Abstract
Intercellular adhesion molecules (ICAMs) bind to leukocyte β2 integrins, which, among other functions, provide costimulatory signals for T-cell activation. ICAM-5 (telencephalin) is expressed in the somadendritic region of neurons of the mammalian brain. The receptor for ICAM-5 is the integrin LFA-1, a major leukocyte integ-rin expressed in lymphocytes and microglia. In conditions of brain ischemia, epilepsy, and encephalitis, the soluble form of ICAM-5 (sICAM-5) has been detected in physiologic fluids. Here, we report that sICAM-5 attenuates the T-cell receptor-mediated activation of T cells as demonstrated by the decreased expression of the activation markers CD69, CD40L, and CD25 (IL-2R). This effect is most clearly seen in CD45ROLow (naive), and not in CD45ROHigh (memory) T cells, and is most effective early in priming, but not in the presence of strong costimulatory signals. Furthermore, sICAM-5 promotes the mRNA expression of the cytokines TGF-β1 and IFN-γ, but not TNF. The formation of sICAM-5 is promoted by activated T cells through the cleavage of ICAM-5 from neurons. This suggests that ICAM-5 is involved in immune privilege of the brain and acts as an anti-inflammatory agent.
Introduction
Inflammation is generally regarded as harmful to the brain because local and infiltrated immune cells, as well as proinflammatory cytokines, exacerbate the neuronal damage in various neurodegenerative and autoimmune diseases.1-4 Healthy brain prevents the penetration of lymphocytes into the brain parenchyma and also down-regulates the activity of infiltrated lymphocytes.5,6 Nevertheless, the brain is neither isolated nor passive in its interactions with the immune system.7 Once neuronal damage occurs, T cells and antigen-presenting cells (APC) accumulate at lesion sites, with concomitant increased expression of the major histocompatibility complex (MHC) and costimulatory molecules, which are necessary for T-cell activation.8-11 It is perceivable that a strict control of the activation of T cells is important to ameliorate their harmful effects on neurons
Optimal activation of T cells by APCs requires at least 2 signals. The first is elicited through recognition of the MHC-peptide complex by T-cell receptor (TCR), and the second is provided by the costimulatory molecules via interaction with their respective receptors on T cells. Costimulatory molecules are key factors required for full activation of the primed T cells, especially the naive T cells. Without costimulation, the primed T cells will turn into unresponsiveness or anergy.12-14
Among the costimulatory molecules, B7 (CD80 or 86), CD40, and intercellular adhesion molecule (ICAM)-1 have all been detected at inflammatory sites in brain.8,10,11 ICAM-1 is known to promote the activation of T cells through binding to its integrin receptor lymphocyte function associated antigen-1 (LFA-1, αLβ2, CD11a/CD18).15-17 LFA-1 and ICAM-1 are involved in the formation of the adhesion ring junction or the peripheral supramolecular activation complex at the immunologic synapse between T cells and APCs.18,19
The ICAMs belong to the immunoglobulin (Ig) superfamily, and 5 ICAM members (ICAM-1-5) have been identified.20,21 In brain, ICAM-1 is expressed in endothelial, epithelial, and glial cells.22 By contrast, ICAM-5 (telencephalin) has been shown to be expressed only in the telencephalic neurons, and its synthesis takes place mainly after birth.23 Structurally, ICAM-5 is a type I membrane protein with 9 Ig-like domains in the extracellular part, a short transmembrane region, and a cytoplasmic tail.23,24 ICAM-5 has been shown to bind to LFA-125-27 and to regulate the morphology of microglia.28 In conditions of brain ischemia,29 epilepsy,30,31 and encephalitis,32 the soluble form of ICAM-5 (sICAM-5) has been detected in physiologic fluids.
Because of its specific expression pattern, and also being a ligand for LFA-1, it is crucial to establish whether ICAM-5 can act as a costimulatory molecule for T cells, as ICAM-1 does, and whether it regulates T-cell activity during brain inflammation. Here, we find that, in contrast to ICAM-1, the expression of ICAM-5 is not up-regulated by the cytokines tumor necrosis factor (TNF) and interferon-γ (IFN-γ). Activated T cells promote the cleavage of ICAM-5 from neurons, which results in the formation of sICAM-5. Whereas sICAM-1 acts costimulatorily, sICAM-5 suppresses the TCR-mediated activation of T cells as indicated by the decreased expression of CD69, CD40L, and CD25 (IL-2R) on T cells, especially CD45ROLow naive T cells. Moreover, sICAM-5 promotes the expression of transforming growth factor-β1 (TGF-β1) and IFN-γ, but not TNF.
Methods
Reagents
TNF, IFN-γ, and bovine serum albumin were from Sigma-Aldrich (St Louis, MO). Avidin was from Pierce Chemical (Rockford, IL). MMP-2/MMP-9 inhibitor II and MMP-9 inhibitor I were from Calbiochem (San Diego, CA). CTT and CTTW/A peptides were gifts of Dr E. Koivunen (Division of Biochemistry, University of Helsinki).
Antibodies
The polyclonal antibodies (pAb) anti-ICAM-5cp and 1000J have been described.33 The actin pAb was from Sigma-Aldrich. The matrix metalloproteinase (MMP)-2 pAb was from Santa Cruz Biotechnology (Santa Cruz, CA). The MMP-9 and glial fibrillary acid protein (GFAP) pAbs, and the β1 integrin monoclonal antibody (mAb) 2253 were from Chemicon (Temecula, CA). The β1 integrin mAb TS2/16 was kindly provided by Prof. T. A. Springer (Immune Institute, Harvard Medical School, Boston, MA). The β2 integrin mAbs R7E4 (CD18), TS1/22, TS2/4, and MEM 83 (CD11a) have been described previously.34 The ICAM-1 mAb 583 was from R&D Systems (Minneapolis, MN). The biotinylated CD3 mAb UCHT1 and control mAb were from Ancell (Bayport, MN). The CD28 mAb, the phycoerythrin-conjugated CD69, CD25, CD11a, and control mAbs were from ImmunoTools (Friesoythe, Germany). The fluorescein isothiocyanate-conjugated CD45RO, and phycoerythrin-conjugated CD40L (CD154) were from BD Biosciences (San Jose, CA). Horseradish peroxidase-conjugated antimouse, antirabbit, and antihuman IgG were from GE Healthcare (Little Chalfont, United Kingdom). Alexa488-conjugated antirabbit IgG was from Invitrogen (Carlsbad, CA). Cy3-conjugated antimouse IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA).
Cell culture
T-cell isolation
Approval was obtained from the Division of Biochemistry, University of Helsinki institutional review board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Buffy coats used for the isolation of human T cells were obtained from the Finnish Red Cross Blood Transfusion Service (Helsinki, Finland). Mononuclear leukocytes were first isolated by Ficoll-Hypaque gradient centrifugation and filtration through a nylon column. T cells were further purified by the MACS pan T cell or CD4+/CD8+ T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany), and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 1% penicillin and streptomycin, and 1% glutamine (Cambrex, Karlskoga, Sweden).
