Abstract
Dendritic cells (DCs) control T cell–based immunity. To do so they need to mature and migrate to sites of T-cell priming. We have previously shown that cognate interactions of human CD4+ T cells with DCs induce DC maturation. We show here that CC chemokines produced during antigen-specific T-DC interactions also induce strong morphologic modifications and migration of immature DCs. These modifications are required for efficient T-cell activation. Moreover, we show that CC chemokines produced during antigen-specific DC–T-cell interactions induce the dissolution of structures involved in cell motility and present on immature DCs (ie, podosomes). We thus propose a model in which chemokines secreted during Ag-specific contact between T cells and DCs induce disassembly of interacting and neighboring immature DC podosomes, leading to recruitment of more immature DCs toward sites of antigenic stimulation and to amplification of T-cell responses.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells with the unique property of inducing priming and differentiation of naive CD4+ and CD8+ T cells into helper and cytotoxic effectors, respectively. Their efficiency is due to their unique ability to process antigen, express costimulatory molecules, secrete cytokines, and migrate to the appropriate sites, in tissues or lymphoid organs, to prime T cells.1
CD4+ helper T cells have been shown to promote the quality of T-cell responses.2,3 There is ample evidence that this helper activity is due to the ability of CD4+ T cells to act directly on DCs in an antigen-dependent manner. Indeed, experiments conducted both in murine and human models have shown that interaction between CD40 and CD40L,4-7 expressed respectively by DCs and activated T cells, as well as T cell–derived cytokines such as IFN-γ and TNF-α, modify the DC properties by inducing expression of costimulatory molecules and IL-12.8-10 This T cell–induced modification of DCs has been called “education”3 or “licensing.”11
As stated above, expression by DCs of costimulatory molecules and secretion of cytokines is not sufficient to induce T-cell activation. DCs also need to be recruited to the appropriate sites to induce T-cell responses. This implies migration of maturing DCs from peripheral tissues toward the draining lymph nodes through lymphatic vessels (reviewed in Randolph et al12 ) as well as mobilization of resident DCs of the lymphoid organs to the T-cell zones13 or recruitment of DCs, mostly immature, to the peripheral inflammatory tissues.14,15
We have previously shown in a human model that antigen-specific interactions of CD4+ T cells with immature DCs induce activation of DCs leading to expression of costimulatory molecules and production of IL-12.10 We show herein that CC chemokines produced during these antigen-specific interactions between CD4+ T cells and DCs also induce specific attraction of immature DCs to the site of interaction. This induced chemotaxis of immature DCs is accompanied by a complete remodeling of the DC actin cytoskeleton, which leads to dissolution of adhesion structures known as podosomes16 and to a drastic change of the morphology of DCs. Actin cytoskeleton remodeling also depends on chemokines suggesting that the disappearance of podosomes and the acquisition of migratory ability by DCs are linked.
Methods
Reagents and antibody
Medium: RPMI 1640 Glutamax, 1% pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen, Cergy Pontoise, France), and 10% FCS (Biowest Nauillé, France). Recombinant human IL-4 and GM-CSF were from BruCells (Anderlecht, Belgium). Recombinant bacterial superantigens were from Toxin Technology (Sarasota, FL). Bordetella pertussis toxin (PTX) and viral CC chemokine inhibitor (VCCI) were from Sigma-Aldrich (St Quentin Fallavier, France), chemokines (CCL3, CCL4, CCL5, CXCL8) from R&D Systems (Minneapolis, MN). Mouse mAbs against human CD4, CD69, CD40, CD80, CD83, CD86 and isotypic controls coupled to fluorochromes were from BD Biosciences (Le Pont de Claix, France). Alexa 488-conjugated F(ab′)2 antimouse Abs, CFSE (Carboxy-fluorescein diacetate succinimidyl ester), DiD (Vybrant DiD cell-labeling solution) and phalloidin coupled to Alexa 543 were from Molecular Probes (Cergy Pontoise, France).
Cells
Dendritic cells were generated from human monocytes of healthy donors as previously described.10 Informed consent was obtained in accordance with the Declaration of Helsinki. Briefly, anti-CD14+ monocytes were sorted positively using magnetic microbeads (Miltenyi Biotec, Paris, France). Monocytes were cultured for 5 days in medium supplemented with 100 ng/mL GM-CSF and 50 ng/mL IL-4. This protocol lead to 98% to 99% of CD1a+/CD14− DC presenting an immature phenotype.10 CD4+ T cells were negatively selected from peripheral blood mononuclear cells (PBMCs), after depletion of CD14+ cells, using the T-cell isolation kit II from Miltenyi Biotec. Sorted CD4+ T cells were 97%-99% CD4+/CD3+.
Preparation of active supernatants
Dendritic cells (1.5 × 105) were cocultured with 3 × 105 T cells in 24-well plates in the presence or absence of superantigen. Supernatants were collected and filtered 24 hours later.
T-cell activation assay and flow cytometric analysis
Dendritic cells were left untreated or pretreated with 200 ng/mL PTX for 2 hours, extensively washed and cocultured overnight with CD4+ T cells in flat or round-bottom 96-well plates.
Cells were stained with APC-coupled anti-CD69 and fluorescein isothiocyanate (FITC)-coupled anti-CD4 mAbs at 4°C for 30 minutes. Samples were analyzed on a FACSCalibur using CellQuest software 5.2.1 (BD Biosciences).
Migration experiments
Chemotaxis of DCs was measured by migration through a polycarbonate filter of 5 μm pore size in transwell chambers (Corning Costar, Lowell, MA). DCs (2 × 105, 150 μL) were seeded in the upper chamber, and control medium or stimuli (250 μl of supernatants or recombinant chemokines at 100 ng/mL) were added to the lower chamber. In some experiments, DCs were pretreated with 200 ng/mL PTX (2 hours at 37°C) or CCI (20 minutes, room temperature) before the assay. After 2 hours at 37°C, 105 beads (Beckman Coulter, Villepinte, France) were added to all lower wells and the number of cells for a given number of beads were counted by flow cytometry. Values are given as percentage of cells in the lower well plus or minus standard deviation (SD).
For the Dunn chamber analysis, both annular wells of the Dunn chamber were filled with control medium and the poly-L-lysin coated coverslip seeded with DCs was inverted onto the chamber. A narrow filling slit at one edge was left to access the outer well. To set up a chemotactic gradient, medium was drained from the outer well and replaced with medium containing the chemoattractant. The slit was sealed with nail polish.17
Cells were left for 20 to 30 minutes in the Dunn chamber before being visualized with a Leica DMI 6000 B (Wetzlan, Germany) under 10× phase contrast objective. Images were captured at 1-minute intervals and analyzed with Metamorph software (Universal Imaging, Downingtown, PA). Average rates of cell movement were calculated based on the total movement of cell centroid over the time of observation.
Directionality of cell movement was analyzed using scatter diagrams of cell displacement. The diagrams were oriented so that the position of the outer well of the chamber was vertically upward (y direction). Each line represented the trajectory of one cell during the recording period (90 minutes) where the starting point of the migration was fixed at the intersection of the 2 axes.
