Abstract
B7-H1 is an immunoglobulin-like immune suppressive molecule broadly detectable on the majority of human and rodent cancers, and its functions have been attributed to delivering an inhibitory signal to its counter-receptor programmed death-1 (PD-1) on T cells. Here we report that B7-H1 on cancer cells receives a signal from PD-1 to rapidly induce resistance against T cell–mediated killing because crippling signaling capacity of B7-H1 but not PD-1 ablates this resistance. Importantly, loss of B7-H1 signaling is accompanied by increased susceptibility to immune-mediated tumoricidal activity. In addition to resistance against T-cell destruction, B7-H1+ cancer cells also become refractory to apoptosis induced by Fas ligation or the protein kinase inhibitor Staurosporine. Our study reveals a new mechanism by which cancer cells use a receptor on immune cells as a ligand to induce resistance to therapy.
Introduction
Cancer cells display altered surface molecular signatures that distinguish them quantitatively and qualitatively from their normal derivatives. These modifications in receptor and ligand expression commonly facilitate tumor growth and progression or to evasion of host defense mechanisms.1,2 For example, some tumor cells down-regulate their cell surface major histocompatibility complex (MHC), which is required for recognition by tumor antigen-specific T lymphocytes.3 As a result, these tumor cells become less recognizable by the immune system and more resistant to immune-mediated destruction. Another example is that during progression, cancer cells frequently overexpress proteases and modify glycosylation of cell surface proteins that are normally involved in tissue repair, remodeling, and homeostasis to facilitate invasion and metastasis.4,5 In general, these modifications in cell membrane ligands and receptors regulate interactions between tumor cells and nontransformed cells in the microenvironment in a fashion that enhances tumor growth, invasion, and immune resistance
We previously identified an immunoglobulin (Ig)–like molecule termed B7-H1,6 which is either constitutively or inducibly expressed by the majority of human and rodent cancer cells.7,8 Ample evidence demonstrates that B7-H1 acts as a ligand for the receptor programmed death-1 (PD-1) to deliver an inhibitory signal to T cells, leading to inhibition of immune responses.9 The mechanisms underlying B7-H1/PD-1–mediated suppression include induction of apoptosis, anergy, unresponsiveness, and exhaustion of T cells.7,10–14 Interaction between B7-H1 and PD-1 is also shown to participate in the suppression of autoimmune diseases and transplantation rejection in animal models.15–18. A recent study suggests that B7-H1, in addition to PD-1, also binds B7-1 (CD80) on T cells to inhibit their activation.19 We and others have observed that B7-H1+ tumor cells are much more resistant to CD8+ cytolytic T cell (CTL)–mediated destruction in vitro than their B7-H1–negative parental cells, and this resistance is correlated with decreased efficacy of immunotherapy in mouse tumor models.20–22 Ablation of B7-H1 and PD-1 interaction by neutralizing antibodies could restore CTL-mediated lysis of tumor cells in vitro, suggesting that B7-H1/PD-1 interaction forms a barrier between tumor cells and CTL, and this phenomenon has been termed “molecular shield.”20 These results have been interpreted as inhibition of CTL activity induced by unidirectional engagement of PD-1 on the T cell by B7-H1 on the tumor cells.
However, there are alternate interpretations for this molecular shield phenomenon. Although interaction between B7-H1+ tumor cells and PD-1 on T cells has been shown to induce T-cell suppression, it is possible that the molecular shield is simply attributable to rapid loss of CTL cytolytic function. However, when B7-H1+ and B7-H1− tumor cells were mixed together with antigen-specific CD8+ CTL in short-term in vitro assays, preferential lysis of B7-H1− cells is observed.20 This experiment suggests that overall cytolytic function of CD8+ CTL upon exposure to B7-H1 in short-term assays is not impaired. Another possibility is that B7-H1 and PD-1 simply form a physical barrier to prevent interaction of T-cell receptor (TCR) and tumor antigen presented in the MHC class I. Finally, it is possible that B7-H1 could act as a receptor to transmit a signal from T cells to tumor cells, leading to resistance of lysis. To test these hypotheses, we specifically engineered B7-H1 and PD-1 molecules with normal binding capacity but impaired ability to transmit signals to tumor cells or T cells, respectively, to examine their effects on molecular shielding of tumor cells from T-cell killing. The results support a mechanism whereby PD-1 on T cells acts as a ligand for B7-H1, whereas B7-H1 acts as a receptor to transmit signals to the tumor cells, thereby enhancing its resistance to apoptosis induction by both immune effectors and proapoptotic drugs.
Methods
Mice and tumor lines
Female DBA/2, C57BL/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD). Age-matched mice, 6 to 10 weeks old, were used for all experiments. 2C transgenic mice (a gift from Dr Larry Pease, Mayo Clinic, Rochester, MN) and PD-1–deficient mice in B6 background (a gift from Dr Tasuko Honjo, Kyoto University, Kyoto, Japan) were described previously.16 The 2C × PD-1KO mice were obtained by backcrossing between 2C transgenic mice and PD-1KO mice. All mice were maintained in the animal facility at Johns Hopkins Hospital under approved protocol by the Institutional Animal Care and Use Committee. P815 mastocytoma cells were purchased from the American Type Culture Collection (Rockville, MD). A subclone, which does not express B7-H1, even in the presence of interferon-γ (IFN-γ) or activated T cells, was selected before transfection. Stable transfectants of P815 lines including mock/P815 and B7-H1/P815 were described previously.20 Renca is an H-2d murine renal cell carcinoma line (a gift from Dr Drew M. Pardoll, Johns Hopkins University, Baltimore, MD).
Monoclonal antibodies, fusion proteins, and flow cytometric analysis
Purified monoclonal antibody (mAb) against mouse H-2Dd and CD95 (clone Jo2) was purchased from BD PharMingen (San Diego, CA) and H-2Ld from BioLegend (San Diego, CA). Rat mAb (clone TY25) against B7-DC, fluorescein isothiocyanate (FITC)– or phycoerythrin-conjugated goat antimouse antibodies and FITC-conjugated goat antihamster antibodies were purchased from eBioscience (San Diego, CA). An Armenian hamster mAb (clone 10B5) against mouse B7-H1,20 a hamster mAb (clone G4) against mouse PD-1,20 a rat mAb (clone 2A) against mouse CD137,23 mouse PD-1Ig fusion protein,20 and mouse B7-H1Ig fusion protein20 were all described previously. Fluorescence was detected by FACScalibur flow cytometry and analyzed with Cell Quest software (BD Biosciences, Mountain View, CA).