Cytokine stimulation
Isolated human T cells (2 × 106 per sample) were either left untreated or treated with 10 μg/mL CD3 and 5 μg/mL CD28 mAbs, with or without TNF or IFN-γ (50 ng/mL), then cocultured with 10- to 14-day-old hippocampal cells in 1 mL/well Neurobasal media (Invitrogen) for 16 hours in a humidified incubator at 37C°. In some cases, MMP inhibitors were added into the culture media. Hippocampal neurons were also treated with TNF or IFN-γ directly. The conditioned media were collected and centrifuged to get rid of cell debris. The attached cells were stripped either for Western blotting or total RNA isolation.
sICAM-5 detection
The conditioned media were concentrated 20-fold by Vivaspin centrifugal concentrators (Sartorius, Epsom, United Kingdom), suspended in Laemmli sample buffer, resolved by 4% to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Invitrogen), and transferred to nitrocellulose membranes (Whatman, Dassel, Germany). The membranes were blotted with anti-ICAM-5, or anti-MMP-9 pAb.
Recombinant protein purification
Recombinant ICAM-5 D1-2-Fc protein was purified from culture media using affinity chromatography with protein A-Sepharose and ÁKTA prime system (GE Healthcare).
T-cell activation assay
For the solid phase stimulation system, 12.5 μg of avidin was coated onto 48-well plates. After blocking with 1% bovine serum albumin/phosphate-buffered saline (PBS), 100 ng of biotinylated anti-CD3 or control mAb was added to the wells. Purified T cells were then added (5 × 105 cells/well) in the presence of 0.8 μg/mL anti-CD28 mAb, 20 to 100 μg/mL soluble ICAM-1-Fc (R&D Systems), ICAM-5 D1–2-Fc protein or control mIgG, and stimulated for 5 to 48 hours. T cells were then stained by phycoerythrin-conjugated CD69, CD40L, CD25, CD11a, and fluorescein isothiocyanate-conjugated CD45RO mAbs, or annexin V (Roche Diagnostics, Basel, Switzerland), and analyzed by FACScan and CellQuest software (BD Biosciences). The stainings were reported as either mean fluorescent values or percentages of expression level. Alternatively, T cells were used for total RNA isolation.
Immunofluorescence microscopy
Primary hippocampal cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% TX-100 in PBS, and blocked with 3% bovine serum albumin in PBS-0.05% Tween-20 buffer, then double-stained for ICAM-1 and GFAP or ICAM-5, followed by Cy3-conjugated antimouse and Alexa 488–conjugated antirabbit IgGs. The fluorescent images were taken at 22°C with glycerol imaging medium by a confocal laser scanning microscope under 63× magnification (Leica TCS SP2 AOBS, HCX PL APO 63×0/1.4-0.6; Leica Microsystems, Wetzlar, Germany) using a Leica CCD camera and the LCSLite software.
Total RNA isolation and cDNA synthesis
Total RNA from hippocampal or T cells was extracted with the RNeasy Protect Mini Kit (Qiagen, Hilden, Germany). Purified RNA was quantitated with a BioPhotometer (Eppendorf Nordic, Copenhagen, Denmark). The RNA quality was evaluated using the A260/A280 ratio and electrophoresis. Residual genomic DNA was removed by DNase I treatment (Invitrogen), which was then inactivated by addition of ethylenediaminetetraacetic acid (EDTA) and heating at 65°C for 10 minutes. The resulting RNA was reverse-transcribed to cDNA using Superscript II reverse transcriptase together with oligo d(T20) and random hexamers (Invitrogen) at 55°C for 50 minutes.
Real-time quantitative PCR
cDNA samples were amplified with ABsolute SYBR Green Master Mix (ABgene, Rochester, NY) and 250 nM of gene-specific primers. Samples were run in triplicate using the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) with the following program: a 10-minute preincubation at 95°C, followed by 40 cycles of 15 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C. The data were normalized relative to expression of CASC3 (T cells),36 and glyceraldehyde-3-P-dehydrogenase (GAPDH) or β-actin (hippocampal cells), using an algorithm based on PCR efficiency and the threshold cycle (Ct).37 Unique amplification products and absence of primer-dimers were evaluated by melting curve analysis. The PCR primers for the genes of interest were, shown in 5′-direction: ICAM-1: CAAGGGCTGTCACTGTTCAA and CTTCAGAGGCAGGAAACAGG; ICAM-5: GCCACAGCTACAGCAAGTGA and GAATTCCCTCCAAGGTGACA; β-actin: GGGAAATCGTGCGTGACATT and GCGGCAGTGGCCATCTC; GAPDH: TGATTCTACCCACGGCAAGTT and TGATGGGTTTCCCATTGATGA; IL-4: CTTTGCTGCCTCCAAGAACA and GGCAGCGAGTGTCCTTCTC; IFN-γ: GGAGACCATCAAGGAAGACATG and TGGACATTCAAGTCAGTTACCGA; TNF: GCCCAGGCAGTCAGATCATC and GCTTGAGGGTTTGCTACAACATG; TGF-β1: ATTGAGGGCTTTCGCCTTAG and CCGGTAGTGAACCCGTTGA; CASC3: GCTCCGGTGGATTCTAGTACAAG and GTATATGGTTGGGCATACCTCGA, respectively.
Statistical analysis
Each experiment was repeated for at least 3 times. Data are presented as mean plus or minus SD of triplicates unless otherwise stated. reverse transcription–polymerase chain reaction data were analyzed by the Mann-Whitney U test, and the other data were analyzed by the Student t test. The level of statistical significance was set at P less than .05.
Results
ICAM-5 mRNA and protein levels are not up-regulated by proinflammatory cytokines
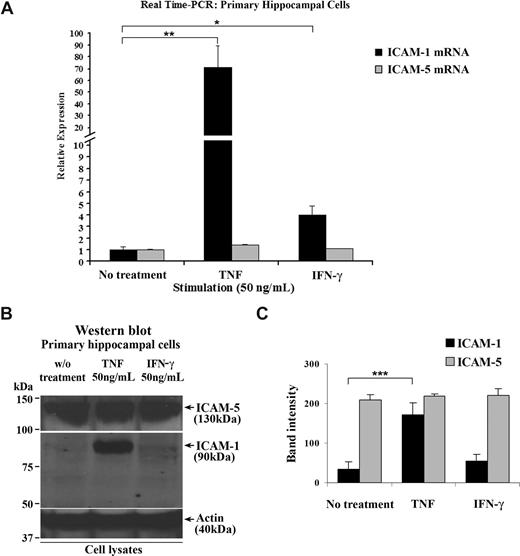
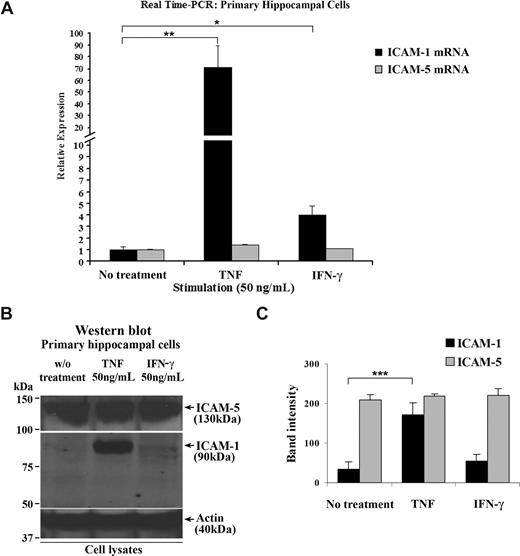
ICAM-5 starts to be expressed in neurons in newborn human, mouse, and rat brains and is maintained at a high level during adulthood.23,24,32,38 By contrast, ICAM-1 is rapidly up-regulated under inflammatory conditions in brain.39 To study the ICAM-5 expression under inflammatory conditions in more detail and because of limited source of human brain tissues, we used 14-day-old cultured rat primary hippocampal cells that contain both neurons and astrocytes. When the cells were treated with 50 ng/mL TNF or IFN-γ, ICAM-1 mRNA was significantly increased, compared with that in resting cells. Unlike ICAM-1, ICAM-5 mRNA was not affected by these cytokines (Figure 1A). Corresponding results were also obtained for the ICAM-1 and ICAM-5 proteins (Figure 1B,C).