Cytokine detection in the supernatant
Chemokine secretion was measured in the supernatants by cytometric bead array (BD Biosciences).
Flow cytometric analysis of chemokine production
Dendritic cells were cocultured overnight with CD4+ T cells in flat-bottom 96-well plates. Cells were then stained with APC-coupled anti-CD4 and Cy-Chrome–coupled anti-CD1a mAbs (BD Biosciences). After surface labeling cells were fixed, permeabilized, and stained for intracellular cytokine production with PE-coupled anti-CCL3, anti-CCL5, or FITC-coupled anti-CCL4 (R&D Systems). Isotypic controls were used as negative controls. Samples were analyzed on a FACSCalibur (BD Biosciences).
Video imaging
Videomicroscopy was performed using a Leica DM IRBE epifluorescence microscope equipped with a 63×/1.32 numeric aperture objective, a cooled charge-coupled device camera (Coolsnap EZ; Photometrics, Kew, Australia for videos S1,2; MicroMax, Princeton Instruments (Evry, France) for videos S3,4 [available on the Blood website; see the Supplemental Materials link at the top of the online article]). Poly-L-lysin coverslips covered with DCs were placed into a chamber on the microscope at 37°C in a 5% CO2 atmosphere. At time 0, T cells were added, and images were recorded every 3 minutes for 5 hours. Data acquisition and analysis were done with MetaMorph v.6.1 software (Universal Imaging).
Confocal microscopy
Dendritic cells and T cells were cocultured on coverslips. In some experiments, cells were stained with CFSE or DiD before culture according to manufacturer's instructions.
After coculture, cells were fixed with 3% paraformaldehyde (Carlo Erba, Val de Reure, France) and incubated in phosphate-buffered saline (PBS) glycine (10 mM) to quench free aldehyde groups. Cells were then permeabilized with 0.1% Triton, stained first with antivinculin Ab (Sigma-Aldrich) and then with Alexa 488–conjugated F(ab′)2 antimouse Ig. F-actin was visualized using Alexa 543–conjugated phalloidin. Coverslips were mounted onto glass slides using Fluoromount-G (Southern Biotechnology Associates, Montrouge, France). Green and red fluorescence were acquired sequentially to prevent leakage of fluorescence from one channel into another.
Images were collected using a Leica TCS SP2 confocal scanning microscope equipped with a 63×/1.4 numeric aperture oil-immersion objective. All quantifications were performed blindly on 8-bit images using MetaMorph software.
Results
Antigen-specific contacts between CD4+ T cells and DCs induce DC mobility and recruitment toward the sites of DC–T-cell interaction
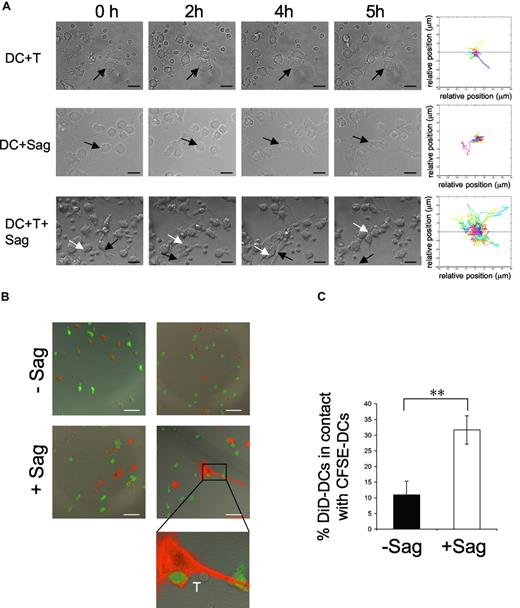
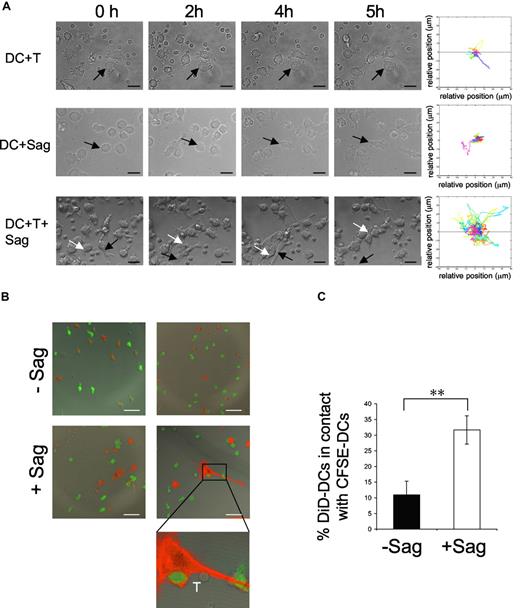
We followed by time-lapse videomicroscopy the first hours of interaction between human immature DCs and CD4+ T lymphocytes. Immature DCs were preincubated with a cocktail of superantigens (TSST1 + SEE + SEA + SEC + SED) and plated on glass coverslips. CD4+ T cells were then added and every 3 minutes a series of 4 xy plane contrast phase images was acquired for 5 hours. In the presence of CD4+ T cells alone, or superantigens alone, DCs neither changed their morphology nor became mobile (Figure 1A; Videos S1,S2). Moreover, as previously described,18 DCs established few interactions with T cells in the absence of antigenic stimulation (Figure 1A; Videos S1). In sharp contrast, in the presence of both T cells and superantigens, immature DCs became mobile, as shown by video and quantification of the images (Figure 1A; Video S3). This DC mobility was accompanied, after 2 to 3 hours of interaction with CD4+ T cells, by striking morphologic changes of the DCs, which acquired an elongated shape, extending and retracting long dendrites. Activated DCs established contacts with CD4+ T cells but also with other DCs, forming dynamic clusters of CD4+ T cells and DCs.
DC mobility increases after contact with T cells and superantigen. Dendritic cells (DCs; 105) were exposed to superantigen (Sag) and seeded on polylysin-coated coverslips. T cells were added (3 × 105) before image recording. Images were recorded every 3 minutes for 5 hours. (A) Still images taken from Videos S1,S2,S3 (DC + T cells, DC + Sag, and DC + T + Sag, respectively). Arrows point to individual DCs. Bar, 20 μm. Right panels, mobility tracks of all DCs present in the fields from Videos S1,S2,S3 are shown. Results are representative of 3 experiments. (B,C) DCs are recruited to the sites of DC–CD4+T cell interaction. DCs stained with DiD (red) pulsed with TSST1 (+ Sag) or left unpulsed (-Sag), were seeded on coverslips together with unpulsed immature CFSE-labeled DCs (green) and CD4+ T cells and cultured for 6 hours. Cells were then fixed and coverslips mounted for confocal microscopy. (B) Representative images are shown. Bar, 40 μm (C) Percentages of DiD-DC in contact with CFSE-DC. Results represent means plus or minus SD of 3 independent experiments (30-40 conjugates analyzed per experiment). Significance assessed by Student unpaired t test (**P < .005).
