Abstract
Second primary malignancies and premature death are a concern for patients surviving treatment for childhood lymphomas. We assessed mortality and second malignant neoplasms (SMNs) among 1082 5-year survivors of non-Hodgkin lymphoma (NHL) in the Childhood Cancer Survivor Study, a multi-institutional North American retrospective cohort study of cancer survivors diagnosed from 1970 to 1986. Standardized mortality ratios (SMRs) and standardized incidence ratios (SIRs) were calculated using US population rates. Relative risks for death and solid tumor SMNs were calculated based on demographic, clinical, and treatment characteristics using Poisson regression models. There were 87 observed deaths (SMR = 4.2; 95% CI, 1.8-4.1) with elevated rates of death from solid tumors, leukemia, cardiac disease, and pneumonia. Risk for death remained elevated beyond 20 years after NHL. Risk factors for death from causes other than NHL included female sex (rate ratio [RR] = 3.4) and cardiac radiation therapy exposure (RR = 1.9). There were 27 solid tumor SMNs (SIR = 3.9; 95% CI, 2.6-5.7) with 3% cumulative incidence between 5 and 20 years after NHL diagnosis. Risk factors were female sex (RR = 3.1), mediastinal NHL disease (RR = 5.2), and breast irradiation (RR = 4.3). Survivors of childhood NHL, particularly those treated with chest RT, are at continued increased risk of early mortality and solid tumor SMNs.
Introduction
Non-Hodgkin lymphoma (NHL) accounts for 6% of cancers before age 20 years in the United States.1 Incidence increases from 0.5 per million at age younger than 5 years to 36.6 per million at ages 15 to 19 years in the United States.2 With 5-year survival estimated as 78% for children diagnosed with NHL during 1992 to 1998,3 the increasing number of patients surviving into middle age and late adulthood has prompted concern for adverse late health effects.4
Systemic chemotherapy (CT) is the mainstay of treatment for NHL,5-7 guided by histology and stage.8 Despite the ongoing evolution of treatment regimens, studies examining delayed health effects must take into account earlier regimens, including radiation therapy (RT) to bulk disease sites,5 central nervous system RT, COMP (cyclophosphamide, vincristine, methotrexate, and prednisone), a leukemia-adapted regimen,9 epipodophyllotoxins,10 doxorubicin, intrathecal CT, and other CT agents, some of which have been associated with second malignancies.7,11-14 An appreciation of factors associated with impaired long-term survival of these patients will aid clinicians in surveillance of childhood NHL survivors.
Predictors of late mortality and life-threatening late disease are not well established for 5-year survivors of pediatric NHL.10,15-17 An increased incidence of leukemia and several solid tumors18-27 as well as cardiomyopathy28-31 was documented in large studies after treatment for NHL in adulthood. The current analysis was undertaken to provide a detailed assessment of survival, cause-specific mortality, and second cancer incidence among 5-year survivors of NHL in the Childhood Cancer Survivor Study (CCSS) as a reference for healthcare providers monitoring survivors of childhood NHL. The CCSS study population, reflecting a large and unique resource, has been the source of a number of scientific publications.32 Previous reports have addressed outcomes relating to late mortality and second cancers in the entire cohort and after several childhood cancer diagnoses. The current report provides results of detailed analyses, using updated data, to provide a more comprehensive assessment of outcomes in an important subgroup of childhood cancer survivors.
Methods
Study population
Subjects with a confirmed diagnosis of NHL in the years 1970 to 1986 were identified through the CCSS, a multi-institutional retrospective cohort study of children diagnosed with cancer before age 21 who survived 5 years from diagnosis (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The overall study design has been described previously.32 For the current analyses, eligibility was limited to subjects with a primary diagnosis of NHL (ICD-O-2 codes 9592.3-9723.3). Of 1532 eligible NHL survivors, 1082 (71%) were enrolled on completing a survey questionnaire.