ICAM-5 is constitutively expressed in hippocampal cells stimulated by proinflammatory cytokines. Fourteen-day-old cultured primary rat hippocampal cells that contain both neurons and astrocytes were treated with 50 ng/mL TNF or IFN-γ for 16 hours. Total RNA from these cells was harvested, and ICAM-1 and ICAM-5 mRNA levels were quantified by real-time PCR (A). ICAM-1 mRNA was significantly increased by TNF or IFN-γ treatment. By contrast, ICAM-5 mRNA was not affected by these 2 cytokines. The protein levels of the 2 ICAMs were also compared (B,C), which showed similar changes as their respective mRNA levels. Data are shown as mean plus or minus SD of triplicates (*P < .05, **P < .01, ***P < .001).
ICAM-5 is constitutively expressed in hippocampal cells stimulated by proinflammatory cytokines. Fourteen-day-old cultured primary rat hippocampal cells that contain both neurons and astrocytes were treated with 50 ng/mL TNF or IFN-γ for 16 hours. Total RNA from these cells was harvested, and ICAM-1 and ICAM-5 mRNA levels were quantified by real-time PCR (A). ICAM-1 mRNA was significantly increased by TNF or IFN-γ treatment. By contrast, ICAM-5 mRNA was not affected by these 2 cytokines. The protein levels of the 2 ICAMs were also compared (B,C), which showed similar changes as their respective mRNA levels. Data are shown as mean plus or minus SD of triplicates (*P < .05, **P < .01, ***P < .001).
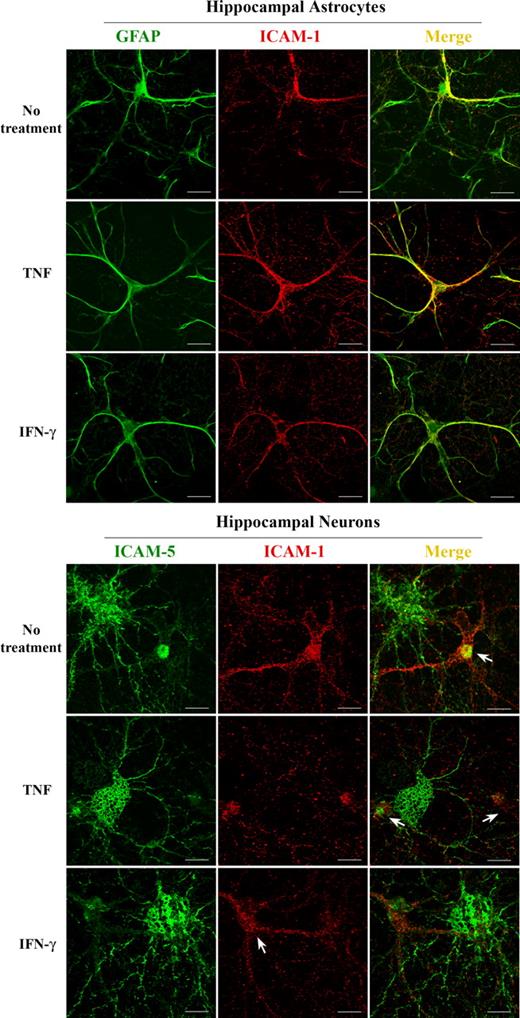
ICAM-5 and ICAM-1 are expressed in neurons and astrocytes, respectively
ICAM-1 is known to be expressed in astrocytes in mammalian brain parenchyma.22 An earlier article showed that human fetal neurons also express ICAM-1.40 For us to clarify the cellular origin of ICAM-1 and ICAM-5, the 2 molecules were immunostained in rat hippocampal cells. We found that ICAM-1 was exclusively expressed in hippocampal astrocytes as indicated by the astrocyte marker GFAP (Figure 2 top panel), whereas no ICAM-1 was detected in hippocampal neurons (Figure 2 bottom panel). On the other hand, ICAM-5 was only expressed in hippocampal neurons, but not in astrocytes, which stained positive for ICAM-1 (Figure 2 bottom panel).
ICAM-1 and ICAM-5 are expressed in hippocampal astrocytes and neurons, respectively. ICAM-1 was immunostained together with GFAP (top panels) or ICAM-5 (bottom panels) in hippocampal cells, and visualized with a confocal fluorescence microscope. ICAM-1 (red) was exclusively expressed in hippocampal astrocytes as indicated by the astrocyte marker GFAP (green, top panels). Coexpression is shown in yellow. ICAM-1 was not detected in hippocampal neurons (bottom panels), whereas ICAM-5 (green, bottom panels) was exclusively expressed in hippocampal neurons, but not in astrocytes (arrows in bottom panels). Bars represent 10 μm.
ICAM-1 and ICAM-5 are expressed in hippocampal astrocytes and neurons, respectively. ICAM-1 was immunostained together with GFAP (top panels) or ICAM-5 (bottom panels) in hippocampal cells, and visualized with a confocal fluorescence microscope. ICAM-1 (red) was exclusively expressed in hippocampal astrocytes as indicated by the astrocyte marker GFAP (green, top panels). Coexpression is shown in yellow. ICAM-1 was not detected in hippocampal neurons (bottom panels), whereas ICAM-5 (green, bottom panels) was exclusively expressed in hippocampal neurons, but not in astrocytes (arrows in bottom panels). Bars represent 10 μm.
The data herein demonstrate that ICAM-1 and ICAM-5 have a different cellular distribution in brain and do not respond to proinflammatory cytokines in a similar fashion. These findings suggest that the 2 molecules may play different roles during inflammation in brain.