DC mobility increases after contact with T cells and superantigen. Dendritic cells (DCs; 105) were exposed to superantigen (Sag) and seeded on polylysin-coated coverslips. T cells were added (3 × 105) before image recording. Images were recorded every 3 minutes for 5 hours. (A) Still images taken from Videos S1,S2,S3 (DC + T cells, DC + Sag, and DC + T + Sag, respectively). Arrows point to individual DCs. Bar, 20 μm. Right panels, mobility tracks of all DCs present in the fields from Videos S1,S2,S3 are shown. Results are representative of 3 experiments. (B,C) DCs are recruited to the sites of DC–CD4+T cell interaction. DCs stained with DiD (red) pulsed with TSST1 (+ Sag) or left unpulsed (-Sag), were seeded on coverslips together with unpulsed immature CFSE-labeled DCs (green) and CD4+ T cells and cultured for 6 hours. Cells were then fixed and coverslips mounted for confocal microscopy. (B) Representative images are shown. Bar, 40 μm (C) Percentages of DiD-DC in contact with CFSE-DC. Results represent means plus or minus SD of 3 independent experiments (30-40 conjugates analyzed per experiment). Significance assessed by Student unpaired t test (**P < .005).
These results show that antigen-specific interactions between CD4+ T cells and DCs induce mobility, morphologic changes, and clustering of DCs.
We then tested whether the acquired DC mobility support their attraction to the site of ongoing DC–T-cell interactions.
To do so, we designed an experiment to visualize the recruitment of immature DCs to the sites of superantigen-specific DC–CD4+ T-cell interactions. DC preparations were separated in 2; one population was stained with the fluorescent lipid DiD and pulsed or not with TSST1 and the other was CFSE-labeled and was not exposed to superantigen. After extensive washing, both populations were seeded on coverslips. CD4+ T cells were then added. Six hours later, coverslips were analyzed by confocal microscopy. Image quantification showed that in the absence of antigen about 10% of DiD-labeled DCs were in contact with CFSE-labeled DCs, whereas in the presence of superantigen, the number of DiD-DC/CFSE-DC interactions was significantly increased (to 31%; Figure 1B,C). These results show that neighboring immature DCs, which did not directly interact with T cells, are recruited to sites of antigen-specific DC-CD4+ T-cell interactions.
Chemokines produced during superantigen-specific DC-CD4+ T-cell interactions induce chemotaxis of immature DCs
We then asked if soluble factors produced during superantigen-induced DC-CD4+ T-cell interactions were sufficient to trigger DC mobility and morphologic changes. Immature DCs were exposed to supernatants from cocultures of DC plus CD4+ T cells plus superantigens and followed by videomicroscopy. As shown in Figure 2A and Video S4, DCs became rapidly mobile and acquired an elongated morphology. This behavior was similar to that observed in the presence of T cells.
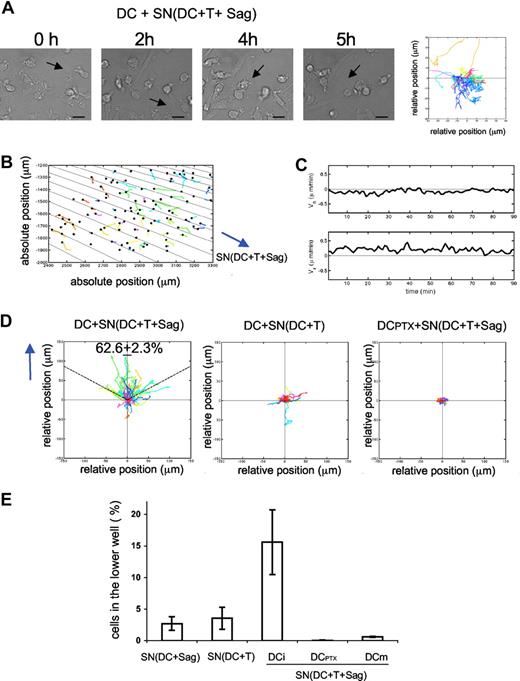
Chemokines produced during DC–T-cell interactions induce DC chemotaxis. (A,B) DCs were seeded on coverslips and exposed to supernatants from 24-hour cocultures of DC plus T cells plus superantigens. Videomicroscopy was performed as in Figure 1. (A) Individual frames from Video S4 and corresponding DC mobility tracks are shown; the results are representative of 4 experiments. Bar, 20 μm. (B-D) Dunn chamber assay. (B) Migration tracks of DCs in a gradient of supernatant from DC plus T cells plus superantigens (one representative experiment). DC individual trajectories in the chamber are represented in colors and direction of the gradient is figured with black lines. (C) Mean horizontal and radial velocity of DCs. (D) DC trajectories are plotted in a scatter diagram with the starting point for each cell at the intersection between the x- and y-axes, and the direction of the gradient vertical upwards. Left panel: DCs exposed to “active” supernatants as in panel B. Percentage of cells that ended up within a 120° arc facing the supernatant source is indicated (mean ± SD of 3 independent experiments with ≥ 400 cells tracked). Middle panel: DCs exposed to supernatant from DC plus T cells. Right panel: PTX-treated DCs exposed to “active” supernatant. (E) Transwell chemotaxis assays. Untreated immature DCs or DCs treated with PTX (DCPTX) or LPS-treated DCs (DCm) were placed in the upper chamber of a transwell, and supernatants from the indicated 24-hour cocultures in the lower chamber. Percentages of DCs attracted to the lower chambers after 2 hours are shown. Data are means plus or minus SD of 3 independent experiments.
Chemokines produced during DC–T-cell interactions induce DC chemotaxis. (A,B) DCs were seeded on coverslips and exposed to supernatants from 24-hour cocultures of DC plus T cells plus superantigens. Videomicroscopy was performed as in Figure 1. (A) Individual frames from Video S4 and corresponding DC mobility tracks are shown; the results are representative of 4 experiments. Bar, 20 μm. (B-D) Dunn chamber assay. (B) Migration tracks of DCs in a gradient of supernatant from DC plus T cells plus superantigens (one representative experiment). DC individual trajectories in the chamber are represented in colors and direction of the gradient is figured with black lines. (C) Mean horizontal and radial velocity of DCs. (D) DC trajectories are plotted in a scatter diagram with the starting point for each cell at the intersection between the x- and y-axes, and the direction of the gradient vertical upwards. Left panel: DCs exposed to “active” supernatants as in panel B. Percentage of cells that ended up within a 120° arc facing the supernatant source is indicated (mean ± SD of 3 independent experiments with ≥ 400 cells tracked). Middle panel: DCs exposed to supernatant from DC plus T cells. Right panel: PTX-treated DCs exposed to “active” supernatant. (E) Transwell chemotaxis assays. Untreated immature DCs or DCs treated with PTX (DCPTX) or LPS-treated DCs (DCm) were placed in the upper chamber of a transwell, and supernatants from the indicated 24-hour cocultures in the lower chamber. Percentages of DCs attracted to the lower chambers after 2 hours are shown. Data are means plus or minus SD of 3 independent experiments.