Plasmids
To generate truncated chimeric murine ΔB7-H1, full-length B7-DC, and PD-1, polymerase chain reaction (PCR) fragments of each gene were digested with XhoI and BglII, XhoI and EcoRI, or BamH1 and XhoI restriction sites, respectively (5′primer for truncated B7-H1; 5′-ccgctcgaggccaccatgaggatatttgctg -3′, 3′primer for truncated B7-H1; 5′-gaagatcttcttgttttctcaagaaga-3′, 5′primer for full-length B7-DC; 5′-ccgctcgaggccaccatgctgctcctgctgccga-3′, 3′primer for full-length B7-DC; 5′-ggaattcctagatcctctttctct-3′, 5′primer for full-length PD-1; 5′-cgcggatccgccaccatgtgggtccggcag-3′ and 3′primer for full-length PD-1; 5′-ccgctcgagtcaaagaggccaagaac-3′). Subsequent ligations of these fragment were performed into XhoI/BamHI-digested pEGFP-N1 (Clontech, Mountain View, CA), XhoI/EcoRI-digested pcDNA3.1 (Invitrogen, Carlsbad, CA) or BamHI/XhoI-digested pHR′ cytomegalovirus (CMV) vectors. Chimeric murine B7-DC/H1 and truncated chimeric murine ΔPD-1 was constructed by 2-step PCR. Overlapping oligonucleotide primers were synthesized and 2 flanking 5′ and 3′ primers were designed to contain XhoI and BglII or BamHI and XhoI restriction sites. Appropriate regions of cDNA were initially amplified using the corresponding overlapping and flanking primers (5′primer for B7-DC; 5′-ccgctcgaggccaccatgctgctcctgctgccga-3′, 3′primer for B7-DC; 5′-cagaagcacccagtgccacgttctggggac-3′, 5′primer for B7-H1; 5′-gtccccagaacgtggcactgggtgcttct-3′, 3′primer for B7-H1; 5′-gaagatctttacgtctcctcgaattgtgt-3′, 5′primer for PD-1; 5′-cgcggatccgccaccatgtgggtccggcag-3′, 3′primer for PD-1; 5′-gcccttgctcaccatcttgttgagcagaagac-3′, 5′primer for enhanced green fluorescent protein (EGFP); 5′-cttctgctcaacaagatggtgagcaagggc-3′ and 3′primer for EGFP; 5′-ccgctcgagttacttgtacagctcgtc-3′). Then, using the flanking 5′ and 3′ primers, fragments for which sequences overlapped were fused together and amplified. PCR products were digested with XhoI and BglII or BamHI and XhoI and ligated into XhoI/BamHI-digested pcDNA3.1 or BamHI/XhoI-digested pHR′ CMV vectors.
Gene transfection and lentivirus-mediated transduction
To generate ΔB7-H1/P815, B7-DC/P815, and B7-DC/H1/P815, P815 cells were transfected with corresponding vectors by using electroporation and cloned by limiting dilution as described previously.20 Recombinant lentiviruses with full-length or truncated PD-1 were used to transduced 2C × PD-1KO CTLs, The 293T cell lines were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum. All recombinant lentiviruses were produced by transient transfection of 293T cells according to standard protocols. Briefly, subconfluent 293T cells were cotransfected with 20 μg of a plasmid vector, 15 μg of pCMV-ΔR8.91, and 5 μg of pMD2G-VSVG by lipofectamine (Invitrogen, Carlsbad, CA). After 48 hours, recombinant lentivirus vectors were harvested. For transduction, 2C × PD-1KO T cells were plated on 24-well plate, and RPMI medium containing 50% medium containing recombinant lentivirus vectors, 10% fetal bovine serum, 25 mM HEPES, 100 units/mL penicillin G, 100 μg/mL streptomycin sulfate, 55 μM 2-ME, and 30 units/mL human interleukin-2 (IL-2) was added. After 48 hours of incubation, cells were washed and split. The procedure was repeated 4 times to increase transduction efficiency. PD-1–positive cells after transduction were purified by magnetic beads (Miltenyi Biotec, Auburn, CA).
CTL growth and functional assay
For the generation of alloreactive CTLs or 2C CTLs, lymph node cells (5 × 106/mL) from C57BL/6 mice were stimulated with irradiated spleen cells (2 × 106/mL) from DBA/2 mice in 24-well plates for 5 days. Cells were incubated with 51Cr-labeled target cells at the indicated effector/target (E/T) ratios for 4 hours as described previously.20 The spontaneous releases of 51Cr are less than 10%. In cold target competition assay, mock/P815 and B7-H1/P815 cells were premixed and incubated with activated 2C CTLs in 24-well plates for 4 hours. Cells were stained with phosphatidylethanolamine-conjugated anti–H-2Dd mAb and 10B5 mAb plus FITC-conjugated goat antihamster Ig antibodies or green fluorescence protein (GFP), and all cells were counted by flow cytometry. Wells containing only target cells were included as controls. To prepare PD-102C CTL clone, the lymph node cells from 2C × PD-1KO mice were maintained in the complete RPMI 1640 medium supplemented with 10% fetal bovine serum, 25 mM HEPES, 100 units/mL penicillin G, 100 μg/mL streptomycin sulfate, 55 μM 2-ME, and 30 units/mL human IL-2 by stimulating with 50 Gy-irradiated spleen cells from DBA/2 mice every 10 to 14 days.
Cell apoptosis assays
PD-1 Ig fusion protein or control IgG were coated on 96 well-plates for 18 hours at 4°C. Full-length B7-H1/P815 cells were cultured with Staurosporine (STP; 1 μM) in the well-coated PD-1Ig or control IgG. Cells were harvested after 24 hours and stained with annexin-V (BD PharMingen) at 5 μL/test, propidium iodide (PI; Sigma-Aldrich, St Louis, MO) at 5 μg/mL for 1 hours and mAbs against H-2Dd. Apoptosis was calculated as the percentage of annexin V+ PI− cells gated in the H-2Dd+ fractions.