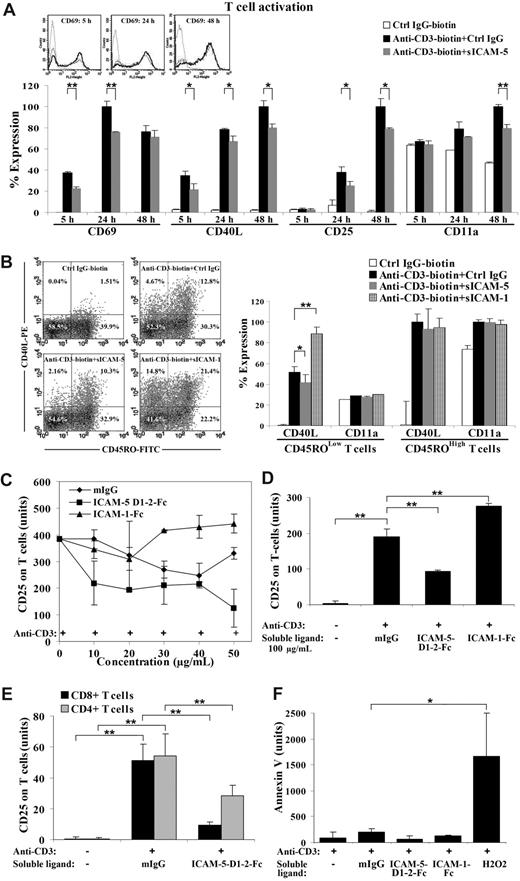
sICAM-5 down-regulates several activation markers on activated T cells induced by TCR ligation
ICAM-1 is known to promote the activation of T cells through binding to LFA-1.17 Similar findings on ICAM-2 and ICAM-3 have also been reported.16,41-43 Considering these effects, we started to wonder whether ICAM-5 would have similar function on infiltrated T cells in inflammatory brain.
To address this question, we used an in vitro T-cell activation assay, where the isolated human peripheral T cells were activated by immobilized anti-CD3 mAb in the absence or presence of sICAM-5. Ligation by anti-CD3 mAb induces TCR signaling and leads to up-regulation of several cell surface molecules, including CD69, CD40L, and CD25, especially on memory T cells.44
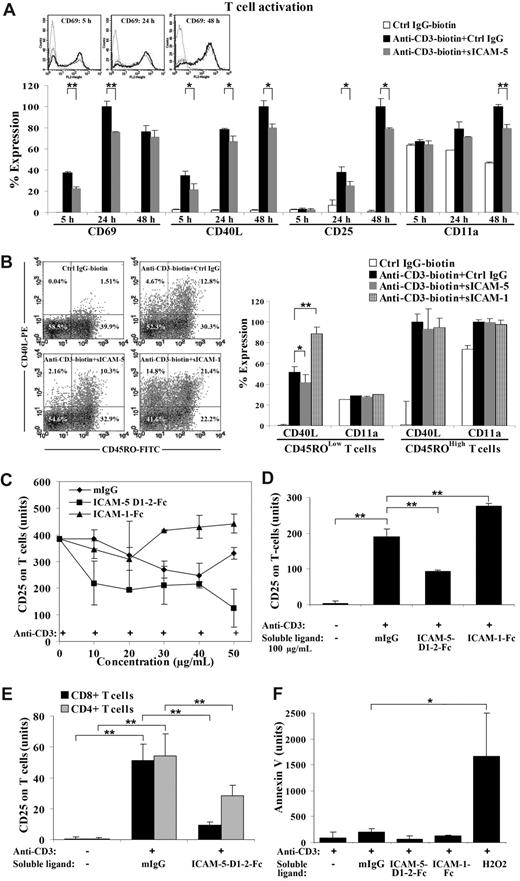
Surprisingly, CD69 and CD40L were significantly decreased within 24 hours in sICAM-5 D1-2-Fc (50 μg/mL) treated activated T cells (Figure 3A). CD40L remained suppressed until the late activation stage (24-48 hours), when CD25 was also decreased (Figure 3A). Meanwhile, the early activation marker CD69 was not significantly altered after 24 hours of stimulation, whereas a suppression of the late activation marker CD25 was most significant after 24 hours (Figure 3A).
sICAM-5 down-regulates the anti-CD3-induced T-cell activation. Ligation of immobilized anti-CD3 mAb induces TCR signaling and leads to up-regulation of CD69, CD40L, and CD25 on human T cells. The expression level of each marker was analyzed by flow cytometry and presented as the percentage of expression level in panels A and B, or as the mean fluorescent value in panels C-F. CD69 and CD40L were significantly decreased in sICAM-5 D1–2-Fc (50 μg/mL) treated T cells within 24 hours. CD40L remained suppressed until the late activation stage (24-48 hours), when CD25 was also decreased (A). The overlaid histograms of CD69 expression by nonactivated T cells (dot line), anti-CD3-activated T cells (solid dark line), and anti-CD3 plus sICAM-5-treated T cells (solid gray line) at 5 hours, 24 hours, and 48 hours are inserted in panel A. Furthermore, T cells were double stained for CD45RO and CD40L after each treatment for 20 hours and represented as dot plots. The percentage of positive cells is marked in each quadrant. sICAM-1-Fc (75 μg/mL) increased, whereas sICAM-5-Fc reduced, the proportion of CD40L+ T cells (panel B left). Moreover, sICAM-1-Fc significantly enhanced, whereas sICAM-5-Fc decreased, CD40L expression in activated CD45ROLow naive, but not in CD45ROHigh memory T cells (panel B right). The level of integrin CD11a polypeptide was not significantly changed by ICAM-1 or ICAM-5 (panel B right). The presence of higher than 30 μg/mL sICAM-1-Fc further promoted the up-regulation of CD25 (C,D). By contrast, sICAM-5 D1-2-Fc decreased CD25 expression at concentrations greater than 10 μg/mL (C,D). Moreover, CD25 expression on activated CD4+ and CD8+ T cells was decreased by 50 μg/mL sICAM-5 D1-2-Fc (E). Neither sICAM-1 nor sICAM-5 induced apoptosis of the treated T cells, as illustrated by annexin V staining (F). Data are shown as means plus or minus SD of triplicates (*P < .05, **P < .01).
sICAM-5 down-regulates the anti-CD3-induced T-cell activation. Ligation of immobilized anti-CD3 mAb induces TCR signaling and leads to up-regulation of CD69, CD40L, and CD25 on human T cells. The expression level of each marker was analyzed by flow cytometry and presented as the percentage of expression level in panels A and B, or as the mean fluorescent value in panels C-F. CD69 and CD40L were significantly decreased in sICAM-5 D1–2-Fc (50 μg/mL) treated T cells within 24 hours. CD40L remained suppressed until the late activation stage (24-48 hours), when CD25 was also decreased (A). The overlaid histograms of CD69 expression by nonactivated T cells (dot line), anti-CD3-activated T cells (solid dark line), and anti-CD3 plus sICAM-5-treated T cells (solid gray line) at 5 hours, 24 hours, and 48 hours are inserted in panel A. Furthermore, T cells were double stained for CD45RO and CD40L after each treatment for 20 hours and represented as dot plots. The percentage of positive cells is marked in each quadrant. sICAM-1-Fc (75 μg/mL) increased, whereas sICAM-5-Fc reduced, the proportion of CD40L+ T cells (panel B left). Moreover, sICAM-1-Fc significantly enhanced, whereas sICAM-5-Fc decreased, CD40L expression in activated CD45ROLow naive, but not in CD45ROHigh memory T cells (panel B right). The level of integrin CD11a polypeptide was not significantly changed by ICAM-1 or ICAM-5 (panel B right). The presence of higher than 30 μg/mL sICAM-1-Fc further promoted the up-regulation of CD25 (C,D). By contrast, sICAM-5 D1-2-Fc decreased CD25 expression at concentrations greater than 10 μg/mL (C,D). Moreover, CD25 expression on activated CD4+ and CD8+ T cells was decreased by 50 μg/mL sICAM-5 D1-2-Fc (E). Neither sICAM-1 nor sICAM-5 induced apoptosis of the treated T cells, as illustrated by annexin V staining (F). Data are shown as means plus or minus SD of triplicates (*P < .05, **P < .01).