We next performed experiments to test if the mobility induced by “active” supernatants was due to chemotaxis (directional movement of cells) or chemokinesis (random cell movement) using a Dunn chemotaxis chamber. This system allows us to study the behavior of cells subjected to linear gradient of chemoattractants.17 Immature DCs were exposed to gradients of “active” supernatants in the Dunn chamber and imaged by time-lapse videomicroscopy under phase contrast. Migration tracks of the DCs were then quantitatively analyzed with a program developed in Matlab (Paris, France). Figure 2B shows cell trajectories from one representative experiment, and direction of the gradient is represented by black lines (the gradient is radial, because of the geometry of the chamber). In Figure 2D, the same trajectories are represented in a scatter diagram, with the gradient directed vertically upwards (y).
We calculated the percentage of migrating immature DCs, which ended up within a 120° arc facing the supernatant source. For totally random migration, we would expect to find 33% of the cells in this region. In contrast, when DCs were exposed to a gradient of “active” supernatant, 62.6% (± 2.3%) of the DCs were found within the 120° arc after 90 minutes of migration, showing a directional migration of DCs toward the supernatant source.
We further characterized the dynamic parameters of T cell–dependent DC migration by measuring the mean velocity of the DCs in the Dunn chamber. In Figure 2C, the radial and the horizontal velocity are plotted as a function of time. The radial velocity (Vr, component of the mean velocity along the gradient), is positive over time, and reaches 0.5 μm/min. In contrast, the values of the horizontal velocity (Vh, component perpendicular to the gradient), oscillate in a small range around zero, indicating that the horizontal cell displacement is random and limited. Control supernatants prepared from DCs cocultured overnight with T cells in the absence of superantigen did not induce any chemotaxis of DCs (Figure 2D middle panel).
We then used PTX, an inhibitor of Gαi protein–coupled receptors, which blocks all Gαi-coupled chemokine receptors, to test the involvement of chemokines. DCs were pretreated with 200 ng/mL PTX for 2 hours and extensively washed before imaging their migration in the Dunn chamber. As shown in Figure 2D right panel, PTX pretreatment of DCs inhibited chemotaxis induced by “active supernatants.”
To confirm that soluble factors secreted during T-DC contacts could promote immature DC migration, we performed transwell chemotaxis assays.19 Medium or supernatants from different cocultures were placed in the lower chamber of the transwell system, and immature DCs were added to the upper chamber. As presented in Figure 2E, “active” supernatants induced significant migration of immature DCs to the lower chamber, whereas supernatants from cocultures of DC plus CD4+ T cells or of DC plus superantigens alone did not. PTX treatment completely abrogated the “active” supernatant-induced DC migration, confirming the results obtained in the Dunn chamber (Figure 2E). Of note, mature DCs obtained by overnight lipopolysaccharide (LPS) treatment did not migrate toward “active” supernatants (Figure 2E), whereas as previously shown20 they migrated toward CCL19 and CCL21 (data not shown), showing that “active” supernatants specifically induced the migration of immature DCs.
Altogether, these results demonstrate that chemokines produced during superantigen-specific interaction of CD4+ T cells with DCs induce chemotaxis in immature DCs, supporting the recruitment of fresh immature DCs toward the site of T cell–DC specific interaction.
CC chemokines produced by DC-CD4+ T-cell conjugates induce migration of immature DCs
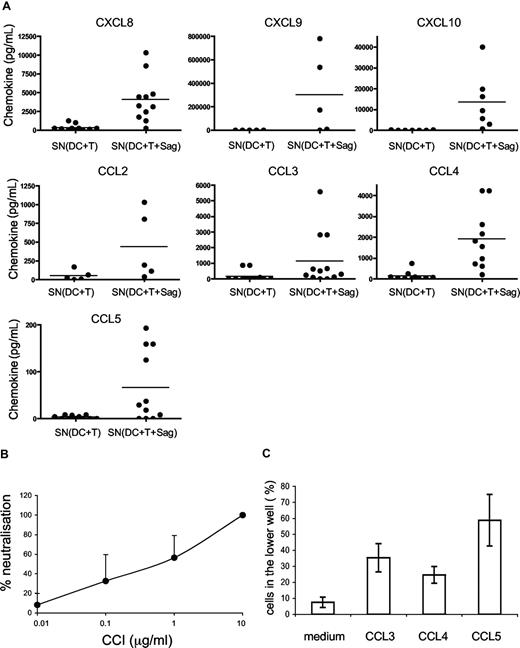
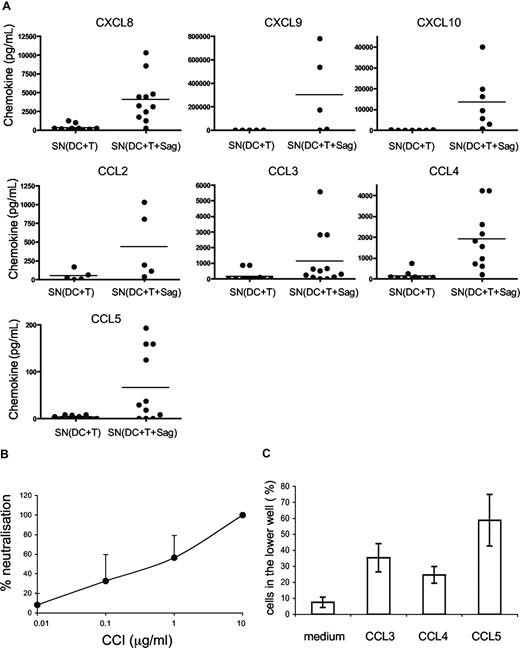
We then studied which chemokines were produced in supernatants of CD4+ T cells cocultured with DCs or DCs pulsed with TSST1, by cytometric bead arrays. As shown in Figure 3A, the following chemokines were secreted in the cocultures of DCs and T cells: CXCL8, CXCL9, CXCL10, CCL2, CCL3, CCL4, CCL5. Most of these chemokines were secreted only in the presence of superantigen.
DC migration is induced by CC chemokines. (A) Chemokines secreted in the cocultures of DC plus T cells with or without TSST1 are quantified by cytometric bead arrays. Each dot represents one donor; mean of chemokine secretion is indicated in each column by the horizontal bar. (B) CCI inhibits DC migration. DCs were pretreated with different concentrations of CCI and migration toward the active supernatant was analyzed. Percentages (mean ± SD) of neutralization of DC migration is calculated as follows: 100 − ((% of CCI-treated DCs in lower chamber/ % of untreated DCs in the lower chamber) × 100). (C) Percentage of DCs attracted toward different recombinant chemokines in transwell chambers are shown. Means plus or minus SD of triplicates from one representative experiment are shown.
DC migration is induced by CC chemokines. (A) Chemokines secreted in the cocultures of DC plus T cells with or without TSST1 are quantified by cytometric bead arrays. Each dot represents one donor; mean of chemokine secretion is indicated in each column by the horizontal bar. (B) CCI inhibits DC migration. DCs were pretreated with different concentrations of CCI and migration toward the active supernatant was analyzed. Percentages (mean ± SD) of neutralization of DC migration is calculated as follows: 100 − ((% of CCI-treated DCs in lower chamber/ % of untreated DCs in the lower chamber) × 100). (C) Percentage of DCs attracted toward different recombinant chemokines in transwell chambers are shown. Means plus or minus SD of triplicates from one representative experiment are shown.