Results
Expression of B7-H1 confers resistance of tumor cells to specific CTL-mediated lysis
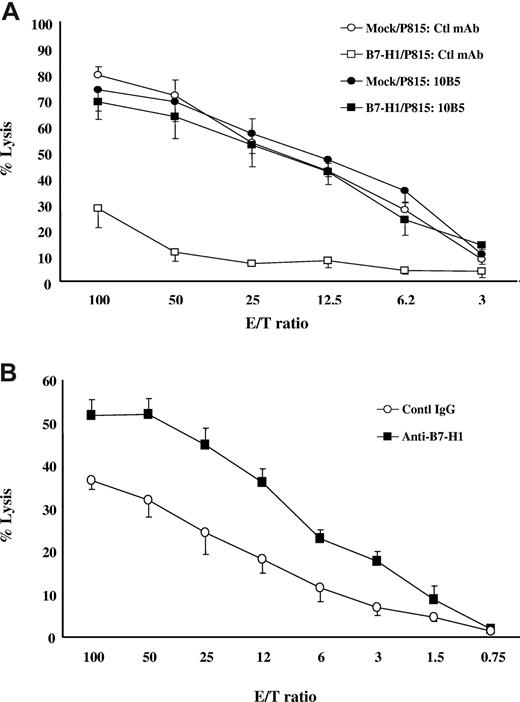
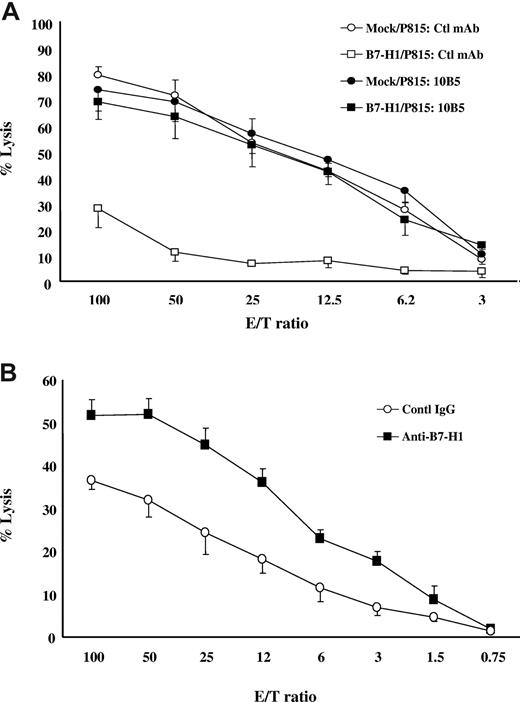
Previous studies showed that expression of B7-H1 rendered P815 cells resistant to tumor-specific CD8+ CTL, and this resistance requires B7-H1 and PD-1 interaction.20 To determine whether this observation could be generalized, we first established an allogeneic CTL line by stimulation of B6-derived T cells with irradiated spleen cells from DBA/2 mice (H-2d) and subsequently examined the susceptibility of mock/P815 and B7-H1/P815 cells to lysis in a 4-hour 51Cr release assay. As predicted, whereas mock/P815 cells were susceptible to lysis by allogeneic CTL in a wide range of E/T ratios, B7-H1/P815 cells were much more resistant. However, this resistance could be completely eliminated by inclusion of murine B7-H1–neutralizing mAb. (Figure 1A). Next, we used Renca cells as targets for lysis by alloantigen-specific TCR transgenic 2C T cells. Renca is a murine renal cell carcinoma and does not constitutively express B7-H1 on cell surface. However, expression of B7-H1 could be induced by IFN-γ (data not shown). After incubation with mouse IFN-γ (5 ng/mL) for 48 hours, Renca cells were cocultured with preactivated 2C T cells for 4 hours in the presence of control or antimurine B7-H1 mAb (clone10B5). 10B5 significantly increased lysis of 2C CTLs against Renca cells in a wide range of E/T ratios (from 3:1 to 100:1; Figure 1B). Taken together, our data indicate that B7-H1–mediated “molecular shield” is not limited to a specific T cell or certain tumor lines.
Molecular shield is observed generally. (A) B7-H1+ P815 cells are resistant to allospecific CTL-mediated lysis. Allospecific T cells were incubated at indicated E/T ratios with 51Cr-labeled mock/P815 or B7-H1/P815 cells in the presence of control IgG or anti-mouse B7-H1 mAb (clone 10B5) for 4 hours. CTL activity was determined in a 51Cr release assay. Each point is the mean of triplicates with SD. The data are representative of 3 experiments. (B) B7-H1+ Renca cancer cells are resistant to allospecific CTL-mediated lysis. Renca cells were incubated with IFN-γ (5 ng/mL) for 48 hours to up-regulate B7-H1 expression (data not shown). Activated 2C CTLs were incubated at indicated E/T ratios with 51Cr-labeled Renca cells with control IgG or antimurine B7-H1 antibody (clone 10B5) for 4 hours. CTLs activity was determined in a 51Cr release assay. Each point is the means of triplicates with SD. The data are representative of 3 experiments.
Molecular shield is observed generally. (A) B7-H1+ P815 cells are resistant to allospecific CTL-mediated lysis. Allospecific T cells were incubated at indicated E/T ratios with 51Cr-labeled mock/P815 or B7-H1/P815 cells in the presence of control IgG or anti-mouse B7-H1 mAb (clone 10B5) for 4 hours. CTL activity was determined in a 51Cr release assay. Each point is the mean of triplicates with SD. The data are representative of 3 experiments. (B) B7-H1+ Renca cancer cells are resistant to allospecific CTL-mediated lysis. Renca cells were incubated with IFN-γ (5 ng/mL) for 48 hours to up-regulate B7-H1 expression (data not shown). Activated 2C CTLs were incubated at indicated E/T ratios with 51Cr-labeled Renca cells with control IgG or antimurine B7-H1 antibody (clone 10B5) for 4 hours. CTLs activity was determined in a 51Cr release assay. Each point is the means of triplicates with SD. The data are representative of 3 experiments.
PD-1 signaling is not required for molecular shielding of tumor cells from T-cell killing
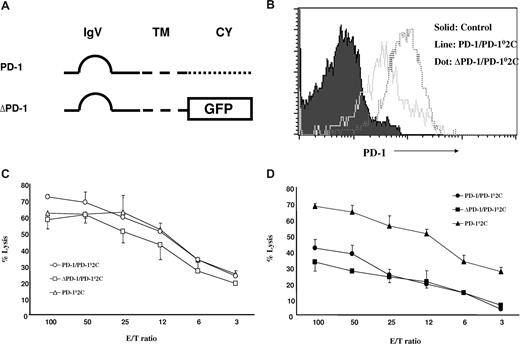
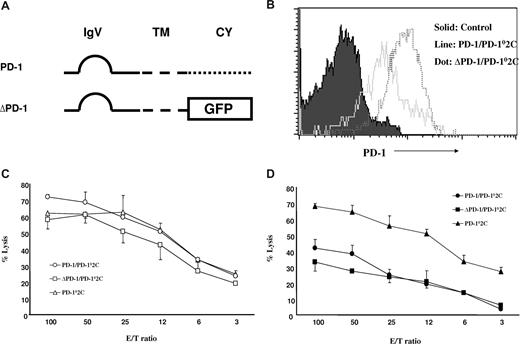
Our previous study showed that interaction between B7-H1 and PD-1 is required for the formation of a molecular shield.20 It is widely accepted that signaling from B7-H1 to PD-1 on T cells delivers a negative signal to suppress T-cell responses.11 Therefore, one possible explanation for molecular shield is tumor-associated B7-H1 signaling through PD-1 into T cells, leading to transient loss of T-cell cytolytic activity. To test this possibility, we first prepared truncated PD-1, in which the intracellular domain of PD-1 was replaced by GFP gene (Figure 2A) to eliminate its intracellular signaling. This truncated PD-1 (ΔPD-1) as well as wild-type PD-1 (PD-1) was inserted into lentiviral vectors for efficient T-cell transduction. To eliminate interference of endogenous PD-1 on T cells, we first backcrossed 2C T cells to PD-1KO mice (2C × PD-1KO) and established PD-1KO (PD-10) 2C CTLs cloned from 2C × PD-1KO mice. A PD-102C CTL clone was transduced with the recombinant PD-1 lentiviruses containing either ΔPD-1 or PD-1 to establish CTL lines. Both PD-1/PD-102C and ΔPD-1/PD-102C lines, which stably expressed cell surface PD-1, were established. Both 2C lines express high-level cell surface PD-1 (Figure 2B). Although ΔPD-1/PD-102C expresses somewhat higher levels of cell surface PD-1 than PD-1/PD-102C, its cytolytic activity against mock/P815 cells is comparable to 2C CTL from PD-1KO mice (Figure 2C). This result indicates that cytolytic activities of these CTL lines are undistinguishable in the absence of B7-H1 on tumor cells. When B7-H1/P815 cells were used as targets, cytolytic activity of PD-1/PD-102C CTLs was decreased to approximately 50% compared with PD-102C T cells. Interestingly, ΔPD-1/PD-102C CTLs demonstrated a virtually identical decrease in cytolytic activity as PD-102C T cells (Figure 2D). Therefore, the intracellular domain of PD-1 is not required for the resistance to CTL lysis. Our results thus support that PD-1 signaling to T cells does not contribute to the molecular shield and suggest a possible role for reverse signaling by B7-H1 into the tumor cell as a mechanism of molecular shielding.