As the peripheral blood T cells represent a mixed population that contributes differently to T-cell responses, we wanted to discern whether the suppressing effect of sICAM-5 is confined to different T-cell populations. For this purpose, we double-stained the activated T cells for CD45RO and CD40L after each treatment for 20 hours (Figure 3B). The CD45ROLow population represents the majority of naive T cells, whereas CD45ROHigh T cells are mostly memory or activated cells. We found that sICAM-5 reduced the proportion of activated CD40L+ T cells in the CD45ROLow population to half (from 4.6% down to 2.1%) but only slightly in the CD45ROHigh population (from 11.3% down to 8.8%). By contrast, sICAM-1 highly increased CD40L+ T cells in both the CD45ROLow (from 4.6% up to 14.8%) and the CD45ROHigh populations (from 11.3% up to 19.9%; Figure 3B dot plots). Interestingly, sICAM-1-Fc significantly enhanced, whereas sICAM-5-Fc decreased, the expression level of CD40L in activated CD45ROLow naive, but not in CD45ROHigh memory T cells (Figure 3B right panel). In comparison, the level of the integrin CD11a polypeptide was not significantly changed by ICAM-1 or ICAM-5 treatment (Figure 3B). Furthermore, the suppressive effect of sICAM-5 was also apparent in naive CD45ROLow T cells that were costimulated by anti-CD28 mAb (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Because the T cells that are able to infiltrate into brain are usually activated at peripheral sites,5 we chose the late T-cell activation marker CD25 to continue our study. We further found that, whereas sICAM-1-Fc synergistically promoted up-regulation of CD25 expression on activated T cells at concentrations greater than 30 μg/mL, sICAM-5 D1-2-Fc led to the opposite effect at concentrations as low as 10 μg/mL (Figure 3C,D). Furthermore, we found that sICAM-5 D1-2-Fc down-regulated the CD25 expression on both CD4+ and CD8+ T cells, especially on CD8+ cells (Figure 3E).
To examine whether the suppressive effect of sICAM-5 D1-2-Fc on T cells is the result of apoptotic death, we stained the treated T cells with annexin V and found that neither sICAM-1 nor sICAM-5 at 50 μg/mL concentration induced T-cell apoptosis (Figure 3F).
Integrin antibodies together with sICAM-5 down-regulate CD25 expression on T cells
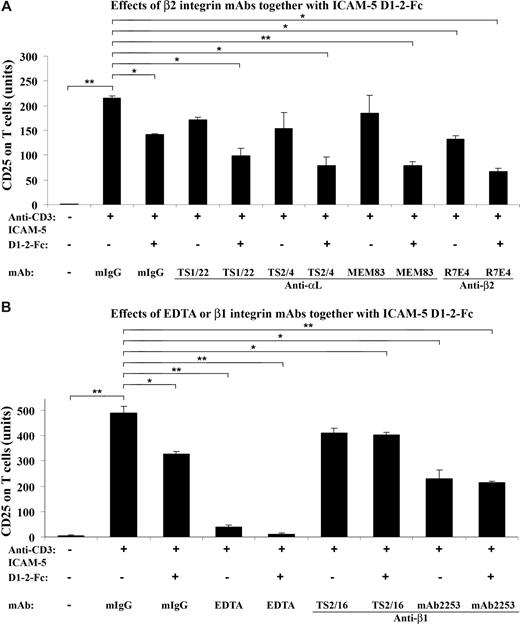
Because ICAM-5 is a ligand for LFA-1,26 we further studied whether mAbs against LFA-1 or other integrins would affect the suppressive effect of sICAM-5 on the CD25 expression. For this purpose, T cells were incubated in the presence of 10 μg/mL sICAM-5 D1-2-Fc together with 5 μg/mL mAbs against αL, β2, and β1 chains, respectively, in anti-CD3–coated plates (Figure 4).
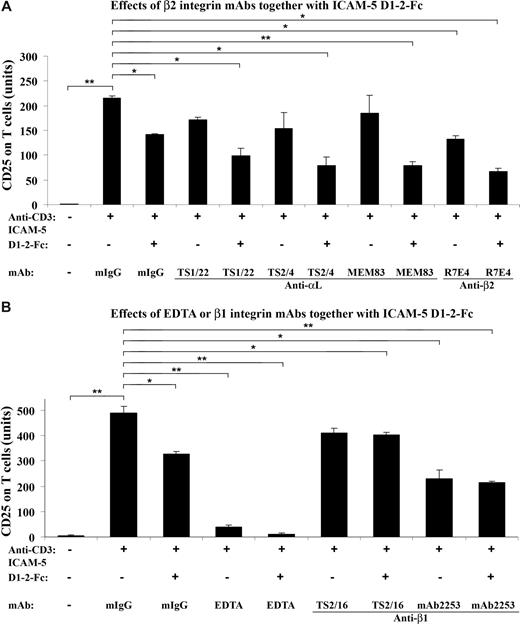
β2 integrin mAbs suppress the CD25 expression synergistically with sICAM-5. T cells were incubated in the presence of 10 μg/mL sICAM-5 D1–2-Fc together with 5 μg/mL of mAbs against integrin αL, β2, and β1 chains, respectively, in anti-CD3–coated plates for 48 hours. The αL mAbs TS2/4 and TS1/22 did not significantly inhibit the CD25 expression on T cells by themselves but showed additional suppressive effects together with sICAM-5. The activating αL mAb MEM83 neither decreased the CD25 expression alone nor counteracted the suppressive effect of sICAM-5. By contrast, the β2 mAb R7E4 inhibited the CD25 expression per se and also showed additional suppressive effects together with sICAM-5 (A). The β1 integrin activating mAb TS2/16 did not inhibit the CD25 expression by itself and significantly counteracted the suppressive effect of sICAM-5. On the other hand, the β1 integrin inhibitory mAb 2253 significantly inhibited the CD25 expression per se but showed no additional suppressive effect when applied together with sICAM-5 (B). Moreover, 5 mM EDTA significantly down-regulated the CD25 expression and acted synergistically with sICAM-5 (B). Data are shown as means plus or minus SD of triplicates (*P < .05, **P < .01).
