26 using a horseradish peroxidase (HRP)–conjugated anti-VWF antibody (Dako, Glostrup, Denmark). Factor VIII (FVIII) coagulant was measured using a one-stage method, with cephaloplastin as activated cephalin, as reported elsewhere.27 VWF collagen binding (VWF:CB) activity was assessed by ELISA using type I and type III collagen diluted in acetic acid solution (95% and 5%, respectively), as described elsewhere.28 Briefly, after overnight coating with collagen, microtiter plates were incubated with plasma VWF for 1 hour at room temperature; bound VWF was evaluated with HRP-conjugated anti-VWF antibody (DAKO). VWF propeptide (VWFpp) concentration was determined with an ELISA test kindly supplied by GTI Diagnostics (Waukesha, WI). All FVIII and VWF assays were performed using a pool of normal plasma for reference.
DDAVP (Emosint, Sclavo, Italy) was administered subcutaneously at a dose of 0.3 μg/kg. Blood samples were collected before and after 15, 30, 60, 120, 180, 240, 360, and 480 minutes, and 24 hours after DDAVP infusion. Time courses of VWF:Ag, VWF:CB, and FVIII plasma concentrations after DDAVP administration were analyzed using a one-compartment model with first-order input and output kinetics,29 also including baseline concentrations, B, and a time lag between DDAVP administration and the increase in plasma concentration, t', as follows:
where A is the y-axis intercept, Kre is the release rate constant, Kel is the elimination rate constant, and t is the time. The model was fitted to each set of concentration-time data by means of the Prism statistical package (GraphPad, San Diego, CA). Goodness of fit was evaluated by r2. Areas under the concentration-time curve (AUC), release (t1/2re), and elimination (t1/2el) half-lives were calculated using standard formulas: AUC = A/Kel − A/Kre; T1/2re = 0,693/Kre; T1/2el = 0.693/Kel.
Using this kinetic model,29 the amount of VWF:Ag released by DDAVP (Q) is: Q = A × VD × (Kre-Kel)/Kel, where VD is the volume of distribution of VWF:Ag.
Likewise, plasma clearance (CL) is CL = Kel × VD, and the baseline rate of release of VWF:Ag (Vre) is Vre = B × CL. The VD of VWF could not be calculated from our data, so the VD reported by Menache et al30 after intravenous. VWF administration (40 mL/kg) was used to obtain an approximate estimate of Q, CL, and Vre in our patients.
ABO subtypes were genotyped by sequencing exons 6 and 7 of the ABO gene. Genomic DNA was extracted from peripheral blood leukocytes using the QIAamp DNA blood Mini Kit (Qiagen, Hilden, Germany). Exons 6 and 7 of the ABO locus were amplified from 100 ng genomic DNA by polymerase chain reaction (PCR) with AmpliTaq Gold (Applied Biosystems, Warrington, United Kingdom) in a thermal cycler (2700; Perkin Elmer Life and Analytical Sciences, Waltham, MA). Primers for amplification and sequencing of exon 6 were ABO-1: 5′-TGGCACCCTGCCAGCTCCAT-3′ and ABO-2: 5′-TCACTCGCCACTGCCTGGGT-3′. Primer sequences of exon 7 were, at the beginning of exon A10: 5′-TTCCT-GAAGCTGTTCCTGGAGACG-3′ and AB2: 5′-GCTCGTAGGTGA-AGGCCTCCC-3′; and at the end of exon ABO-6: 5′-GGGAGGCCT-TCACCTACGAGC-3′ and ABO-5: 5′-GCTGCCGGCAGCCCTCC-CAGAG-3′. The amplified fragments were sequenced with the dideoxy method using the Big Dye terminator sequencing kit (Applied Biosystems). We referred to Yamamoto's ABO allele classification,31 where the A101 allele was the reference sequence. We compared all sequences with particular nucleotide positions of various alleles at these 2 exons of the human ABO locus. To simplify, we chose to consider 5 major subtypes, that is, 2 A alleles (A1 was the reference allele and A2 associated with a C deletion at position 1060), because all the other A alleles were rare; only one B allele (differing from the A1 allele in 7 single base substitutions), since the other B alleles were rare (< 1%) and have so far only been reported in the Japanese32 ; and 2 O alleles (O1 associated with a G deletion at position 261, and O2, a nondeletion allele). O2 alleles include either a G>A substitution at the position 802, or the G insertion at the position 800. Laboratory data and pharmacokinetic parameters were expressed as means plus or minus SE. The t test was used to compare all the results, and a correlation analysis was conducted to evaluate the association between the parameters. A modified t test was used when variances were not equal. P values below .05 were considered statistically significant.
