Abstract
The concept of introducing genes into human cells for therapeutic purposes developed nearly 50 years ago as diseases due to defects in specific genes were recognized. Development of recombinant DNA techniques in the 1970s and their application to the study of mouse tumor viruses facilitated the assembly of the first gene transfer vectors. Vectors of several different types have now been developed for specific applications and over the past decade, efficacy has been demonstrated in many animal models. Clinical trials began in 1989 and by 2002 there was unequivocal evidence that children with severe combined immunodeficiency could be cured by gene transfer into primitive hematopoietic cells. Emerging from these successful trials was the realization that proto-oncogene activation by retroviral integration could contribute to leukemia. Much current effort is focused on development of safer vectors. Successful gene therapy applications have also been developed for control of graft-versus-host disease and treatment of various viral infections, leukemias, and lymphomas. The hemophilias seem amenable to gene therapy intervention and informative clinical trials have been conducted. The hemoglobin disorders, an early target for gene therapy, have proved particularly challenging although ongoing research is yielding new information that may ultimately lead to successful clinical trials.
Introduction
The concept of gene therapy, namely the use of a genetic element to correct a deficiency in an important cellular constituent, emerged in the early 1960s. Knowledge that DNA was the primary genetic material, recognition that hereditary traits were based on specific genes, and the demonstration that defects or deficiencies in specific proteins caused disease were the elements that led to the initial conceptualization of gene therapy. The scientific and ethical issues arising out of proposals for genetic manipulation in humans were already widely discussed in the early 1970s with a general conclusion that gene therapy for severe disorders for which there was no corrective treatment would be ethically acceptable and analogous to the use of drugs to treat specific diseases.1,2
Over the past 35 years there have been progressive advances so that now curative gene therapy for severe combined immunodeficiency (SCID) is a clinical reality.3-6 Genetically modified cells were first infused into patients in 1989 when a retroviral vector was used to mark tumor-infiltrating lymphocytes administered to patients with melanoma.7 In September 1990, a 4-year-old child with SCID secondary to adenosine deaminase (ADA) deficiency was the first patient with a genetic disorder to be treated with gene therapy using a retroviral vector.8 In the ensuing 17 years, the field has evolved and continues to hold substantial promise for broad application of gene transfer in the treatment of human disease. At times progress has seemed slow but the evolution of the field should be put in the context usually required to achieve significant therapeutic advances. For example, chemotherapeutic agents active against childhood acute lymphoid leukemia were identified and organized into multiagent chemotherapy regimens combined with central nervous system irradiation in the 1960s.9 However, it required an additional 40 years of effort to progress from a 5% to 10% cure rate to current levels of 80% to 90%.10 Similarly, attempted clinical bone marrow transplantation for malignancy was first reported in 1957 and success was reported for immunodeficiencies in 1968,11,12 but success for leukemia was not reported until 1970 and many years of effort were required before it became a therapeutic modality that could be broadly applied for the treatment of bone marrow failure, hematologic malignancies, and other disorders.13 In this review the use of gene transfer in the context of treatment of immunodeficiencies, graft-versus-host disease, viral infections, lymphomas and leukemias, hemophilia, and the hemoglobin disorders illustrate the realized and potential future applications of gene therapy for the treatment of blood disorders. My goal is to provide a historical account of how the field has evolved over the past 35 years with examples that highlight the therapeutic potential of gene transfer.
Seminal advances
The discovery of retroviral reverse transcriptase in 1970 initiated a series of methodologic and conceptual advances that, over the next decade, created the potential for gene therapy. In a review published in 1988, Harold Varmus described the impact of retrovirology on many fields of science.14 The ability of reverse transcriptase15,16 to transcribe any RNA molecule into DNA made possible the synthesis of complementary DNA (cDNA) copies of messenger RNA. This advance was followed in a few years by molecular cloning of cDNAs for globin17 and later of genomic sequences encoding globin.18 Over the ensuing years many genes were cloned and studied including those mutated in human genetic diseases. The first transfer of a functional gene involved the introduction of Herpes simplex thymidine kinase gene into mouse fibroblasts in the form of a purified DNA fragment isolated from viral DNA.19 Entry was accomplished via a calcium phosphate precipitation method developed to introduce viral DNA into cells20 and later was also accomplished by microinjection.21,22 Only rare cells integrated the transferred genes so that both processes were too inefficient to make potentially therapeutic applications feasible. A report in 1980 of DNA transfer from a drug-resistant cell line into hematopoietic cells of mice leading to methotrexate resistance in vivo23 was neither substantiated by molecular analysis nor reproduced in other laboratories.