β2 integrin mAbs suppress the CD25 expression synergistically with sICAM-5. T cells were incubated in the presence of 10 μg/mL sICAM-5 D1–2-Fc together with 5 μg/mL of mAbs against integrin αL, β2, and β1 chains, respectively, in anti-CD3–coated plates for 48 hours. The αL mAbs TS2/4 and TS1/22 did not significantly inhibit the CD25 expression on T cells by themselves but showed additional suppressive effects together with sICAM-5. The activating αL mAb MEM83 neither decreased the CD25 expression alone nor counteracted the suppressive effect of sICAM-5. By contrast, the β2 mAb R7E4 inhibited the CD25 expression per se and also showed additional suppressive effects together with sICAM-5 (A). The β1 integrin activating mAb TS2/16 did not inhibit the CD25 expression by itself and significantly counteracted the suppressive effect of sICAM-5. On the other hand, the β1 integrin inhibitory mAb 2253 significantly inhibited the CD25 expression per se but showed no additional suppressive effect when applied together with sICAM-5 (B). Moreover, 5 mM EDTA significantly down-regulated the CD25 expression and acted synergistically with sICAM-5 (B). Data are shown as means plus or minus SD of triplicates (*P < .05, **P < .01).
Although the αL mAbs TS2/4 and TS1/22 did not significantly inhibit the CD25 expression on T cells per se, both mAbs showed additional suppressive effects when present with sICAM-5, whereas the activating mAb MEM83 neither had any effect on the CD25 expression alone nor counteracted the suppressive effect of sICAM-5 (Figure 4A). By contrast, the β2 integrin mAb R7E4 not only inhibited the CD25 expression but also showed an additional suppressive effect when applied together with sICAM-5 (Figure 4A).
The β1 integrin activating mAb TS2/16 did not inhibit the CD25 expression and significantly counteracted the suppressive effect of sICAM-5 (Figure 4B). On the other hand, the inhibitory mAb 2253 significantly down-regulated the CD25 expression per se but did not show additional suppressive effect when applied together with sICAM-5 (Figure 4B).
Besides, we found that EDTA at 5 mM concentration significantly down-regulated the CD25 expression and also acted synergistically with sICAM-5 (Figure 4B). The above data demonstrate that the β2 integrin LFA-1 is involved in the costimulation of TCR-mediated activation of T cells, and that sICAM-5 suppresses T-cell activation by binding to LFA-1. β1 integrins may also play a role here.
sICAM-5 promotes the expression of TGF-β, but not TNF mRNA in activated T cells
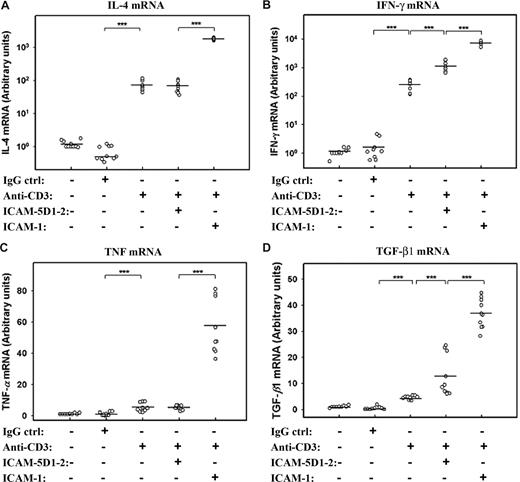
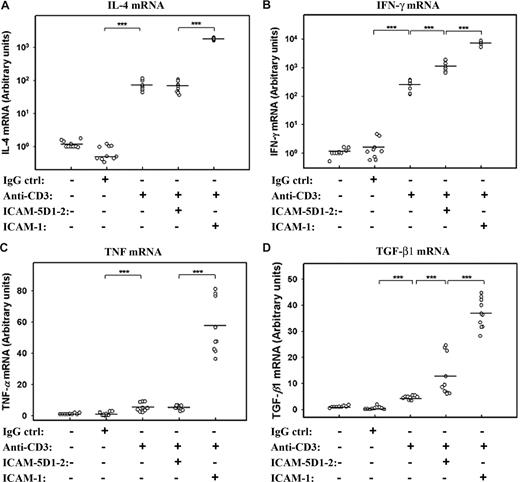
These experiments show an attenuating effect of sICAM-5 on the strength of T-cell activation. However, the brain-specific regulation of T cells may also involve a qualitative modulation of their response. We therefore measured the effect of sICAM-5 on cytokine mRNA synthesis in anti-CD3–treated T cells. We found that anti-CD3 stimulation strongly increased the expression of IL-4 and IFN-γ mRNAs, which were synergistically boosted by sICAM-1 (Figure 5A,B). On the contrary, sICAM-5 stimulated IFN-γ, but not IL-4 mRNA in T cells. The expression of TNF and TGF-β1 mRNAs were not stimulated to a great extent by anti-CD3 mAb alone, whereas the sICAM-1 was strongly costimulatory (Figure 5C,D). sICAM-5, on the other hand, did not have an effect on TNF mRNA (Figure 5C) but significantly increased the expression of TGF-β1 mRNA (Figure 5D).
sICAM-5 and sICAM-1 differentially regulate the cytokine expression in T cells. sICAM-1 potentiated the anti-CD3–induced activation on IL-4 (A), IFN-γ (B), TNF (C), and TGF-β1 (D) mRNAs, whereas sICAM-5 had no effect on IL-4 (A) and TNF (C) mRNAs but significantly promoted the anti-CD3–induced activation on IFN-γ (B) and TGF-β1 (D) mRNAs. Data are dot plots with mean values shown (n = triplicates from 3 donors; ***P < .001).
sICAM-5 and sICAM-1 differentially regulate the cytokine expression in T cells. sICAM-1 potentiated the anti-CD3–induced activation on IL-4 (A), IFN-γ (B), TNF (C), and TGF-β1 (D) mRNAs, whereas sICAM-5 had no effect on IL-4 (A) and TNF (C) mRNAs but significantly promoted the anti-CD3–induced activation on IFN-γ (B) and TGF-β1 (D) mRNAs. Data are dot plots with mean values shown (n = triplicates from 3 donors; ***P < .001).
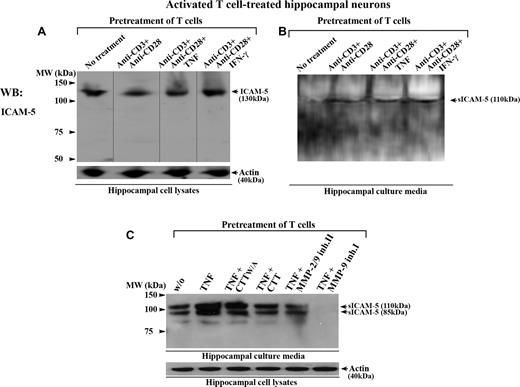
Activated T cells induce ICAM-5 shedding from neurons
sICAM-5 has been detected under inflammatory conditions in brain.29,32 We have recently reported that active MMP-2 and MMP-9 specifically cleave full-length ICAM-5 from neurons, which produces sICAM-5.33 Because activated T cells secrete large amounts of MMP-2 and MMP-9 during inflammation, we therefore wanted to examine whether T cells themselves could contribute to the release of sICAM-5 and thereby generate a feedback loop for their activation.