Results
Forty-seven healthy subjects (14 males and 33 females) between 24 and 70 years old (mean, 39.9 ± 2.0 years) were investigated; 28 were blood group O and 19 were non-O (15 A, 3 B and 1 AB). There was no difference in mean age (non-O: 38.2 ± 3.2 years; O group: 41.0 ± 2.7 years) or gender distribution between the 2 groups. Allele composition showed a prevalence of O1O1 in the O group (82%), and of A1O1 and BO1 in the non-O group (53% and 16%, respectively; Table 1). As expected, mean VWF:Ag values were lower in the O group (79.2 ± 5.8 U/dL vs 114.2 ± 8.6 U/dL in non-O cases; P < .05) and so were VWF:CB levels (79.0 ± 7.5 U/dL vs 115.9 ± 10.3 U/dL; P < .005); the mean VWF:CB/VWF:Ag ratio was not statistically different in O versus non-O (1.01 ± 0.03 and 1.12 ± 0.07, respectively). Moreover, there were no significant ABO-related differences in FVIII levels (100.2 ± 7.4 U/dL in O vs 109.2 ± 6.6 U/dL in non-O).
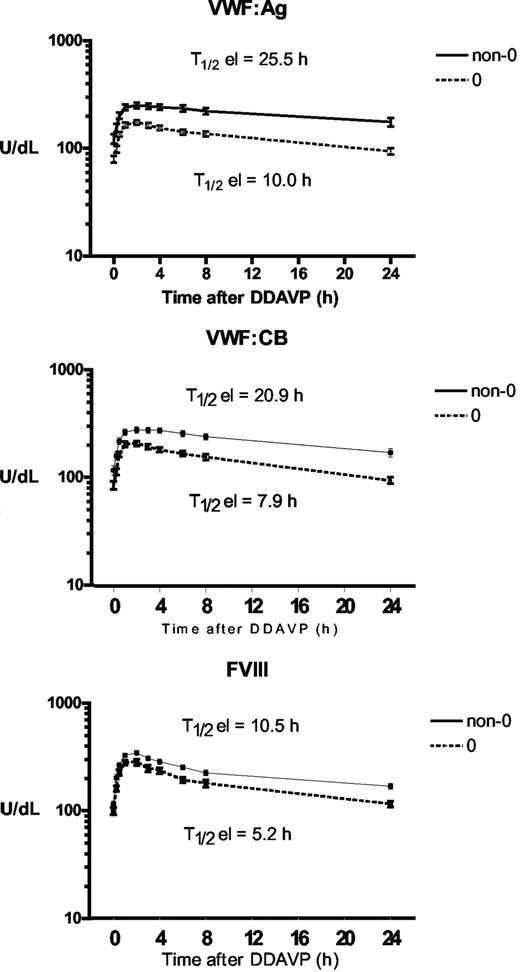
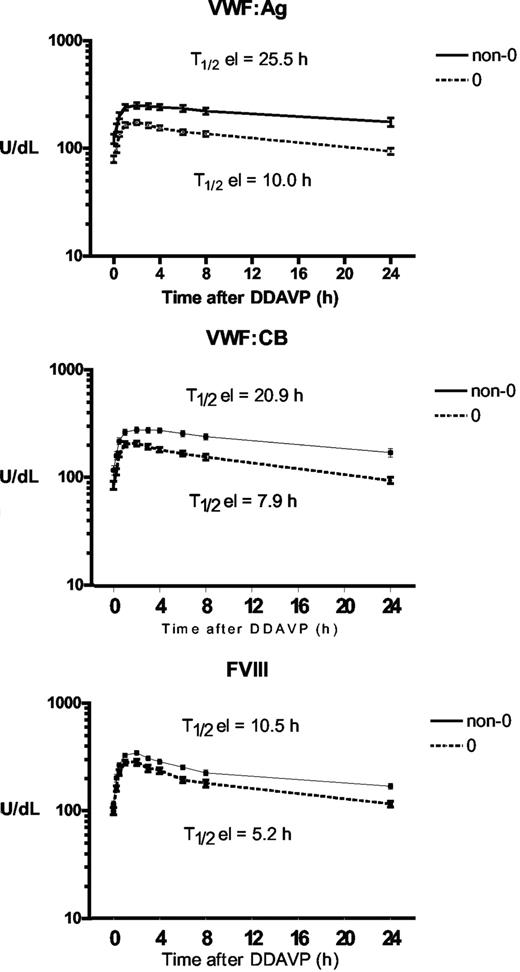
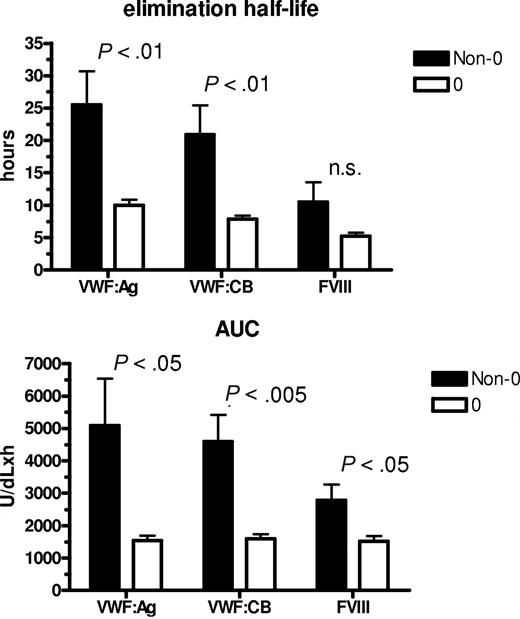
To assess whether ABO group influences VWF half-life, time courses of FVIII, VWF:Ag, and VWF:CB were analyzed after DDAVP administration, using a one-compartment model with first-order input and output kinetics, and including a time lag between DDAVP administration and the increase in plasma VWF concentration. The mean FVIII, VWF:Ag, and VWF:CB time courses in O and non-O subjects are shown in Figure 1. A significantly shorter T1/2el of VWF:Ag was demonstrable in O group individuals (10.0 ± 0.8 hours vs 25.5 ± 5.3 hours in the non-O cases; P < .01) and a similar behavior was seen for the T1/2el of VWF:CB (7.9 ± 0.5 hours vs 20.9 ± 4.5 hours; P < .01) and for FVIII, though it lacked statistical significance, (5.2 ± 0.5 hours vs 10.5 ± 0.3 hours; P = .094; Figure 2 top panel). The post-DDAVP mean VWF:CB/VWF:Ag ratio