To better characterize which chemokines were involved in DC migration, we used vCCI (viral CC chemokine inhibitor), a viral component that specifically inhibits chemokine receptors belonging to the CC family.21,22 As expected, CCI inhibited DC migration toward CCL5, but not toward CXCL12 (Figure S1). Pretreatment of DCs with CCI inhibited dose-dependently DC migration toward “active supernatants” (Figure 3B) showing that CC chemokines are involved in the migration of immature DCs.
We thus tested the CC chemokines present in the “active” supernatants for their ability to induce immature DC migration. As shown in Figure 3C, CCL3, CCL4, and CCL5 induced migration of immature DCs in the transwell assay.
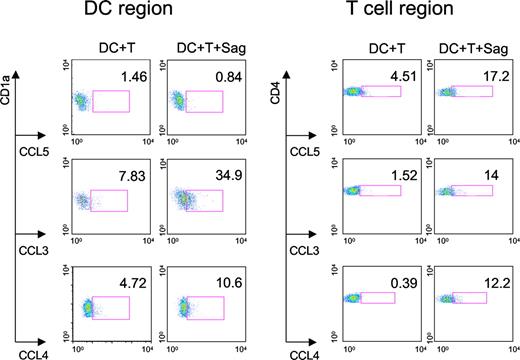
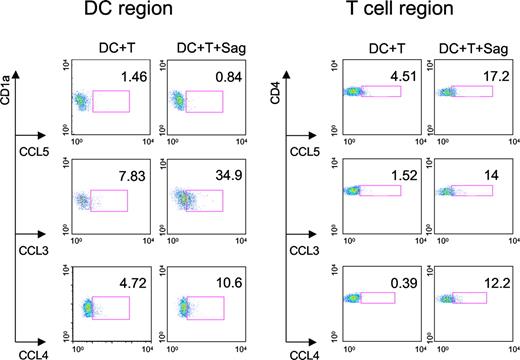
We then characterized the cell types that produce these 3 CC chemokines. Intracellular chemokine stainings were performed on CD4+ T cells and DCs cocultured overnight with TSST1. As shown in Figure 4, both DCs and T cells produced CCL3 and CCL4, whereas CCL5 was only detected in T cells.
Production of CC chemokines by DCs and CD4+ T cells. DCs and CD4+ T cells were cocultured with or without TSST1 (Sag) for 24 hours. FACS analysis was performed to detect intracellular chemokine production (CCL3, 4, and 5) in DCs gated on CD1a expression and CD4+ T cells gated on CD4 expression. Percentage of DCs or T cells producing chemokines are indicated in each panel. One representative experiment of 3 is shown.
Production of CC chemokines by DCs and CD4+ T cells. DCs and CD4+ T cells were cocultured with or without TSST1 (Sag) for 24 hours. FACS analysis was performed to detect intracellular chemokine production (CCL3, 4, and 5) in DCs gated on CD1a expression and CD4+ T cells gated on CD4 expression. Percentage of DCs or T cells producing chemokines are indicated in each panel. One representative experiment of 3 is shown.
Altogether, these results demonstrate that CC chemokines produced during antigenic stimulation by both CD4+ T cells and DCs induce migration of immature DCs.
Dendritic cell responses to chemokines enhance T-cell activation
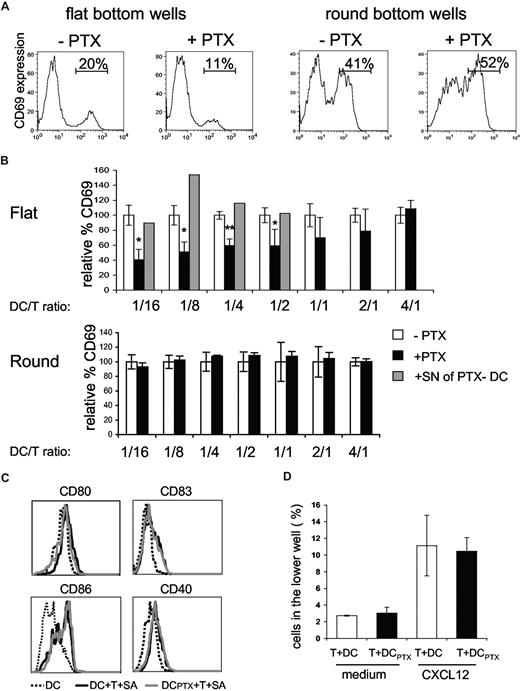
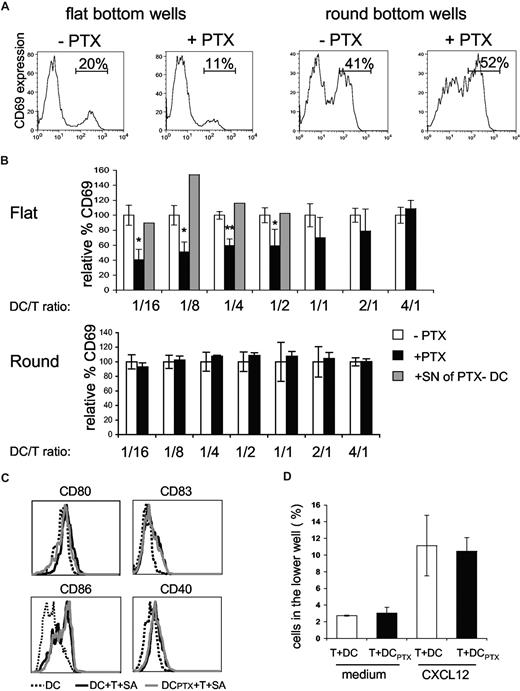
We then tested if DC responses to chemokines, produced during superantigen-induced interaction of CD4+ T cells with DCs, would influence T-cell responses in return. To do so we cultured overnight CD4+ T cells together with untreated or PTX-pretreated DCs at different ratios in the presence of TSST1. We then followed CD69 expression by T cells (Figure 5A,B).
Pertussis toxin–treated DCs are impaired in the ability to activate CD4+ T cells. (A,B) CD4+T cells (4 ×104) were incubated with DCs, pulsed with 100 ng/mL of TSST1, pretreated (+PTX) or not (−PTX) with PTX. CD69+ expression on CD4+ T cells was analyzed by FACS. (A) Histograms of CD69 expression on CD4+ T cells cocultured with DCs at a DC/T ratio of 1:4 in round or flat-bottom wells; percentages of CD69+/CD4+ T cells are indicated. (B) For each DC/T cell ratio, data were normalized to the percentage of CD69+/CD4+ T cells obtained in the cocultures of untreated DCs (= 100%). Means and SD of 3 experiments are shown. For the indicated ratio, overnight supernatants from PTX-pretreated DCs were added to the cocultures (▒) (C) Flow cytometric analysis of DC maturation markers in the flat-bottom cocultures. (D) CD4+ T cells were added to the upper chamber of a transwell system together with untreated or PTX-treated DCs (DCPTX) and migration toward CXCL12 was analyzed. Significance was assessed by Student unpaired t test (*P < .05; **P < .005). Error bars represent SD.