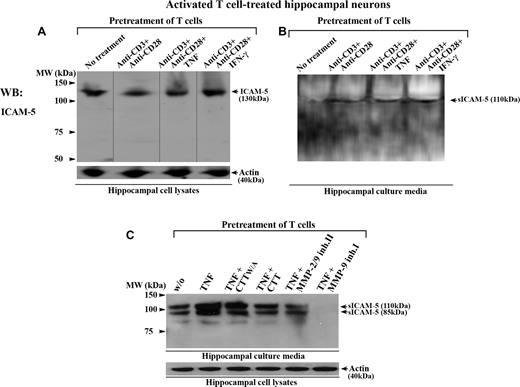
To study this, we incubated human T cells together with rat hippocampal neurons. Rat ICAM-5 has 97.5% and 85.9% overall sequence identity with the mouse and human homologs, respectively (NCBI HomoloGene database). We have earlier shown that activated human T cells bind to murine hippocampal neurons through a LFA-1/ICAM-5 interaction.27 Here, when T cells were first costimulated by anti-CD3/CD28 with or without TNF or IFN-γ, and subsequently incubated with hippocampal neurons for 16 hours, the activated T cells promoted the cleavage of membrane-bound ICAM-5 from neurons (Figure 6A) and resulted in the release of sICAM-5 into the culture media (Figure 6B). Similar results were observed when T cells were activated by the cytokine TNF alone (Figures 6C,S2), and this effect was prevented by several inhibitors of MMP-2 and MMP-9 (Figure 6C). We also checked whether the aforementioned cytokines directly induced the cleavage of ICAM-5 from hippocampal neurons, and found no such effects (Figure S2).
Activated T cells promote the cleavage of ICAM-5 from neurons. Human T cells were either left untreated or treated with anti-CD3 plus anti-CD28 mAbs, with or without TNF or IFN-γ (50 ng/mL), and incubated with 10- to 14-day-old rat hippocampal neurons for 16 hours. The conditioned media and hippocampal cell lysates were processed for Western blotting. ICAM-5 was detected by pAbs against the ICAM-5 cytoplasmic tail (A) or ectodomains (B,C), respectively. Full-length ICAM-5 is 130 kDa (A). Vertical lines are inserted to indicate repositioned gel lanes. Both TCR- (B) and cytokine- (C) mediated activation of T cells induced the release of sICAM-5 fragments (85-110 kDa) into the culture media. The cleavage was blocked by the MMP-2/9 inhibitor II (25 μM), MMP-9 inhibitor I (25 μM), and CTT peptide (100 μM), but not by the CTTW/A peptide (C).
Activated T cells promote the cleavage of ICAM-5 from neurons. Human T cells were either left untreated or treated with anti-CD3 plus anti-CD28 mAbs, with or without TNF or IFN-γ (50 ng/mL), and incubated with 10- to 14-day-old rat hippocampal neurons for 16 hours. The conditioned media and hippocampal cell lysates were processed for Western blotting. ICAM-5 was detected by pAbs against the ICAM-5 cytoplasmic tail (A) or ectodomains (B,C), respectively. Full-length ICAM-5 is 130 kDa (A). Vertical lines are inserted to indicate repositioned gel lanes. Both TCR- (B) and cytokine- (C) mediated activation of T cells induced the release of sICAM-5 fragments (85-110 kDa) into the culture media. The cleavage was blocked by the MMP-2/9 inhibitor II (25 μM), MMP-9 inhibitor I (25 μM), and CTT peptide (100 μM), but not by the CTTW/A peptide (C).
Discussion
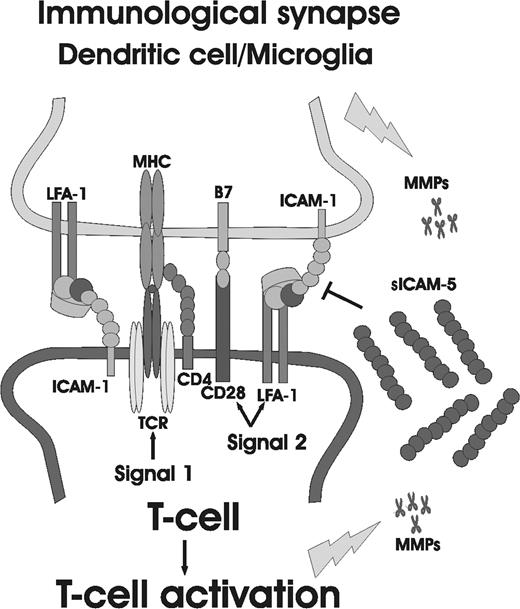
Here we demonstrate that the neuronal cell adhesion molecule ICAM-5, an Ig superfamily member, participates in the cross-talk between TCR and the costimulatory receptor LFA-1, and surprisingly unlike the other studied ICAMs, down-regulates the TCR-mediated T-cell activation, especially in CD45ROLow naive cells. This provides a possible mechanism by which ICAM-5 regulates the activity of the immunologic synapse in brain, as depicted in Figure 7. We show that ICAM-5 expression is different from ICAM-1 in cellular origin. When activated T cells interact with neurons, ICAM-5 is cleaved. This results in the production of sICAM-5, which in turn decreased the expression of CD69, CD40L, and CD25 (IL-2R), and up-regulate the cytokines TGF-β1 and IFN-γ synthesis in T cells. Therefore, neurons can restrict the activation of antigen-specific T cells that infiltrate the brain, especially at their early priming stage.
The involvement of ICAM-5 in regulation of the immunologic synapse in brain. Dendritic cells and/or microglia are the local APCs in the brain. In the case of brain inflammation, they control the activation of invaded T cells through TCR and costimulatory molecules, such as CD28 and LFA-1. Activated T cells or microglial cells secrete MMPs, which in turn cleave the ectodomains of ICAM-5 from neurons, producing sICAM-5. sICAM-5 may compete with ICAM-1 at the early T-cell priming stage and neutralize the costimulatory signal induced by ICAM-1 for T-cell activation.
The involvement of ICAM-5 in regulation of the immunologic synapse in brain. Dendritic cells and/or microglia are the local APCs in the brain. In the case of brain inflammation, they control the activation of invaded T cells through TCR and costimulatory molecules, such as CD28 and LFA-1. Activated T cells or microglial cells secrete MMPs, which in turn cleave the ectodomains of ICAM-5 from neurons, producing sICAM-5. sICAM-5 may compete with ICAM-1 at the early T-cell priming stage and neutralize the costimulatory signal induced by ICAM-1 for T-cell activation.