remains similar in O and non-O individuals, with no significant difference with respect to basal values (1.28 ± 0.05 in O and 1.17 ± 0.05 in non-O at the peak, and 1.18 ± 0.03 in O and 1.13 ± 0.05 in non-O at 480 minutes). These findings suggest a faster clearance of all VWF components, including the larger multimers, in O group individuals. The mean AUC for VWF:Ag was also consistently lower in O group cases, (1541 ± 145 U/dLxh vs 5091 ± 1449 U/dLxh in non-O, P < .05), and the same was true of VWF:CB (1570 ± 143 U/dLxh vs 4591 ± 824 U/dLxh, P < .005) and FVIII (1516 ± 161 U/dLxh vs 2786 ± 478 U/dLxh, P < .05; Figure 2 bottom panel). On the other hand, no ABO-related differences emerged for the other kinetic parameters, apart from the Kel changes, which paralleled those of T1/2el. Details of all the kinetic parameters of VWF:Ag measured in O and non-O subjects are given in Table 2. The significant differences between O and non-O subjects involve parameters related more to VWF clearance (ie, T1/2el, Kel, and AUC) than to VWF release (ie, A, Kre, T1/2re, t'), which would entitle us to claim that VWF synthesis and release are unchanged, but the rate of clearance is higher in people with the O than in those with the non-O blood group. To further support this hypothesis, the amount of VWF released from endothelial cells (Q), the baseline rate of VWF release (Vre), and VWF clearance (CL) were calculated using the VWF volume of distribution (VD) estimated by Menache et al (40 mL/kg)30 and the equations reported in the “Methods.” We found similar Q and Vre values in O and non-O cases, but VWF clearance was significantly greater in O than in non-O blood group cases (3.24 ± 0.25 mL/h/kg vs 1.64 ± 0.20 mL/h/kg, P < .05; Table 3). These findings confirm that the influence of O blood group on circulating VWF levels is mainly attributable to a faster elimination.