PD-1 signaling to T cells is not required for molecular shield. (A) Schematic of wild-type PD-1 (PD-1) and intracellular domain-truncated PD-1 (ΔPD-1) genes. IgV indicates IgV domain; TM, transmembrane domain; CY, cytoplasmic domain, GFP, gene encoding for GFP. (B) The expression of PD-1 on 2C × PD-1KO T cells upon transduction. PD-1KO (PD-10) 2C T cells were transduced with lentiviruses containing either wild-type PD-1 (PD-1) or truncated PD-1 (ΔPD-1), and each cell line was examined for PD-1 expression by flow cytometry using anti-mouse PD-1 mAb (G4). (C,D) Cytolytic activity of PD-1 transduced PD-102C against P815 tumor cells. Activated PD-1/PD-102C, ΔPD-1/PD-102C, or nontransduced PD-102C CTL was incubated at indicated E/T ratios with 51Cr-labeled mock/P815 (C) or B7-H1/P815 (D) cells in the presence of control IgG or anti-mouse B7-H1 mAb (10B5) for 4 hours. CTL activity was determined in a 51Cr release assay. Each point is the mean of triplicates with SD. Data are representative of 3 experiments.
PD-1 signaling to T cells is not required for molecular shield. (A) Schematic of wild-type PD-1 (PD-1) and intracellular domain-truncated PD-1 (ΔPD-1) genes. IgV indicates IgV domain; TM, transmembrane domain; CY, cytoplasmic domain, GFP, gene encoding for GFP. (B) The expression of PD-1 on 2C × PD-1KO T cells upon transduction. PD-1KO (PD-10) 2C T cells were transduced with lentiviruses containing either wild-type PD-1 (PD-1) or truncated PD-1 (ΔPD-1), and each cell line was examined for PD-1 expression by flow cytometry using anti-mouse PD-1 mAb (G4). (C,D) Cytolytic activity of PD-1 transduced PD-102C against P815 tumor cells. Activated PD-1/PD-102C, ΔPD-1/PD-102C, or nontransduced PD-102C CTL was incubated at indicated E/T ratios with 51Cr-labeled mock/P815 (C) or B7-H1/P815 (D) cells in the presence of control IgG or anti-mouse B7-H1 mAb (10B5) for 4 hours. CTL activity was determined in a 51Cr release assay. Each point is the mean of triplicates with SD. Data are representative of 3 experiments.
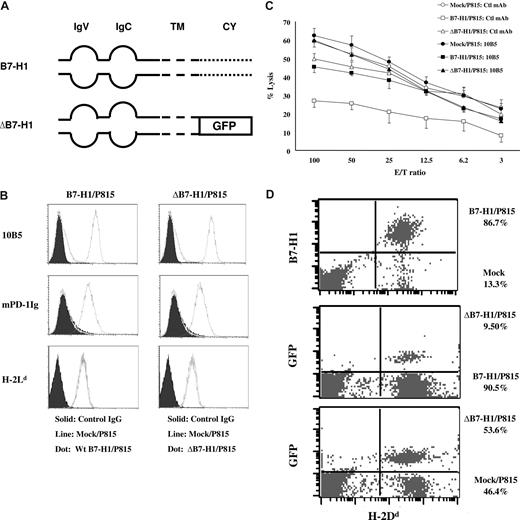
Intracellular domain of B7-H1 is required for molecular shielding
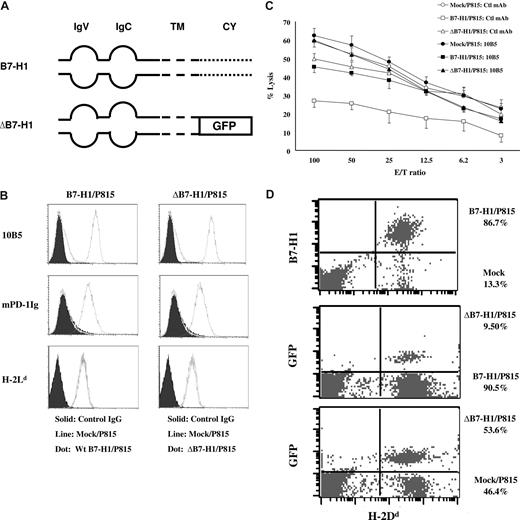
To determine the role of tumor-associated B7-H1 signaling in the formation of the molecular shield, we constructed a vector to express truncated B7-H1 (ΔB7-H1), in which the intracellular domain of B7-H1 was replaced by GFP gene (Figure 3A). A stable P815 line expressing ΔB7-H1 was established, and the expression of B7-H1 and H-2 Ld (Figure 3B) as well as the ability to bind PD-1Ig fusion protein or anti–B7-H1 antibody are comparable to the line expressing wild-type B7-H1 (B7-H1; Figure 3B). We also confirmed that both ΔB7-H1/P815 and B7-H1/P815 cells could inhibit the proliferation of T cells in vitro (data not shown). These data indicate that ΔB7-H1 has normal binding to PD-1 and induces functional PD-1 in T cells. We next determined if P815 cells expressing ΔB7-H1 could still confer tumor resistance to CTL lysis. Whereas B7-H1/P815 cells were resistant to lysis by 2C T cells, ΔB7-H1/P815 cells lost resistance and were lysed equally as mock/P815 cells by 2C T cells. In addition, the inclusion of 10B5 did not affect the lysis (Figure 3C), indicating that the intracellular domain of B7-H1 is critical for the formation of molecular shield. To validate this, we did 1:1 mixes of mock/P815:B7-H1/P815, mock/P815:ΔB7-H1/P815, and B7-H1/P815:ΔB7-H1/P815 followed by coincubation with 2C CTLs for 4 hours, as described previously. The cytolysis for each cell was analyzed by flow cytometry. The ratios of B7-H1/P815 versus mock/P815, B7-H1/P815 versus ΔB7-H1/P815, and ΔB7-H1/P815 versus mock/P815 were 6.51, 9.50, and 1.15, respectively (Figure 3D). These results indicate that ΔB7-H1, similar to mock/P815, fails to confer molecular shielding to P815 and could not induce cytolytic function of 2C T cells in vitro.