The entry of immune cells into the brain is highly regulated. Although the antigen-specific T cells can reach the brain parenchyma, they are not easily activated to exert their effector functions (immune privilege).6 Neurons constitutively express high level of Fas ligand that induces T-cell apoptosis.45,46 If T cells survive, they are required to recognize their cognate antigen in the context of MHC. However, the constitutive expression of MHC is minimal in the normal brain.47 Moreover, neurons and astrocytes secrete several soluble factors, such as TGF-β, to down-regulate T-cell activity.6
Despite this, T cells can still initiate disease or contribute to pathogenicity in brain, as the MHC and costimulatory molecules are strongly up-regulated in a multitude of pathologic conditions, including infection, ischemia, axonal degeneration, traumatic nerve injury, malignant transformation, and neurodegenerative disorders, such as multiple sclerosis, HIV-associated dementia, and Alzheimer, Parkinson, and Creutzfeldt-Jakob diseases.8-11 Thus, the interaction of T cells and APCs through the immunologic synapses should be strictly controlled to prevent the cytotoxic effects of T cells on neurons. But how MHC and costimulatory molecules cross-talk with TCR and coreceptors and how the cross-talk is regulated in brain are still poorly understood. Our findings help to better understand the underlying mechanisms.
It is known that ICAM-1 is rapidly up-regulated under inflammatory conditions in the brain.39 We found that ICAM-5 expression was refractory to cytokine stimulation, which resembles the behavior of ICAM-2 in endothelial cells.48 ICAM-1, ICAM-2, and ICAM-3 have been shown to promote T-cell activation through LFA-1 binding.16,41-43 We think that the constitutive expression pattern of ICAM-5 and its suppressive effect on T cells help neurons to strengthen the immune privilege in brain, which corroborates with the concept that brain is typically hostile to infiltrated T cells.
In our T-cell activation assay, sICAM-1-Fc significantly enhanced, whereas sICAM-5-Fc decreased, CD40L expression in activated naive, but not in memory T cells. This suggests that naive cells are more dependent on costimulatory signals delivered by ICAM-1. We also found that the β2 integrin LFA-1, and possibly β1 integrins, are involved in the costimulation of TCR-mediated T-cell activation. The β2 integrin mAbs and EDTA enhanced the suppressive effects of ICAM-5 on the CD25 expression, suggesting that when ICAM-5 binds to LFA-1, it acts synergistically with the β2 integrin mAbs. Soluble ICAM-1 and ICAM-2 are potent activators of β2 integrins, and recent data show that the integrin phosphorylation is needed for activation.34
Our data on the time-dependent effect of ICAM-5 on CD69, CD40L, and CD25 expression indicates that ICAM-5 does not interfere with the expression kinetics of these surface markers but only lowers the magnitude of the ultimate T-cell activation. The molecular mechanism for ICAM-5-induced inhibition of T-cell activation may be the result of its binding to an inhibitory site on LFA-1, thus preventing the costimulatory signal provided by other ligands, such as ICAM-1 (Figure 7). Obviously, further studies are needed to illuminate the underlying mechanisms.
sICAM-5 is increased under several pathologic conditions of the brain.29,31,32 In a recent study, we have characterized the MMP-2/MMP-9-induced cleavage of ICAM-5 after stimulation of glutamate receptors. The active MMP-2/MMP-9 molecules cleave ICAM-5 in the second and ninth extracellular Ig-like domains, resulting in soluble fragments.33 Recent findings suggest that both MMP-2 and MMP-9 are important for myelin-specific T cells to pass through the brain vascular endothelium and induce experimental allergic encephalomyelitis (EAE).49,50 The cleavage of neuronal ICAM-5 by T cell–induced MMPs and the consequent release of sICAM-5 may act as a local feedback mechanism. This could raise the threshold level for the activation of nonmemory T cells, especially for brain antigen-specific T cells, without preventing a necessary memory type response to proceed.
We also found that sICAM-5 inhibited the activation of both CD4+ and CD8+ T cells. CD4+ T cells can differentiate into at least 2 subsets of effector cells: Th1 cells, which secrete predominantly IFN-γ and TNF, and Th2 cells, which secrete IL-4, IL-5, and IL-10. In our cytokine profiling experiment, neither ICAM-1 nor ICAM-5 showed any preference in regulating the 2 subsets of Th cells. ICAM-1 boosted both Th1 and Th2 types of cytokines, whereas ICAM-5 promoted the synthesis of IFN-γ and TGF-β1, but not TNF and IL-4. In this regard, our data indicate that neurons themselves may provide neuroprotective effects by regulation of cytokine synthesis in T cells through ICAM-5. Several neuronal molecules, such as CD22, CD47, CD200, fractalkine, and various neuropeptides and neurotransmitters, have been shown to have such neuroprotective effects so far.6 Our current discovery adds new knowledge into this paradigm.
In brain, TNF is mostly regarded as a proinflammatory molecule that promotes inflammation-related neuronal damage mediated through TNF receptor 1.51,52 TGF-β is mainly anti-inflammatory and neuroprotective53,54 and is a key mediator of autoimmune-suppressive functions exerted by CD4+CD25+ regulatory T cells, which are currently under intensive investigation.55,56
Interestingly, previous observations on EAE models showed that TNF deficiency led to failed regression of myelin-specific T-cell reactivity and prolonged survival of activated T cells, resulting in exacerbation of demyelination.57,58 Deficiency of IFN-γ also worsened the EAE59,60 and even permitted disease development in otherwise resistant mouse strains.61 Likewise, TGF-β has recently been shown to promote EAE.62 These novel findings suggest that inflammatory cytokines may act as a double-edged sword, being both immunopromotive and immunosuppressive, depending on the phase of disease.51,52
In conclusion, our findings suggest that ICAM-5 is involved in the immune privilege of the brain and could be a useful anti-inflammatory agent for the treatment of various inflammatory brain diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Patrick Kilgannon for rat ICAM-5 mAbs and pAb 1000J, Dr Yoshihiro Yoshihara for providing the anti-ICAM-5cp pAb, Dr Erkki Koivunen for MMP inhibitory peptides, Dr Jose Casasnovas for CHO cell lines, Dr Michael Stefanidakis for helpful discussions, Seija Lehto, Outi Nikkilä, Leena Kuoppasalmi, and Maria Aatonen for technical assistance, and Yvonne Heinilä for secretarial help.
This study was supported by the Sigrid Jusélius Foundation, the Academy of Finland, the Finnish Cultural Foundation, the Magnus Ehrnrooth Foundation, the Finnish Cancer Society, the Finnish Medical Association, and the Liv och Hälsa Foundation.
Authorship
Contribution: L.T. designed the research and experiments, performed some experiments, analyzed the data, and wrote the manuscript; J.L. helped to design the T-cell activation assay and real-time PCR assay, analyzed the data, and revised the manuscript; M.A. helped to design the research and experiments; S.H. performed the T-cell activation assay and real-time PCR assay; H.R. financed part of the study and revised the manuscript; C.G.G. planned and financed the major part of the study and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Li Tian, Division of Biochemistry, Department of Biologic and Environmental Sciences, Faculty of Biosciences, University of Helsinki, Viikinkaari 5, FIN-00014, Helsinki, Finland; e-mail: li.tian@helsinki.fi.