Time courses of plasma VWF:Ag, VWF:CB, and FVIII concentrations observed before and after the subcutaneous administration of DDAVP (0.3 μg/kg) in O (○) and non-O (▵) blood group healthy individuals. Each time point represents the mean (± SE) of all the values obtained from the subjects investigated.
Time courses of plasma VWF:Ag, VWF:CB, and FVIII concentrations observed before and after the subcutaneous administration of DDAVP (0.3 μg/kg) in O (○) and non-O (▵) blood group healthy individuals. Each time point represents the mean (± SE) of all the values obtained from the subjects investigated.
Influence of ABO blood group on the mean T1/2el and AUC values. Observed for VWF:Ag, VWF:CB, and FVIII in O (□) and non-O (■) healthy individuals after DDAVP administration.
Influence of ABO blood group on the mean T1/2el and AUC values. Observed for VWF:Ag, VWF:CB, and FVIII in O (□) and non-O (■) healthy individuals after DDAVP administration.
After demonstrating that ABO blood group influences VWF clearance, we attempted to establish whether VWF elimination rate affects its basal circulating levels. A direct linear correlation emerged between VWF:Ag and T1/2el values (r = 0.72, P < .001), that is, individuals with a longer T1/2el also have higher VWF levels. On grouping individuals by blood group, a correlation