B7-H1 signaling to tumor cells is required for molecular shield. (A) Schematic of the wild-type B7-H1 (B7-H1) and cytoplasmic domain-truncated B7-H1 (ΔB7-H1) genes. IgV indicates IgV domain; IgC, IgC domain; TM, transmembrane domain; CY, cytoplasmicdomain; GFP, gene encoding GFP. (B) The expression of wild-type B7-H1 (B7-H1; top left) or truncated B7-H1 (ΔB7-H1) on P815 cells (top right) after transfection was determined by flow cytometry using anti-mouse B7-H1 mAb (10B5) or murine PD-1Ig fusion protein. These cell lines were also stained by anti–H-2Ld mAb. (C) Activated 2C CTLs were incubated at indicated E/T ratios with 51Cr-labeled mock/P815, B7-H1/P815, or ΔB7-H1/P815 cells in the presence of control (Ctl) IgG or anti-mouse B7-H1 mAb (10B5) for 4 hours. CTL activity was determined in a 51Cr release assay. Each point is the mean of triplicates with SD. The data are representative of at least 3 experiments. (D) Equal mix of mock/P815 and B7-H1/P815 cells (top), ΔB7-H1/P815, and B7-H1/P815 cells (middle), or mock/P815 and ΔB7-H1/P815 (bottom) were coincubated with 2C CTLs for 4 hours. The E/T ratio is 2.5:1. Cells were harvested and analyzed by flow cytometry. The numbers in the graph indicate the percentage of cells in H-2Dd+ population. The data are representative of at least 3 experiments.
B7-H1 signaling to tumor cells is required for molecular shield. (A) Schematic of the wild-type B7-H1 (B7-H1) and cytoplasmic domain-truncated B7-H1 (ΔB7-H1) genes. IgV indicates IgV domain; IgC, IgC domain; TM, transmembrane domain; CY, cytoplasmicdomain; GFP, gene encoding GFP. (B) The expression of wild-type B7-H1 (B7-H1; top left) or truncated B7-H1 (ΔB7-H1) on P815 cells (top right) after transfection was determined by flow cytometry using anti-mouse B7-H1 mAb (10B5) or murine PD-1Ig fusion protein. These cell lines were also stained by anti–H-2Ld mAb. (C) Activated 2C CTLs were incubated at indicated E/T ratios with 51Cr-labeled mock/P815, B7-H1/P815, or ΔB7-H1/P815 cells in the presence of control (Ctl) IgG or anti-mouse B7-H1 mAb (10B5) for 4 hours. CTL activity was determined in a 51Cr release assay. Each point is the mean of triplicates with SD. The data are representative of at least 3 experiments. (D) Equal mix of mock/P815 and B7-H1/P815 cells (top), ΔB7-H1/P815, and B7-H1/P815 cells (middle), or mock/P815 and ΔB7-H1/P815 (bottom) were coincubated with 2C CTLs for 4 hours. The E/T ratio is 2.5:1. Cells were harvested and analyzed by flow cytometry. The numbers in the graph indicate the percentage of cells in H-2Dd+ population. The data are representative of at least 3 experiments.
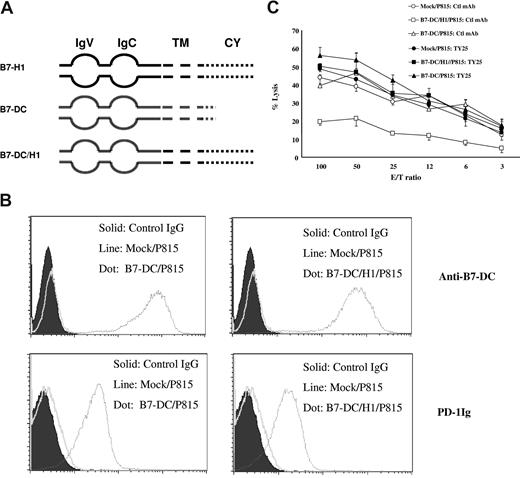
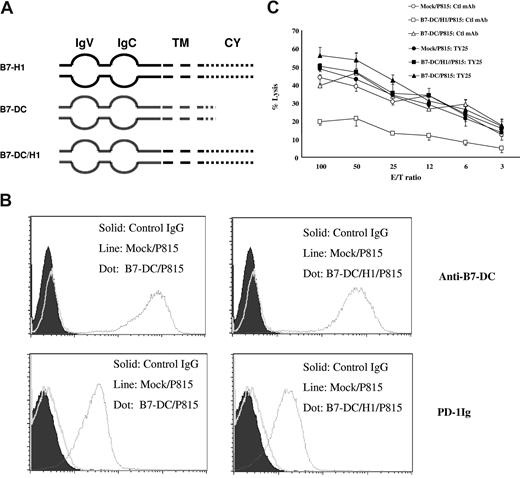
Although our previous data suggest that intracellular domain of B7-H1 is important in determining the formation of molecular shield, it could not be excluded that the extracellular domain of B7-H1 may also contribute to the formation of molecular shield. To address this issue, we replaced the extracellular domain of B7-H1 with corresponding region from B7-DC, another counter-receptor for PD-124 (Figure 4A). The chimeric gene B7-DC/H1 was used for the transfection to establish stable B7-DC/H1/P815 line. P815 cells expressing wild-type B7-DC (B7-DC/P815) were also established as the control. The expression of B7-DC and H-2 Ld (data not shown) as well as ability of these cell lines to bind PD-1Ig was the same in both B7-DC/P815 and B7-DC/H1/P815 lines based on flow cytometry analysis (Figure 4B). As shown in Figure 4C, B7-DC/P815 cells could be lysed equally as well as mock/P815 cells by 2C T cells. This result indicates that B7-DC is not capable of forming a molecular shield even though B7-DC is capable of engaging PD-1 on T cells with affinity at least as high as B7-H1. In contrast, B7-DC/H1/P815 cells were resistant to 2C CTLs, and this resistance was completely blocked by anti–B7-DC mAb (clone TY25). Our findings thus demonstrate that intracellular but not extracellular domain of B7-H1 has unique character to form molecular shield against T-cell lysis.