Pertussis toxin–treated DCs are impaired in the ability to activate CD4+ T cells. (A,B) CD4+T cells (4 ×104) were incubated with DCs, pulsed with 100 ng/mL of TSST1, pretreated (+PTX) or not (−PTX) with PTX. CD69+ expression on CD4+ T cells was analyzed by FACS. (A) Histograms of CD69 expression on CD4+ T cells cocultured with DCs at a DC/T ratio of 1:4 in round or flat-bottom wells; percentages of CD69+/CD4+ T cells are indicated. (B) For each DC/T cell ratio, data were normalized to the percentage of CD69+/CD4+ T cells obtained in the cocultures of untreated DCs (= 100%). Means and SD of 3 experiments are shown. For the indicated ratio, overnight supernatants from PTX-pretreated DCs were added to the cocultures (▒) (C) Flow cytometric analysis of DC maturation markers in the flat-bottom cocultures. (D) CD4+ T cells were added to the upper chamber of a transwell system together with untreated or PTX-treated DCs (DCPTX) and migration toward CXCL12 was analyzed. Significance was assessed by Student unpaired t test (*P < .05; **P < .005). Error bars represent SD.
When DCs were pretreated with PTX, the percentages of activated CD4+ T cells were significantly lower than the percentages obtained with untreated DCs, for DC/T cell ratios less than 1:2, indicating that DC response to chemokines increases T-cell activation. In contrast, for DC/T ratio more than 1:2, PTX treatment of DCs had not significant effects, suggesting that DC response to chemokines is less critical at high cell concentration.
To confirm our interpretation, we performed similar experiments in round-bottom wells, wherein contacts between cells are forced by the geometry of the well. Under these conditions, PTX pretreatment of DCs did not affect T-cell activation.
We have previously shown that DCs cocultured with CD4+T cells and superantigen expressed higher amounts of CD40, CD80, CD83, and CD86.10 This enhanced expression of costimulatory molecules may influence T-cell activation. We thus tested if PTX pretreatment of DCs inhibited the T cell–driven DC maturation. As shown in Figure 5C, PTX treatment did not inhibit DC maturation, demonstrating first that PTX does not grossly perturb DC function and second that the inefficiency of PTX pretreated DCs to activate T cells is not due to a lesser ability to mature.
Because T cells are highly sensitive to PTX, we controlled that PTX-treated DCs did not release PTX in the supernatants, which could directly affect T-cell activation. We thus added overnight supernatants from PTX-pretreated DCs to cocultures of untreated DC plus CD4+ T cells plus TSST1. These supernatants did not inhibit T-cell activation (Figure 5B gray histograms), showing that PTX was not released in the medium. We also tested if direct contact of PTX-pretreated DCs with T cells could affect T-cell migration. PTX-pretreated DCs were thus added to the upper well of a transwell together with T cells and migration toward CXCL12, which has been shown to be PTX sensitive,23 was measured after 4 hours and 30 minutes. T-cell migration toward CXCL12 was not affected by PTX-treated DCs (Figure 5D).
Overall our results strongly suggest that chemokine-induced DC modification plays a role in T-cell activation.
The actin cytoskeleton of immature DCs is modified by cognate interaction with CD4+ T cells, and this depends on chemokines
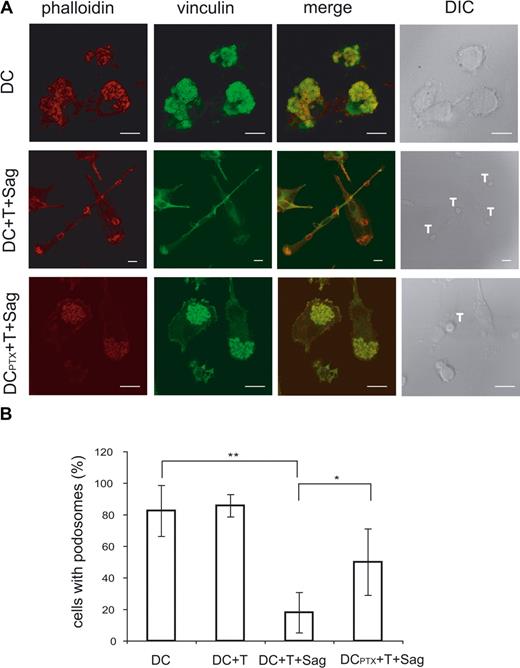
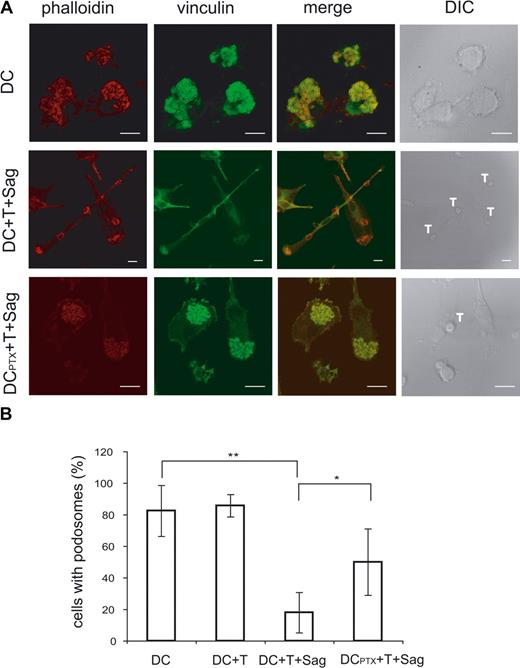
As shown in Figure 1 and Videos S3 and S4, the T cell–induced mobility of DCs was accompanied by striking morphologic changes of the DCs. To further characterize these changes and gain some insights into the mechanisms involved in mobility of immature DCs, we studied the modifications of the DC cytoskeleton induced by CD4+ T cells. Because podosomes have been involved in DC mobility,24,25 we followed these structures in DCs exposed to CD4+ T cells in the presence or absence of superantigen. When plated on glass coverslips, most immature DCs presented the characteristic podosomes, composed of an actin core surrounded by vinculin, on their ventral face (80%, mean of 4 independent experiments, Figure 6A). The percentage of DC-presenting podosomes drastically decreased from 80% to 20% in DCs exposed to antigen-specific interaction with CD4+ T cells. Moreover, when exposed to T cells in the presence of superantigen, DCs showed an organization of the polymerized actin in filaments and actin enrichment at the contact with T cells (Figure 6A).
Ag-specific interactions between CD4+ T cells and DCs induce podosome dissolution in DC. (A) Untreated or PTX-treated DCs were cocultured with CD4+T cells on coverslips in the presence or absence of TSST1. Cells were then fixed and labeled with antivinculin Ab (green) and phalloidin (red). Bar, 10 μm. (B) DC-presenting podosomes were quantified, plotted to the total number of cells present in the field, and presented as percentages. Means plus or minus SD of 4 independent experiments are shown; at least 25 total cells were examined for each condition in each experiment. Significance was assessed by Student unpaired t test (*P < .05; **P < .005).