between T1/2el and basal VWF:Ag values emerged only for the non-O blood group cases; however, P was less than .001. Findings were similar for VWF:CB, while this correlation was not seen in the case of FVIII, the circulating values of which must be influenced by other factor(s).
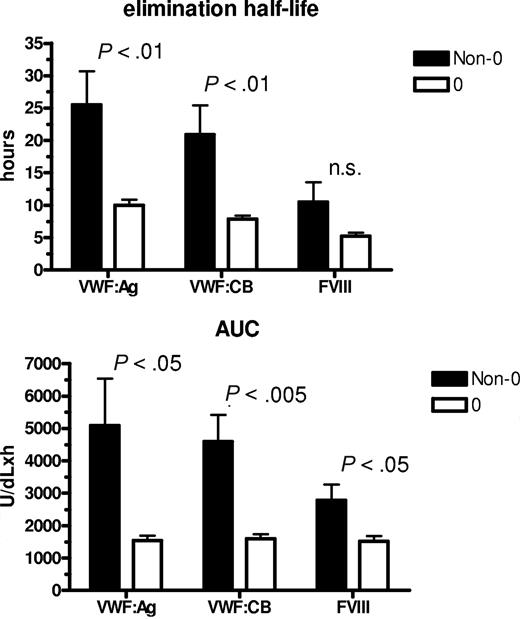
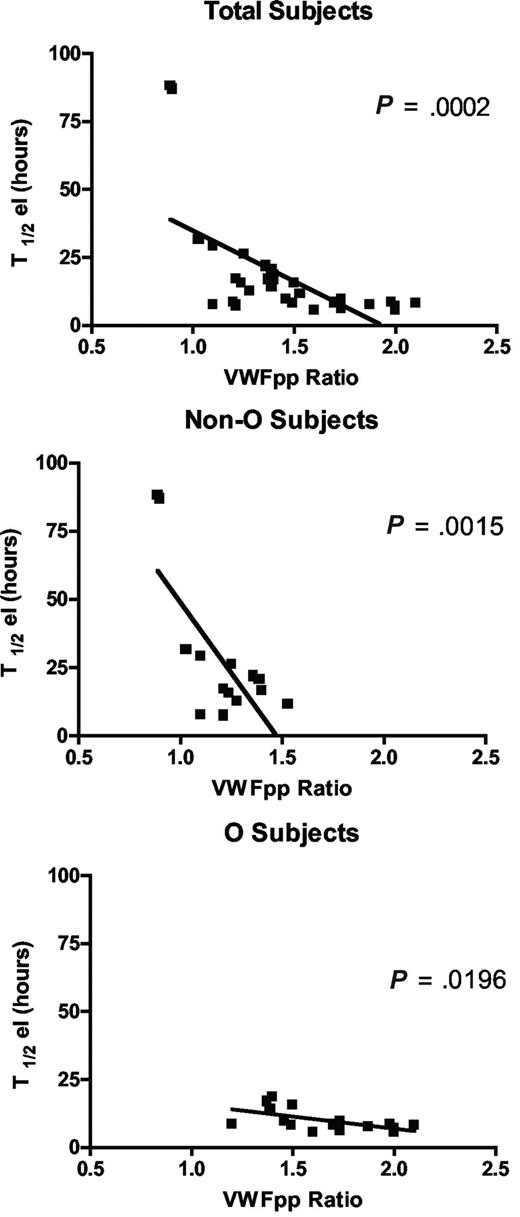
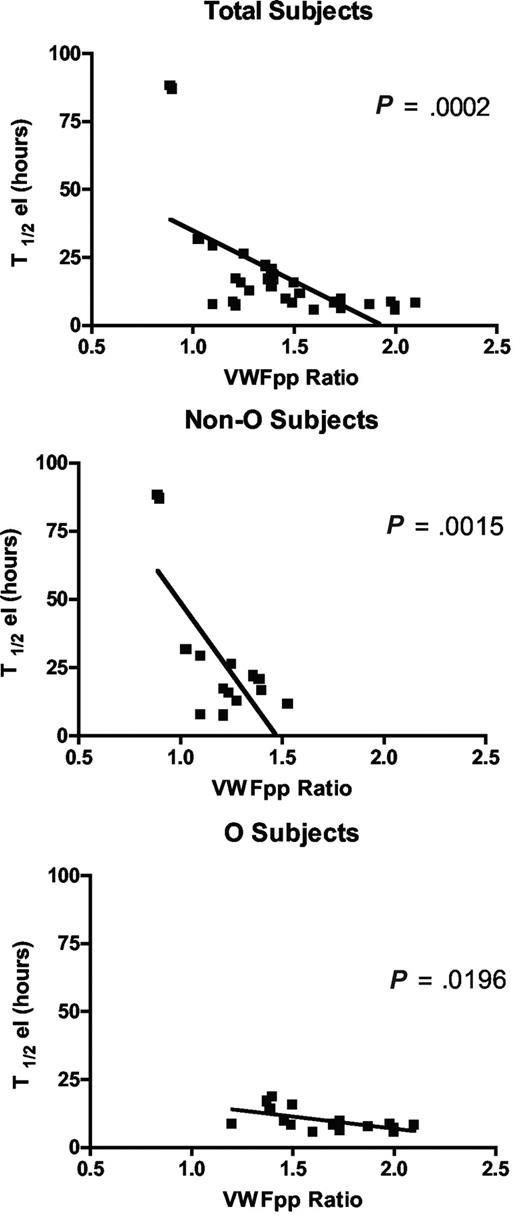
Since circulating VWFpp/VWF:Ag ratio (VWFpp ratio) is thought to correlate with VWF clearance,33 and to confirm the shorter VWF survival observed in our O blood group subjects, we measured VWFpp. Mean VWFpp concentrations in our subjects as a whole were 104.2 (± 4.93) U/dL, without significant difference between cases with blood group O versus non-O (99.7 ± 7.9 U/dL and 109.2 ± 5.49 U/dL, respectively). Instead, there was a significant difference (P < .001) in the VWFpp ratio between O (1.6 ± 0.1) and non-O (1.2 ± 0.05) subjects. An inverse linear correlation was found between T1/2el and the VWFpp ratio, both taking all subjects together (P < .001) and grouping them as O and non-O (P < .02; Figure 3). The highest VWFpp ratios coincided with the shortest VWF survival, confirming both the shorter VWF survival seen in O blood group cases and the contribution of the VWFpp ratio to investigate variations in VWF half-life.