The human subjects committee at each participating institution reviewed and approved the CCSS protocol and questionnaire. Study subjects or parents (if study subject was < 18 years of age at contact) gave written informed consent to participate in the study and for release of medical records in accordance with the Declaration of Helsinki. Subjects were mailed a questionnaire requesting information on demographic characteristics, health practices, and medical conditions. Study questionnaires and medical records abstraction forms are available at www.stjude.org/ccss.
Using ICD-O site and morphology codes, primary site of disease was categorized as lymph nodes/hematopoietic (hereafter referred to as lymph nodes), gastrointestinal, head/neck (including brain), bone, mediastinum/heart (hereafter referred to as mediastinum), unknown, or other sites. NHL histopathology was characterized as Burkitt or Burkitt-like (hereafter referred to as Burkitt); lymphoblastic, including small lymphocytic or small cleaved cell (hereafter referred to as lymphoblastic); diffuse large cell, including immunoblastic, lymphosarcoma and reticulosarcoma (hereafter referred to as diffuse large cell); or other specified or unspecified. Stage information was limited to an assessment of metastases at the time of diagnosis for 750 of 1082 patients. Medical records of initial NHL treatment were reviewed using a standardized data abstraction protocol for 926 of 977 study subjects who consented to medical records review. Treatment details were missing in 5 of 926 subjects, leaving 921 subjects for analyses of therapy.
Abstracted treatment data included cumulative doses (mg/m2) and routes of administration for 28 CT agents32 ; surgical procedures related to NHL bulk disease; bone marrow transplantation; and NHL relapse. From individual patients' radiation records, including diagrams, photographs, treatment plans, daily records, and follow-up notes, collaborating medical physicists determined body regions (brain, head, neck, chest, abdomen, spine, pelvis, limbs, and total body) included in any treatment field. The breasts, heart, and thyroid were considered irradiated if any part were included in a treatment field.
Ascertainment of mortality and second malignant neoplasms
Analyses of mortality among 5-year NHL survivors were based on searches of the National Death Index in 1996 and 2002 for all eligible subjects. Among 1082 study participants, there were 89 confirmed deaths. Eighty-seven subjects with known date of death were included in analyses of all-cause mortality. Underlying cause of death was obtained from 79 death certificates and from 3 families and was not determined for 5 deaths.
Analyses of second malignant neoplasms (SMNs) were based on self-reported questionnaire responses through December 31, 2002, with review by study pathologist. Only second, and not third or subsequent, malignancies were included in analyses. SMNs that arose less than 5 years after initial NHL diagnosis date were not included. In cases in which the SMN could have represented recurrence of the original condition, the pathology reports were compared and slides were requested for review. If the determination could not be made, a conservative approach was taken and the case was classified as a recurrence.
Statistical analyses
SMRs were calculated as ratios of observed (O) to expected (E) numbers of deaths, calculated based on age-, sex-, and calendar time-specific mortality rates for the United States, obtained from the National Center for Health Statistics. Person-time at risk of mortality in survivors was considered to extend from 5 years after NHL diagnosis until the earliest of the date of death, most recent vital status assessment, or close of study follow-up (December 31, 2002). Excess absolute risks (EARs) were calculated as (O − E)/10 000 person-years.
Standardized incidence ratios (SIRs) were calculated as ratios of observed to expected numbers of new incident cancers, based on incidence rates from the US Surveillance, Epidemiology, and End Results (SEER) population-based cancer registries adjusted for age, sex, race, and calendar time period.3 Person-time at risk for second malignancies lasted from 5 years after NHL diagnosis until the earliest of the date of first SMN diagnosis, death, or most recent follow-up questionnaire, if completed by December 31, 2002. The SEER*Stat program (Information Management Systems, Rockville, MD) was used to calculate SMRs, SIRs, and EARs within strata of demographic, clinical, and treatment characteristics. The cumulative incidence of second cancers, adjusted for competing risks, was computed according to the method of Gooley et al.33 The Kaplan-Meier method was used to calculate proportional survival. Because cumulative incidence of SMNs has been calculated in some other studies using the Kaplan-Meier method, which does not adjust for competing risks, we also used this method to enhance comparability between studies. Analyses of SMNs focused on solid cancers arising in organs other than bone marrow or lymph nodes, excluding lymphoma and leukemia.