Analysis of chimera of B7-H1 and B7-DC in molecular shield. (A) Schematic of the full-length B7-H1 (B7-H1), full-length B7-DC (B7-DC), and the chimera of B7-H1 and B7-DC (B7-DC/H1) genes. IgV indicates IgV domain; IgC, IgC domain; TM, transmembrane domain; CY, cytoplasmic domain. (B) The expression of B7-DC/B7-H1 chimeric genes on P815 cells. The expression of B7-DC on B7-DC/P815 (top left) or B7-DC/H1/P815 (top right) were determined by flow cytometry analysis using anti–mouse B7-DC mAb (clone TY25). The ability of B7-DC/P815 (bottom left) or B7-DC/H1/P815 (bottom right) to bind PD-1Ig fusion protein was also determined similarly. (C) Extracellular domain swap between B7-H1 and B7-DC does not affect molecular shield. Activated 2C CTLs were incubated at indicated E/T ratios with 51Cr-labeled mock/P815, full-length B7-DC/P815, or B7-DC/H1/P815 cells in the presence of control IgG or anti–mouse B7-DC mAb for 4 hours. CTL activity was determined in a 51Cr release assay. Each point is the mean of triplicates with SD. The data are representative of at least 3 experiments.
Analysis of chimera of B7-H1 and B7-DC in molecular shield. (A) Schematic of the full-length B7-H1 (B7-H1), full-length B7-DC (B7-DC), and the chimera of B7-H1 and B7-DC (B7-DC/H1) genes. IgV indicates IgV domain; IgC, IgC domain; TM, transmembrane domain; CY, cytoplasmic domain. (B) The expression of B7-DC/B7-H1 chimeric genes on P815 cells. The expression of B7-DC on B7-DC/P815 (top left) or B7-DC/H1/P815 (top right) were determined by flow cytometry analysis using anti–mouse B7-DC mAb (clone TY25). The ability of B7-DC/P815 (bottom left) or B7-DC/H1/P815 (bottom right) to bind PD-1Ig fusion protein was also determined similarly. (C) Extracellular domain swap between B7-H1 and B7-DC does not affect molecular shield. Activated 2C CTLs were incubated at indicated E/T ratios with 51Cr-labeled mock/P815, full-length B7-DC/P815, or B7-DC/H1/P815 cells in the presence of control IgG or anti–mouse B7-DC mAb for 4 hours. CTL activity was determined in a 51Cr release assay. Each point is the mean of triplicates with SD. The data are representative of at least 3 experiments.
Formation of molecular shield is correlated with tumor resistance to immunotherapy
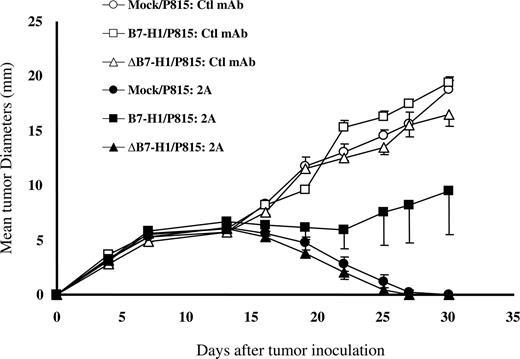
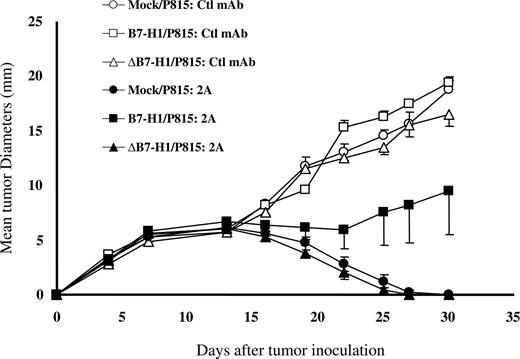
We next evaluated whether reverse signaling by B7-H1 conferred resistance to immune killing of tumors in vivo. We showed previously that B7-H1/P815 tumors were resistant to the treatment by anti-CD137 mAb, whereas mock/P815 or wild-type P815 was sensitive in vivo.20 Anti-CD137 immunotherapy of tumors has been shown to be attributable to enhanced stimulation of endogenous tumor-specific CD8 T cells in vivo. Because ΔB7-H1/P815 fails to be shielded from CTL lysis in vitro, we determined whether or not this line is also resistant to CD137 mAb therapy in vivo. To do so, we first inoculated ΔB7-H1/P815 cells into syngeneic DBA/2 mice to induce progressive growth of subcutaneous tumors. Mock/P815 and B7-H1/P815 cells were also inoculated into a group of mice as controls. As expected, all tumors treated with control mAb grew equally well and eventually killed the mice because growth of P815 tumor itself is not sufficient to induce significant antitumor immunity. However, treatment by anti-CD137 mAb (clone 2A) induced complete regression of mock/P815 tumors after a transient growth period, whereas B7-H1/P815 tumors continued to grow and eventually killed the mice. These data indicate that B7-H1–mediated molecular shielding operates to prevent immune attack in vivo. In contrast, P815 cells expressing ΔB7-H1 regressed completely, a result identical to mock/P815 (Figure 5). Our results thus further support that the intracellular domain of B7-H1 is required for the formation of a molecular shield, which correlates with tumor resistance in vivo.
Loss of molecular shield is associated with increased efficacy of anti-CD137 mAb therapy. Groups of 10 DBA/2 mice were given subcutaneous injections of 5 × 104 mock/P815, B7-H1/P815 or ΔB7-H1/P815 cells on day 0. Mice were then treated intraperitoneally with 200 μg control IgG or anti–mouse CD137 mAb (clone 2A) at days 7 and 10. Each point is the mean of 10 with SD. The data are representative of at least 3 experiments.
Loss of molecular shield is associated with increased efficacy of anti-CD137 mAb therapy. Groups of 10 DBA/2 mice were given subcutaneous injections of 5 × 104 mock/P815, B7-H1/P815 or ΔB7-H1/P815 cells on day 0. Mice were then treated intraperitoneally with 200 μg control IgG or anti–mouse CD137 mAb (clone 2A) at days 7 and 10. Each point is the mean of 10 with SD. The data are representative of at least 3 experiments.