The correlation between the VWFpp/VWF:Ag ratio and T1/2el in the sample as a whole and divided into O and non-O blood groups. An inverse linear correlation was seen whichever way the patients were grouped.
The correlation between the VWFpp/VWF:Ag ratio and T1/2el in the sample as a whole and divided into O and non-O blood groups. An inverse linear correlation was seen whichever way the patients were grouped.
Discussion
For a long time, ABO blood groups have been known to influence circulating VWF levels, but until now the underlying mechanisms have remained elusive. Our study demonstrated that ABO blood groups influence VWF half-life and that people with blood group O have a shorter VWF survival than those with the other blood groups. This is entirely the effect of an increased VWF clearance, since neither the amount nor the rate of VWF release from endothelial cells are influenced by ABO groups.
Plasma VWF concentration varies widely in healthy individuals, and ABO blood group is its main genetic modulator.34 The mechanisms responsible for the effects of blood group on VWF have been debated in recent years: it has been suggested that ABO influences the synthesis/secretion or clearance/catabolism of VWF, and it has been established that ABO blood group influences VWF levels directly, via a functional effect of the ABO locus.10 Platelet VWF content is currently seen as a marker of VWF synthesis, so finding that different ABO blood groups have a similar platelet VWF content ruled out the possibility that the ABO determinant could affect VWF synthesis.20 Starting from the finding that ABO structures exist on the N-linked oligosaccharide chains associated with VWF and bearing in mind that glycosylation may influence the rate of clearance of proteins like erythropoietin and interferon, mediated by a specific hepatic asialoglycoprotein receptor,35 we worked on the assumption that ABO blood groups might influence VWF half-life. We tested our hypothesis by analyzing the time course of VWF plasma concentrations in healthy volunteers, following the administration of DDAVP, a vasopressin analog capable of inducing the release of VWF from endothelial cell storage sites. The kinetic model used to describe VWF release and elimination is an improved version of previous models of ours28 and other authors,30 in that it also considers the rising plasma concentration phase and a possible time lag between DDAVP administration and the increase in VWF plasma level. Unlike the case of most previous studies,36-38 moreover, baseline VWF levels (B) are taken into account, thus avoiding an important bias in half-life determination.
Post-DDAVP VWF survival proved significantly shorter in O than in non-O individuals, the mean VWF half-life in the former being about 40% of the time in the latter (10 hours vs 25.5 hours). Our results are consistent with the observation that O blood group hemophilic patients have a shorter survival of exogenous FVIII36 than blood group A hemophiliacs, and with a more recent paper by Davies et al37 reporting the variable clearance of VWF according to ABO blood group. Indeed, the VWF survival recorded appears longer, especially in the non-O group, than the one reported in the literature. Apart from the clear contribution of ABO blood group, we believe this difference may be due to the well-known interindividual variability in VWF survival times (hinting at other, as yet unknown factors) and the long observation time we used to calculate VWF half-life. Most previously published studies considered a 4-hour observation time, which is definitely too short for the VWF survival generally reported and may consequently have been a cause of error.
In keeping with these findings, the mean VWF plasma clearance that we calculated from the Kel measurements and the published VD values30 was nearly twice as high in non-O as in O subjects (3.24 vs 1.64 mL/h/kg). It is noteworthy that the CL values we found are comparable with those reported by Dobrkovska et al38 in 5 healthy volunteers after administering an intravenous bolus of VWF (range, 1.49-2.83 mL/h/kg). On the other hand, none of the kinetic parameters quantifying VWF release from the endothelial cells (Kre, Vre, t', Q) differed significantly between the 2 groups, meaning that the shorter VWF survival in O group subjects is attributable to a faster elimination of VWF.