Poisson regression was used to calculate rate ratios (RRs) for all-cause mortality and solid SMN incidence among NHL survivors.34 Regression analyses were performed using the routine GENMOD (SAS version 9.1.3; SAS Institute, Cary, NC) incorporating external reference rates from SEER registries. Cohort members without medical records abstraction were excluded from analyses of therapeutic exposures; however, they contributed to analyses of demographic and disease characteristics. Variables examined for potential confounding or effect modification included age at NHL diagnosis, calendar year of NHL diagnosis, sex, race/ethnicity, and smoking. Likelihood ratio tests were conducted using 2-sided P values, and 95% likelihood-based confidence intervals (CIs) were calculated. Regression analyses were performed using SAS PROC PHREG (SAS Institute).
Results
Study subjects who were 5-year survivors of pediatric NHL were 70% male and 86% non-Hispanic white (Table 1). The median age at NHL diagnosis was 10 years. For mortality analyses, follow-up ranged from 0 to 27 years (mean, 17 years) from eligibility to death or the study close. Predominant lymphoma subtypes were Burkitt, lymphoblastic, and diffuse large cell, and the most common location was in lymph nodes (Table 1).
The proportion of subjects receiving CT rose from 67% to 95% during the study years 1970 to 1986, whereas RT declined from 85% to 53%. The proportion receiving 35 Gy or higher maximum tumor dose declined from 48% to 11%. Overall, 65% received combination CT and RT and 30% received CT only. More than 70% of all subjects received COMP. Doxorubicin and bleomycin were used more in older children (age, 15-20 years) with diffuse large cell lymphoma. Epipodophyllotoxins were used more often for lymphoblastic lymphoma than overall (17% vs 9%).
During 18 261 person-years of follow-up, 87 deaths occurred at a median age of 24 years and a median of 13 years after NHL diagnosis. Death occurred 4 times more often than expected among NHL survivors (SMR = 4.2; EAR = 36.3/10 000 person-years) (Table 2), with a higher SMR in female (SMR = 8.1; 95% CI, 5.3-12.0) than male (SMR = 3.5; 95% CI, 2.7-4.4) subjects. The cause-specific mortality rate ratio and difference were elevated for death from NHL, leukemia, solid tumors, cardiac diseases (primarily cardiomyopathy), and pneumonia, but not from external causes, including accidents, suicide, and homicide. The mortality rate ratio for death from all causes was highest 5 to 9 years after NHL diagnosis and was largely attributable to NHL deaths. Observed deaths remained higher than expected 20 or more years from NHL diagnosis for mortality from NHL, solid tumor SMNs, and cardiac diseases. Absolute risk (EAR) of death from circulatory disease increased with time since diagnosis. EAR for death from NHL declined and then later rose with increasing time since diagnosis. EARs for death from solid tumors were first significantly elevated 10 years from NHL diagnosis and remained relatively constant. There was only 1 death from a solid tumor within years 5 to 10. EARs for death from any cause declined and then increased with increasing time since diagnosis. Among 5-year survivors, proportional survival at 20 years after NHL diagnosis was 0.91.