B7-H1 transmits an antiapoptotic signal to tumor cells
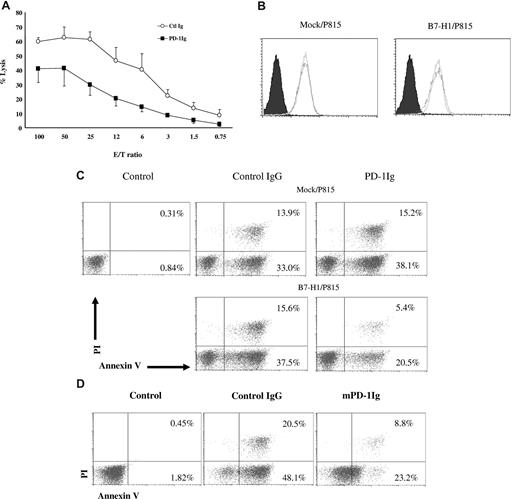
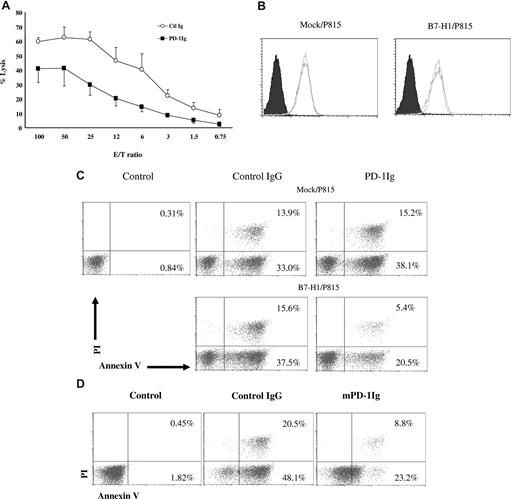
To explore the mechanism of molecular shielding by B7-H1 signaling, we developed a T cell–free system to induce resistance of tumor cells to CTL lysis. In this system, PD-1Ig was immobilized on plastic plates and subsequently incubated with tumor cells to engage B7-H1. Twenty-four hours later, tumor cells were incubated with PD-102C T cells to test susceptibility of tumor cells to lysis. After 4 hours of incubation, the lysis of PD-1Ig–treated B7-H1/P815 cells by PD-102C T cells was significantly lower than control IgG-treated B7-H1/P815 cells in a wide range of E/T ratios (Figure 6A). This provides a simple system to explore the mechanism of B7-H1–induced molecular shielding in the absence of T cells in the induction phase.
B7-H1 transmits an antiapoptotic signal to tumor cells. (A) B7-H1/P815 were cultured in the wells, which were precoated with either control IgG (Ctl Ig) or murine PD-1Ig (PD-1Ig) at 5 μg/mL for 18 hours. After extensive washing, cells were labeled with 51Cr and further incubated with activated PD-102C CTLs at indicated E/T ratios for 4 hours. CTL activity was determined in a 51Cr release assay. Each point is the mean of triplicates with SD. The data are representative of at least 3 experiments. (B) PD-1 stimulation of B7-H1+ tumor cells does not change the expression of Fas. The expression of Fas on mock/P815 or B7-H1/P815 cells before and after culture with immobilized murine PD-1Ig was determined by antimurine Fas mAb in flow cytometric analysis as described above. (C) PD-1 stimulation reduced the susceptibility of tumor cells to Fas mAb-mediated apoptosis. Mock/P815 or B7-H1/P815 cells were cultured in the presence of immobilized control IgG or murine PD-1Ig (5 μg/mL) for 18 hours. After extensive washing, cells were then transferred to the wells coated with anti-mouse Fas antibody (5 μg/mL). After 48 hours of culture, the cells were subjected to an annexin-V and PI binding assay. Mock/P815 without treatment was served as negative control (control). (D) PD-1 stimulation reduced the susceptibility of tumor cells to STP-induced apoptosis. B7-H1/P815 cells were cultured in the presence of immobilized control IgG or murine PD-1Ig (5 μg/mL) for 18 hours. After extensive washing, cells were treated with STP at 0.25 μM for 8 hours and subjected to an annexin-V binding assay. The numbers in the graph indicate the percentage of annexin-V+ or annexin-V+ and PI+ cells.
B7-H1 transmits an antiapoptotic signal to tumor cells. (A) B7-H1/P815 were cultured in the wells, which were precoated with either control IgG (Ctl Ig) or murine PD-1Ig (PD-1Ig) at 5 μg/mL for 18 hours. After extensive washing, cells were labeled with 51Cr and further incubated with activated PD-102C CTLs at indicated E/T ratios for 4 hours. CTL activity was determined in a 51Cr release assay. Each point is the mean of triplicates with SD. The data are representative of at least 3 experiments. (B) PD-1 stimulation of B7-H1+ tumor cells does not change the expression of Fas. The expression of Fas on mock/P815 or B7-H1/P815 cells before and after culture with immobilized murine PD-1Ig was determined by antimurine Fas mAb in flow cytometric analysis as described above. (C) PD-1 stimulation reduced the susceptibility of tumor cells to Fas mAb-mediated apoptosis. Mock/P815 or B7-H1/P815 cells were cultured in the presence of immobilized control IgG or murine PD-1Ig (5 μg/mL) for 18 hours. After extensive washing, cells were then transferred to the wells coated with anti-mouse Fas antibody (5 μg/mL). After 48 hours of culture, the cells were subjected to an annexin-V and PI binding assay. Mock/P815 without treatment was served as negative control (control). (D) PD-1 stimulation reduced the susceptibility of tumor cells to STP-induced apoptosis. B7-H1/P815 cells were cultured in the presence of immobilized control IgG or murine PD-1Ig (5 μg/mL) for 18 hours. After extensive washing, cells were treated with STP at 0.25 μM for 8 hours and subjected to an annexin-V binding assay. The numbers in the graph indicate the percentage of annexin-V+ or annexin-V+ and PI+ cells.
Fas has been shown to participate in the death of some cancer cells. Mock/P815 and B7-H1/P815 cells express comparable levels of Fas on cell surface based on flow cytometric analysis using specific mAb against murine Fas. Incubation of these tumor lines in the presence of PD-1Ig does not affect expression of Fas (Figure 6B). Treatment of mock/P815 and B7-H1/P815 cells by anti-Fas mAb (and control Ig) also induced a comparable apoptosis (54.9% vs 57.2%). However, pretreatment by PD-1Ig significantly decreased the death of B7-H1/P815 cells (35.8%), whereas the death of mock/P815 cells remained the same (52.4%; Figure 6C). This represents 32% inhibition.
We next tested whether B7-H1 confers resistance to drugs that induce apoptosis of tumor cells. STP (Streptomyces staurospores) is a relatively nonselective protein kinase inhibitor. STP is often used as a general method for inducing apoptosis of tumor cells.25 PD-1Ig pretreatment for 8 hours drastically decreased apoptosis of B7-H1/P815 cells (16.9%) compared with control Ig-treated cells (74.9%; Figure 6D). Taken together, our results support that B7-H1 is an antiapoptotic receptor against death of cancer cells.
Discussion
Our study provides the evidence that B7-H1 can act as a receptor to transmit antiapoptotic signal to cancer cells, leading to resistance to cytolysis by CTL as well as Fas and drug-induced apoptosis. In addition, we demonstrate that elimination of the intracellular domain of B7-H1 can ablate cancer resistance to immune destruction, which is accompanied with regression of tumors in mouse models. In the context of existence of multiple ligands or counter-receptors for B7-H1 in hematopoietic cells, our findings suggest a new mechanism contributing to tumor escape from immune destruction in vivo.