The increase in the VWF elimination rate seems to concern all multimer size components, since ultralarge VWF oligomers (as seen by VWF:CB) and VWF molecules in toto (as seen by VWF:Ag) have similar pharmacokinetic parameters, and no difference in the VWF:CB/VWF:Ag ratio was observed between the O and non-O groups, before or after DDAVP. These results are consistent with the observation of a similar clearance rate for high- and low-molecular-weight VWF multimers infused in VWF-deficient mice38 and enable us to hypothesize that, after DDAVP administration, nonproteolytic VWF clearance seems to prevail over proteolysis in humans, despite the presence of DDAVP-induced unusually large multimers in the circulation.
Further confirmation of a shorter VWF survival coinciding with the O blood group comes from this group's higher VWFpp ratio by comparison with non-O individuals, confirming data recently reported by Montgomery's group.33 VWFpp is a large fragment (with 741 amino acids) yielded by mature VWF and stored in the platelet alpha granules and Weibel-Palade bodies of endothelial cells not covalently associated with mature VWF. After continuous or regulated secretion from endothelial cells, VWFpp circulates as a dimer with a half-life of 2 to 3 hours, instead of the 12 to 14 hours of mature VWF. Albeit in different concentrations, in a steady state, plasma VWFpp and VWF:Ag levels represent the balance between secretion and clearance, which is why the VWFpp ratio has recently been recommended as a tool for investigating VWF survival. In our healthy subjects, the VWFpp ratio correlated well with VWF survival, that is, an inverse relationship emerged between VWFpp ratio and T1/2el, higher ratios being associated with a lower T1/2el, confirming that this ratio is sensitive in detecting differences in VWF survival, not only in VWD patients but also under normal conditions.
Different domains seem to be involved in VWF clearance, but a key role seems to be attributable to the D'-D3 as demonstrated by in vitro animal models, employing deleted VWF fragments39 and as suggested by the shorter VWF half-life observed in type Vicenza VWD patients.40 This variant, characterized by the Arg1205His mutation in the D3 domain,41 has the shortest VWF survival among all forms of VWD.40 Though the whereabouts of the ABO determinants on the N-linked chains are not known, it is worth noting that 2 of the 12 N-linked carbohydrate chains that are potential sites of ABO binding are located in D3 domains, where the type Vicenza mutation occurs.
Our results are consistent with the observation that O blood group hemophilic patients have a shorter survival of exogenous FVIII36 than blood group A hemophiliacs and with a more recent paper by Davies et al,37 reporting the variable clearance of VWF according to ABO blood group. We conclude that the lower plasma VWF levels characteristic of individuals in the O blood group are due to a shorter VWF survival, mainly attributable to a faster clearance, and that circulating levels of VWF are greatly influenced by its half-life. These findings obviously do not rule out the possibility of other glycosylation systems, like the O-linked one,42 affecting the clearance of VWF, nor do they preclude the possibility of other, as yet unidentified modifiers being involved. Our results help to shed light on the mechanisms underlying the effects of ABO determinants on circulating VWF levels.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Telethon Foundation and Ministero Università e Ricerca Scientifica 06 (Rome, Italy).
Authorship
Contribution: L.G. performed the hemostatic analysis and genetic characterization of the ABO blood groups; M.G.C. followed up the DDAVP administration and took part in the hemostatic analysis; M.S. performed the VWF propeptide assays; R.P. performed the pharmacokinetic analysis; F.S. took part in the hemostatic analysis and data analysis; E.P. followed up the DDAVP administration; V.D. performed the statistical analysis; A.P. organized the study and discussed the results; A.C. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandra Casonato, Dept of Medical and Surgical Sciences, Via Ospedale Civile 105, Padua, Italy; e-mail: sandra.casonato@unipd.it.