Female sex, cardiac RT exposure, doxorubicin, and bleomycin were associated with increased relative risk of mortality from causes other than NHL in a multivariate model adjusted for RT and histopathology (Table 3). In a multivariate model including all of these factors, only female sex (RR = 2.8; 95% CI, 1.5-5.1) and cardiac RT exposure (RR = 2.1; 95% CI, 1.1-3.9) retained significant associations. No excess mortality risk was associated with alkylating agents as a group or with exposure to daunorubicin, cyclophosphamide, ifosfamide, hydroxyurea, methotrexate, vincristine, steroids, 6-mercaptopurine, L-asparaginase, 6-thioguanine, carmustine, or surgical procedures. Treatment with Ara-C, doxorubicin, epipodophyllotoxins, bleomycin, platinum drugs, and bone marrow transplantation was associated with an increased mortality rate ratio for deaths attributable to NHL (Table 3). No excess risk was associated with RT to the total body, abdomen, pelvis, brain, neck, spine, or limb. Cardiac RT exposure was nonsignificantly associated with death from cardiac diseases (RR = 4.6; 95% CI, 0.9-33.2). Of 3 deaths from pneumonia, 2 bacterial pneumonias occurred in subjects with history of splenectomy and 1 viral pneumonia occurred in a subject without history of splenectomy. During the later study years, there was a suggestion of a decreasing RR for causes other than NHL; however, this trend did not reach statistical significance (P =.098).
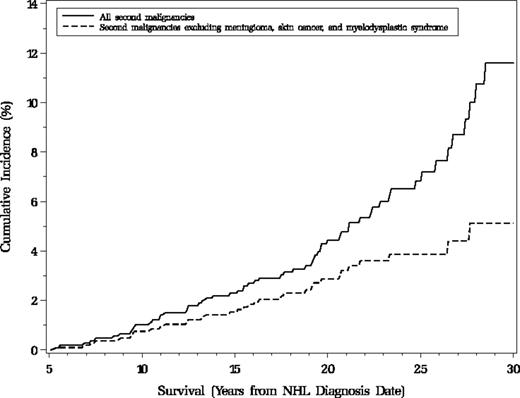
There were 31 self-reported and histologically confirmed malignant cancers among 5-year survivors, including 27 solid tumor malignancies, 3 lymphomas, and 1 leukemia (Table 4). The incidence of new malignancies was more than 3 times the expected rate (SIR = 3.5; 95% CI, 2.5-4.0) with significant excesses of cancer of the breast, thyroid, oral cavity and pharynx, bone, brain, and bladder. Figure 1 presents the cumulative incidence for second malignancies and other nonmalignant second tumors beyond 5 years from NHL diagnosis.
Cumulative incidence of second neoplasms among 1074 5-year survivors of pediatric non-Hodgkin lymphoma, Childhood Cancer Survivor Study, North America. The dashed line represents cumulative incidence of all malignant diagnoses, including bladder transitional cell carcinoma in situ, whereas the solid line includes diagnoses listed above in addition to 4 meningiomas (ICD-O-2: 9532.0, 953.0), 21 skin cancers (ICD-O-2: 8090.3, 8094.3), and one myelodysplastic syndrome (ICD-O-2: 9989.1). Only the first of any of these neoplasms is counted for each subject. Subjects were those alive 5 years after NHL diagnosis, excluding 6 who experienced secondary neoplasms within the first 5 years of NHL and 2 with unknown date of death.
Cumulative incidence of second neoplasms among 1074 5-year survivors of pediatric non-Hodgkin lymphoma, Childhood Cancer Survivor Study, North America. The dashed line represents cumulative incidence of all malignant diagnoses, including bladder transitional cell carcinoma in situ, whereas the solid line includes diagnoses listed above in addition to 4 meningiomas (ICD-O-2: 9532.0, 953.0), 21 skin cancers (ICD-O-2: 8090.3, 8094.3), and one myelodysplastic syndrome (ICD-O-2: 9989.1). Only the first of any of these neoplasms is counted for each subject. Subjects were those alive 5 years after NHL diagnosis, excluding 6 who experienced secondary neoplasms within the first 5 years of NHL and 2 with unknown date of death.