Ag-specific interactions between CD4+ T cells and DCs induce podosome dissolution in DC. (A) Untreated or PTX-treated DCs were cocultured with CD4+T cells on coverslips in the presence or absence of TSST1. Cells were then fixed and labeled with antivinculin Ab (green) and phalloidin (red). Bar, 10 μm. (B) DC-presenting podosomes were quantified, plotted to the total number of cells present in the field, and presented as percentages. Means plus or minus SD of 4 independent experiments are shown; at least 25 total cells were examined for each condition in each experiment. Significance was assessed by Student unpaired t test (*P < .05; **P < .005).
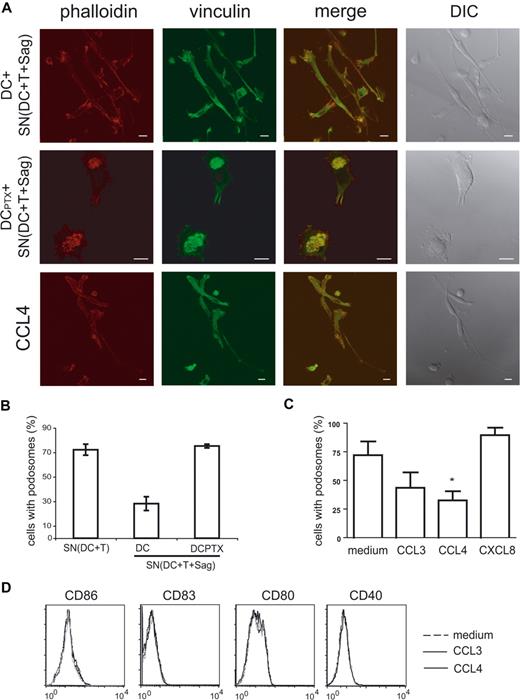
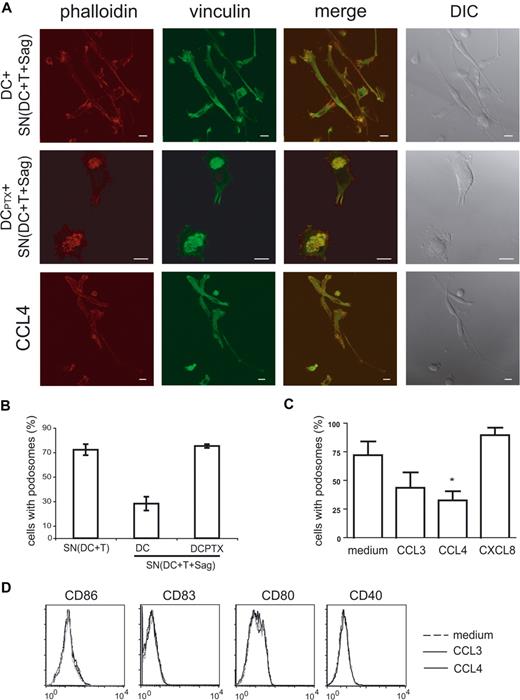
We then studied the cytoskeleton of immature DCs treated for 6 hours with “active” supernatants. As shown in Figure 7A and quantified in Figure 7B, the percentage of DC-presenting podosomes was drastically reduced in DCs submitted to “active” supernatants (28% vs 75%).
Chemokines induce podosome dissolution in DCs. (A) Untreated or PTX-treated DCs were exposed to the indicated supernatants, or to recombinant chemokines (CCL3, CCL4), and then labeled with antivinculin Ab (green) and phalloidin (red). Bar, 10 μm. (B,C) Cells presenting with podosomes were quantified as in Figure 6. Means plus or minus SEM of 4 independent experiments are shown; at least 25 total cells were examined for each condition in each experiment. Significance was assessed by Student paired t test (*P < .05). (D) Flow cytometric analysis of DC maturation markers in response to chemokines.
Chemokines induce podosome dissolution in DCs. (A) Untreated or PTX-treated DCs were exposed to the indicated supernatants, or to recombinant chemokines (CCL3, CCL4), and then labeled with antivinculin Ab (green) and phalloidin (red). Bar, 10 μm. (B,C) Cells presenting with podosomes were quantified as in Figure 6. Means plus or minus SEM of 4 independent experiments are shown; at least 25 total cells were examined for each condition in each experiment. Significance was assessed by Student paired t test (*P < .05). (D) Flow cytometric analysis of DC maturation markers in response to chemokines.
We then examined the role of chemokines on this striking remodeling of the actin cytoskeleton. As shown in Figures 6 and 7, pretreatment of DCs with PTX inhibited podosome dissolution induced by T cells (Figure 6A,B) or “active” supernatants (Figure 7A,B), strongly suggesting that chemokines are involved in the modifications of DC cytoskeleton induced by T cells. To confirm the role of chemokines in these modifications, we treated immature DCs with several CC chemokines and studied the modification of the cytoskeleton. As shown in Figure 7A and quantified in Figure 7C, CCL3 and CCL4 induced podosome dissolution and morphologic changes in immature DCs that were very similar to the modifications observed with the “active” supernatants. In contrast, CXCL8, which binds CXCR1 expressed by immature DCs (data not shown), did not significantly decrease the number of DCs presenting with podosomes (Figure 7C).
Some chemokines have been shown to induce DC maturation26-28 and DC maturation has been shown to induce podosome dissolution.25,29 We thus tested if CCL3 and CCL4, which induced podosomes dissolution (Figure 7A-C), also induced DC maturation. As shown in Figure 7D, no increase of CD86, CD83, CD80, and CD40 expression was observed, although as shown in Figure 5C “active” supernatants did induce increased expression of these markers. These results show that podosome disassembly induced by CC chemokines does not require DC maturation.
Altogether, these results demonstrate that chemokines produced during antigen-specific interaction between CD4+ T cells and DCs induce actin cytoskeleton remodeling in immature DCs, leading to podosome dissolution.
Discussion
Dendritic cells are key cells in the regulation of T-cell responses. Their efficiency depends on their state of activation, which regulates DCs' ability to process antigen and to express costimulatory molecules and cytokines.1 Activation of DCs also controls DC migration to the appropriate sites, in tissues or lymphoid organs, wherein they prime T cells.30,31 CD4+ T cell–dependent signals have been shown to play a crucial role in DC activation by inducing their licensing to prime CTL or their ability to polarize TH cell responses (reviewed in Behrens et al32 and de Jong et al33 ). CD4+ T cell–dependent signals induce expression by DCs of costimulatory markers as well as production of cytokines.8-10 Regulated migration of DCs, implying both migration of DCs from the periphery to the T-cell zones of lymphoid organs and migration inside the tissues or lymphoid organs, is central to the induction of physiologic immune responses and probably to the maintenance of tolerance.34,35 We thus addressed the question of the potential modulation by human CD4+ T cells of DC migratory ability.
We show herein that CC chemokines produced at sites of CD4+ T cell–DC interaction induce recruitment of immature DCs and formation of DC clusters at these sites. Some of these CC chemokines are produced both by DCs and CD4+ T cells (Figure 4), suggesting that both cell types contribute to the attraction of immature DCs to the site of interaction.