Several previous studies by us and others indicate that B7-H1 can evade immune destruction by engaging inhibitory receptor(s) on T cells.20–22 These studies are particularly intriguing because up-regulation of B7-H1 is found in the majority of human cancers. However, previous studies focused on the function of B7-H1 as a ligand (ie, on interaction with the PD-1 receptor on T cells, inhibitory signal would be delivered to T cells, leading to apoptosis, suppression, anergy, and exhaustion).7,10–13 This immune inhibitory function occurs during the induction of T-cell activation in lymphoid organs and/or in the effector phase after migration of activated T cells to peripheral organs.15,22 However, the current study reveals a completely different mechanism for B7-H1 inhibition of antitumor immune responses by transmitting an antiapoptotic signal to tumor cells. However, in these in vitro CTL assays, PD-1 signaling does not seem to suppress cytolytic function of CTL. It is possible that PD-1–mediated dysfunction of CTL may require more than 4 to 6 hours of exposure, which, however, is sufficient for B7-H1 to deliver antiapoptotic function on tumor cells. Although it appears that the predominant mechanism in vitro appears to be B7-H1–mediated tumor resistance, both B7-H1–mediated tumor resistance and PD-1–mediated T-cell dysfunction could play the roles simultaneously in vivo. For example, immunotherapy of established tumors induced by ΔB7-H1/P815 was only partially active compared with tumors induced by wild-type B7-1/P815 (Figure 5), suggesting a role of PD-1–mediated immune dysfunction in vivo. B7-H1 has multiple ligands or counter-receptors. One of the ligands, PD-1, is found to be expressed on T cells, B cells, and a lymphoid/myeloid progenitor cell line.26,27 A recent study shows that in addition to PD-1, B7-H1 could also bind CD80, which is found in the majority of hematopoietic cells as well as stromal cells.19 Because immune cells including T and B cells are often found to infiltrate tumors, our study suggests a new mechanism by which cancer cells could use receptors of immune system to escape from destruction even by nonimmunologic agents.
The intracellular domain of B7-H1 has 30 amino acids and does not contain any obvious motifs for signaling to known antiapoptotic molecules. One possible explanation is that intracellular domain of B7-H1 binds adaptor molecules that deliver antiapoptotic signal. By preparation of truncated B7-H1, our data demonstrate that intracellular domain of B7-H1 on cancer cells is required for the formation of molecular shield. In contrast, expression of truncated PD-1 or wild-type PD-1 into PD-1−/− 2C transgenic T cells confers CTL resistance compared with nontransduced PD-1−/− 2C transgenic T cells (Figure 2D). An alternative interpretation is that transduced PD-1 may interfere with the TCR–MHC interaction. However, this is considered unlikely because PD-1−/− 2C transgenic T cells expressing truncated or wild-type PD-1 could lyse B7-H1− P815 equally well (Figure 2C). Taken together, our data indicate that PD-1 acts as a ligand in this system to stimulate B7-H1. This notion is further supported by the experiments showing that B7-DC, which is capable of binding to PD-1, on tumor cells does not induce resistance of tumor cells to CTL lysis, whereas replacement of its intracellular domain with that from B7-H1 does (Figure 4C). By comparison with B7-H1, murine B7-DC has a very short cytoplasmic tail (4 amino acids) and would not be expected to signal equivalently to B7-H1. Finally, incubation of B7-H1+ tumor cells in the presence of immobilized PD-1 is sufficient to induce resistance to apoptosis (Figure 6). It has been shown that binding to B7-H1 to PD-1 induces an inhibitory signal toward T cells. It is unclear why this effect is not evident in our coculture systems. It is likely that molecular shield occurs very quickly within a couple of hours after exposure, whereas induction of T-cell suppression through PD-1 as a receptor requires more time. This is supported by our previous study showing that induction of apoptosis of human tumor antigen-specific T cells by B7-H1+ cancer cells required 2 to 3 days.7
Although our data demonstrate a role of B7-H1 in the induction of antiapoptotic mechanism on cancer cells, underlying biochemical pathways remain to be defined. We examined several major antiapoptotic and apoptotic pathways using the Apoptosis Oligo GEArray (Superarray Bioscience Corporation, Frederick, MD) and did not find major difference between control Ig versus PD-1Ig treatment on B7-H1/P815 cells in the expression of apoptosis-related genes, including tumor necrosis factor (TNF) receptor family, Bcl-2 family, caspase family, integrin-associated protein family, TNF receptor–associated factor family, caspase recruitment domain family, death domain family, death effector domain family, CIDE family and antiapoptosis genes, etc (T.A., unpublished data, 2007). It has been shown that CTL-mediated lysis of target cells is mediated by at least 2 mechanisms: granule-mediated cytolysis and membrane receptor-mediated apoptosis. After contact of target cells, perforin could be released from CTL to form channels on target cells, which allows passage of important molecules such as granzyme B. Granzyme B will activate caspase 3 and/or cause mitochondrial disruption after cleavage of Bid,28 leading to apoptosis. In addition, cell surface apoptotic receptors such as Fas or TNF–related apoptosis-inducing ligand may also induce apoptosis of target cells after engaging their ligands or counter-receptor.29,30 B7-H1 appears to have a broad role in the resistance of apoptosis of tumor cells, including CTL-mediated death as well as Fas- and STP-mediated apoptosis. It is thus possible that B7-H1 represents an early signal in the cascade to induce the inhibition of both pathways. In the context of broad expression of B7-H1 on tumor cells, this mode of antiapoptosis may play significant role in the prevention of tumor cell death by immune therapy and chemotherapy.
Our findings have important implications in design of cancer therapy. Because B7-H1 has more than one receptor, blockade of a single receptor such as anti–PD-1 mAb may not be sufficient to eliminate the molecular shield. When designing blocking mAb to B7-H1, it is desirable that this mAb should block all receptor binding sites that could trigger formation of molecular shield. Because the role of B7-H1–mediated molecular shield is mediated through prevention of apoptosis, these findings support a combined therapy using blockade of B7-H1 and apoptosis-inducing drugs. Finally, it is desirable to design small molecule inhibitors that could block the signal pathway mediated by the intracellular domain of B7-H1. Our findings thus provide an excellent opportunity to enhance cancer immunity and to prevent tumor-mediated escape of host immune responses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Drew M. Pardoll for his careful reading and comments and Jennifer Osborne for editing this manuscript. We would also like to extend a special thanks to those who provided us with mice, cell lines, and reagents, and we cite their gifts in the text.
This study was supported in part by National Institutes of Health grants CA85721, CA97085, and CA113341.
National Institutes of Health
Authorship
Contribution: T.A., S.Y., G.Z., A.S.F., and S.J.F. performed the experiments. T.A. and L.C. wrote this paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lieping Chen, Johns Hopkins Medical Institutions, 209 David H. Koch Cancer Research Building, 1550 Orleans St, Baltimore, MD 21231; e-mail: lchen42@jhmi.edu.