The SIR for solid tumor SMNs was higher in female than male subjects (Table 5) and remained significantly elevated in women after excluding breast cancer (SIR = 6.4; 95% CI, 3.3-11.1). Five of 6 thyroid cancer SMNs occurred in women, including 4 papillary carcinomas. One case of infiltrating ductal carcinoma of the breast was diagnosed in a man. No solid tumor SMNs were diagnosed after Burkitt lymphoma or NHL diagnosis before the age of 5 years. The SIR for solid tumor SMNs was significantly elevated for attained ages 5 to 19, 20 to 29, and 30 to 39 years. Overall cumulative incidence of solid tumor SMNs from 5 to 20 years after NHL diagnosis was 0.03 (95% CI, 0.02-0.05).
In a Poisson regression model adjusted for any RT and sex, female sex and mediastinal site of NHL were significantly associated with solid tumor SMNs. There was a trend to increasing SMN diagnosis among patients treated in more recent years (P trend = .032). The only CT agent significantly associated with solid tumor SMNs was Ara-C (Table 5). Shielded or unshielded breast exposure to RT was associated with a 7-fold increased risk of solid tumor SMNs, and exposure of the thyroid was associated with a more than 2-fold increased risk. RT involving the total body, abdomen, pelvis, brain, spine, or limb was not significantly associated with solid SMNs. In a multivariate model, female sex, mediastinal disease, and breast RT remained associated with solid tumor SMNs. Sensitivity analyses including 3 additional cancers ascertained from death certificates (where death occurred before the last questionnaire, which was completed by a family member) did not yield a meaningful change in SIR or RR estimates.
Discussion
Our findings among a cohort of 1082 five-year NHL survivors in the CCSS indicate a 3-fold overall risk of SMNs and 4-fold risk of mortality relative to the general U.S. population. There were higher than expected SMRs for death from NHL, solid tumor SMNs, leukemia, cardiac disease, and pneumonia. There was a higher than expected relative risk of death from causes other than NHL in female subjects and after cardiac RT exposure. Despite the higher relative risk of SMNs among patients diagnosed in the later vs earlier years of the 1970 to 1986 study period, the RR of death was not increased in later years, perhaps indicating advances in survival from some treatment-related late effects.
All-cause mortality after NHL was lower than among 5-year survivors of other childhood cancers followed in the CCSS through 1996.35 Our study extends follow-up of NHL diagnoses through the end of 2002 and incorporates primary NHL histopathology categories. We found elevated death rates from second cancers and cardiac and pulmonary diseases, particularly cardiomyopathy and pneumonia, as has been seen after other childhood cancers35-37 and after adult NHL treatment.28-31 The increased risk of death associated with chest RT suggests that NHL treatment may convey some risks similar to those seen after Hodgkin's lymphoma.38 Based on a small number of cases in our cohort, death from nonviral pneumonia occurred only after splenectomy. Our finding of increased risk of death from causes other than NHL among female subjects is consistent with sex-specific differences in anthracycline-associated cardiac damage and certain other health conditions reviewed by Armstrong et al.39 Similarly, in a Nordic population-based study of 13 711 five-year survivors of 13 pediatric cancers, including 781 NHL survivors, the all-cause mortality was significantly higher in females than in males.40 However, no SMR estimates were provided by sex or treatment risk factors for NHL patients specifically as in our study. Despite seeing only 87 deaths in our cohort, our data raise the possibility that 5-year survivors of NHL appear to remain at considerable risk of death from NHL even years after diagnosis.