Because the chemokine receptors CCR1, CCR2, CCR3, CCR5, and CCR6 are expressed by immature DCs,36,37 they may be involved in the chemotaxis we report herein. CCR6 can be excluded because its ligand CCL20 did not induce migration of immature DCs (data not shown). The role of CCR7 can also be excluded because, as reported by others,37 the immature DCs we used in this study did not express any mRNA for CCR7, even 4 hours after exposure to the supernatants, and did not migrate toward CCL19 or CCL21 (data not shown). It is worth noting that the chemokines present in our conditioned supernatants did not induce any migration of LPS-matured DCs (Figure 2E), showing that chemokines produced during antigen-specific interaction of DCs with CD4+ T cells specifically induced mobility of immature DCs.
Time lapse and confocal microscopy images of DCs that have been interacting for 6 to 18 hours with T cells in the presence of superantigens show striking changes of the DCs' morphology, which correlated with the acquisition of their migratory ability. In these conditions, 25% to 30% of the immature DCs elongated, changing from a roundish shape of 10 to 15 μm in diameter to a “neuron-like” shape of 50 to 80 μm in length. These modifications were also CC chemokine–dependent and were not observed when immature DCs were exposed to LPS or poly IC (data not shown) although these TLR ligands induced nonpolarized extension of thin transient dendrites around the cellular body of DCs. These elongated DCs established contact with both CD4+ T cells and other DCs (Figures 1,6,7) and tended to form a network of interdigitating DCs after 4 to 6 hours of antigen-specific contact with T cells. This chemokine-induced DC-DC interaction may favor the transfer of material between DCs, which has recently been shown to amplify T cell responses.38
Morphologic changes observed in immature DCs were accompanied by striking modifications of the actin cytoskeleton. As previously reported,29,39 the majority of immature DCs displayed big clusters of podosomes on their ventral face. When cocultured with CD4+ T cells and superantigen or with “active supernatants,” DCs showed actin filaments along the plasma membrane, as well as polymerized actin-rich “cups” in the contact zones with T cells, but only sparse podosomes on both ends (Figures 6,7). Podosomes are highly dynamic structures consisting of a dense actin core surrounded by a ring of vinculin found on several cells from the myeloid lineage.40 These structures have been shown to be involved in cell migration, tissue invasiveness,41 and diapedesis.42 We show herein a strong inverse correlation between the presence of podosomes and DC migration. Indeed, as reported by others, podosomes expressed by immature DCs may restrict their speed of migration by increasing their interaction with the substrate.25
In our study, we show for the first time that chemokines, which induce DC migration, also induce podosome disassembly. Several maturation-inducing agents such as LPS,29,43 PGE2, and TNF-α,25 have been shown to induce podosome dissolution, suggesting that this dissolution is an integral part of the DC maturation process. Results reported herein suggest that these 2 phenomena can be dissociated because PTX pretreatment of DCs blocks podosome dissolution but does not affect phenotypic DC maturation (Figure 5C), whereas CC chemokines induce podosome dissolution without inducing DC maturation (Figure 7D).
Yet the resulting redistribution of the actin pool trapped in podosomes toward different sites may be required for mature DC functions, such as the maturation of endosomes44 or the recruitment of transmembrane receptors to the contact zone with interacting T lymphocytes.45
Chemokines can enhance and modulate T-cell activation by DCs in more than one fashion. DC presentation of cognate antigen to CD4+ T cells in lymph nodes induces the local production of CCR5 ligands, which attract CD8+ T cells nearby DC-CD4 conjugates, thus facilitating T-cell help.46 Chemokines also modulate T-cell response independently of their chemoattractant activities47 (ie, they bind DC membranes inducing T-cell adhesion48 and may induce DC maturation).26-28 Our data suggest that chemokines produced during DC presentation of cognate antigen to CD4+ T cells may also increase T-cell response by modifying immature DCs.
Indeed, at low cell density PTX-pretreated DCs are less efficient than untreated DCs at inducing T-cell activation (Figure 5). This lower ability of PTX-treated DCs to induce T-cell activation may be due to their inability to spread (Figures 6,7), thus offering less surface of contact with T lymphocytes. Alternatively, it may be due to their defective mobility. This decreased activation of CD4+ T cells was not due to “bystander” effect of PTX released in the medium on T cells (Figure 5B,D). Moreover, it was not due to an absence of secretion of chemokines known to attract T cells, because the same amount of CCL3 and CCL4 was present in supernatants from cocultures of PTX-treated DCs or untreated DCs with CD4+ T cells and superantigen (data not shown). Finally, it was not related to an inhibitory effect of PTX on DC maturation because PTX-treated DCs or untreated DCs cocultured with T cells and superantigen show a comparable increased expression of CD40, CD80, CD83, and CD86 (Figure 5C).
Altogether, our data suggest the following model: antigen-specific interaction between CD4+ T cells and DCs induces the secretion of chemokines by both cell types. This will attract more immature DCs, providing a source of “fresh” nonexhausted DCs that still have the ability to capture antigen and produce IL-12. These findings may be physiologically relevant in different settings. First in inflammatory tissue, Ag-specific interactions of memory or effector T cells with DCs will induce production of chemokines and cytokines that will attract and induce maturation of surrounding or circulating immature DCs. These newly recruited DCs will capture the Ag (from the environment or from the mature DCs), migrate to the draining lymph node, and constitute a new pool of Ag-presenting DCs. Second, in the secondary lymphoid organs, recruitment of some of the numerous resident DCs to sites of antigen-specific DC–T cell interaction may be crucial for the inter-DC Ag transfer recently reported.38 This transfer of Ag to resident DCs would be induced by T-cell signals and accompanied by a T cell–dependent maturation of these resident DCs that are mostly immature at the steady state.49 This would in turn increase T-cell activation by amplifying Ag presentation through a larger network of resident DCs. Our data are compatible with such a model because as shown herein, chemokines produced by antigen-specific DC-CD4+ T cell conjugates induce mobility of DCs as well as direct contact between DCs, potentially enabling transfer of material between DCs. Third, even in the absence of Ag transfer between DCs, efficient self-presentation by newly recruited resident DCs that will also receive T cell–derived activation signal may increase the activation of T cells responding to limiting amount of foreign antigen.50
We are currently developing in vivo models to test these hypotheses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank P. Guermonprez, C. Théry, A.M. Lennon and S. Hugues for critical reading of the manuscript and/or discussion; F. Waharte for assistance with microscopy imaging; and E. Labruyère (Institut Pasteur, Paris) for help with the Dunn Chamber assay.
This work was supported by grants from Institut Curie, Inserm, ARC (Association pour la Recherche contre le Cancer). C. N. is a fellow of FRM (Fondation pour la Recherche Medicale).
Authorship
C.N. performed research, analyzed and interpreted data, and drafted the manuscript; M.L., M.T., S.D., and F.M. performed research; K.C. analyzed data; G.O. contributed vital new reagents or analytical tools; S.A. analyzed and interpreted data; and C.H. designed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Claire Hivroz, Inserm Unité 653, Institut Curie, 26 Rue d'Ulm, 75248 Paris Cedex 05, France; e-mail: claire.hivroz@curie.fr.