We focused on solid tumor SMNs as the primary type of SMN seen among 5-year survivors and as pathologically distinct from NHL recurrence. Risk factors associated with solid tumor SMNs in our study population were female sex and thoracic exposure to RT. Four prior publications have reported SIRs between 3.9 and 12 for SMNs after pediatric NHL.10,41-44 Higher SIRs were seen in an analysis of 456 French and British 3-year survivors of pediatric NHL44 and in a St Jude study of SMNs occurring after NHL diagnosis among 497 pediatric NHL patients.10 Both studies included neoplasms diagnosed within the first 5 years, and allowed lymphoma44 or lymphoma and leukemia10 diagnoses to be counted, probably raising the SIRs. Analyses in the US SEER cancer registry indicated a nearly 4-fold (SIR = 3.9) significantly elevated risk of solid tumor SMNs after NHL diagnosis before the age of 18 years, beginning 2 months after NHL diagnosis.42 With extended follow-up in the registry, SIR was 5.3 for all SMNs after NHL diagnosis.43 The SIRs reported after pediatric NHL are higher than after adult NHL, for which SIR estimates for all SMNs range from 0.9 to 1.6,19,20,22,27 and demonstrate a decline with increasing age at NHL diagnosis.18,20
The SIR was increased for solid tumor SMNs at all attained ages from 5 to 39 years. The mean age of living NHL survivors in our study population was only 32.5 years on December 31, 2002, and risks at older ages cannot be directly predicted from these data. Persistently elevated SMRs more than 20 years after diagnosis are concerning and should be confirmed when cohorts of pediatric NHL survivors reach later adulthood. With the evolution of treatments for pediatric NHL and decreasing use of RT, we anticipate changes in the incidence of severe or life-threatening late effects.
Limitations of the current study include the possibility that some associations may have resulted from chance, given small numbers of deaths and second cancers. However, there are few available large populations in which to estimate cumulative and RRs of SMNs after rare childhood cancer diagnosis. Self-reporting of SMNs via periodic mailed questionnaires may underestimate SMN incidence; however, we found no meaningful change in SIRs or RRs for solid tumor SMNs by including additional cancers ascertained from death certificates. Another limitation, restricting study eligibility to 5-year survivors, prevented evaluation of therapy-related leukemia, which more often occurs within the first 5 years of diagnosis. For analyses of RT effects, our study did not calculate absorbed dose to regions of interest or incorporate scatter from nearby treatment areas. Lastly, whereas we included only second primary cancers, the SEER data with which our observed cases were compared included third and subsequent cancers, possibly leading us to underestimate the relative incidence of SMNs in NHL survivors.
The strength of our findings derives from the comparatively large cohort of children with NHL and detailed treatment information available from medical records using the unique resource of the CCSS. We add to a small literature reporting SMRs, SIRs, and predictors of death and SMNs after pediatric NHL. Separate analyses of relative risks for death from NHL and from other causes distinguishes the adverse effects of therapies from the high risk of death in subjects receiving aggressive regimens. Persistently elevated mortality rates beyond 20 years after NHL diagnosis suggests that healthcare providers responsible for this high-risk population should remain alert to second cancers and cardiac disease among long-term survivors, particularly women and those treated with chest RT. Future studies in the CCSS cohort and in aging populations of pediatric NHL survivors are needed to assess additional nonfatal age-related conditions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Childhood Cancer Survivor Study institutions and investigators for their participation in this study. The authors thank Jeremy Miller of Information Management Systems for data preparation.
This work was supported by the NCI (grant U24 CA55727, L.L.R., Principal Investigator), the University of Minnesota from the Children's Cancer Research Fund, and St Jude Children's Research Hospital from the American Lebanese Syrian Associated Charities; and by the Intramural Research Program of the NIH, NCI, Division of Cancer Epidemiology and Genetics.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: E.C.B. designed and performed analyses and wrote the paper; P.D.I. and C.R. designed analyses; C.R., R.J.H., P.D.I., S.S.D., and A.T.M. contributed to interpretation of data; C.R., P.D.I., A.T.M., L.L.R., and S.H. edited the paper; J.P.N., M.S., A.C.M., J.A.W., and P.A.M. collected and prepared data; M.S. performed radiation dosimetry analyses; J.D.B. prepared and analyzed data and made the figure; L.L.R. designed the overall CCSS research project.
A complete list of the participating institutions and investigators of the Childhood Cancer Survivor Study can be found in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elizabeth C. Bluhm, Radiation Epidemiology Branch, Division of Cancer Epidemiology and Genetics, NCI, 6120 Executive Boulevard, MSC 7238, Rockville, MD 20892-7238; e-mail: bluhme@mail.nih.gov.