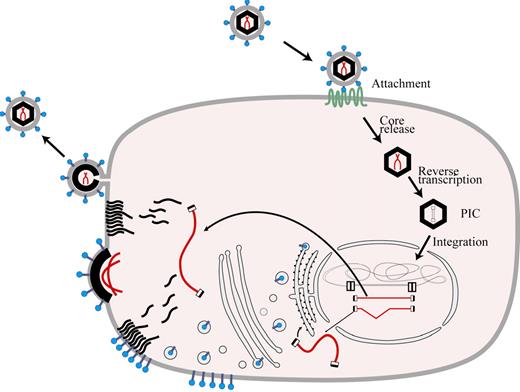
A growing understanding of the retroviral life cycle (Figure 1) made possible by molecular biologic techniques led to definition of the organization of the retroviral genome (Figure 2) and, along with increasing knowledge about the mechanisms in gene regulation, created momentum building toward development of vectors capable of gene transfer into primary hematopoietic cells. Although potentially prone to rearrangement or mutation during passage, retroviruses have the distinct advantage that their genomes are integrated by a specific mechanism that usually leads to a predictable genomic structure in the target cell DNA in which the coding portions of the retroviral genome are flanked by long terminal repeats (LTRs).14 Much of the early study of retroviruses focused on mouse tumor viruses because of their potential relevance to understanding cancer pathogenesis. Because they were available, murine retroviral genomes were employed in making the first gene transfer vectors.
Retroviral life cycle. Entry of a retrovirus into a cell is initiated by interaction of its envelope proteins with cellular receptors followed by internalization through membrane fusion or phagocytosis. The vector core is released and the RNA serves as a template for reverse transcription and formation of the preintegration complex (PIC). The PIC gains access to chromatin during mitosis or, in the case of lentiviruses, by ingress through the nuclear membrane. Following integration into host cell DNA, the retroviral genome is expressed in RNA molecules which are transported to the cytoplasm to serve as a template for synthesis of new viral proteins and, in the case of unspliced RNA species, as a substrate for formation of new viral particles.
Retroviral life cycle. Entry of a retrovirus into a cell is initiated by interaction of its envelope proteins with cellular receptors followed by internalization through membrane fusion or phagocytosis. The vector core is released and the RNA serves as a template for reverse transcription and formation of the preintegration complex (PIC). The PIC gains access to chromatin during mitosis or, in the case of lentiviruses, by ingress through the nuclear membrane. Following integration into host cell DNA, the retroviral genome is expressed in RNA molecules which are transported to the cytoplasm to serve as a template for synthesis of new viral proteins and, in the case of unspliced RNA species, as a substrate for formation of new viral particles.
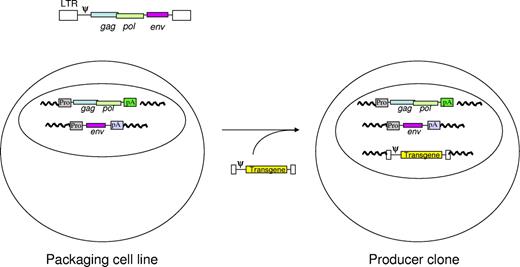
Production of retroviral vector particles. This figure illustrates the concept of the strategy used to derive retroviral vector preparations free of replication competent virus. The diagram at the top shows the organization of a retroviral genome. Murine oncoretroviruses have coding sequences for matrix proteins (GAG), reverse transcriptase (POL), and envelope proteins (ENV). To reduce the risk of recombination and generation of replication competent particles, these coding sequences are separated on 2 or more expression cassettes before transfer into a tissue-culture cell line. The coding sequences for viral proteins are replaced by the coding sequences for the intended therapeutic protein in the vector. Various strategies are used for introducing the vector genome into the packaging cell, which initiates production of replication defective retroviral vector particles that can be used to introduce the therapeutic transgene into target cells.
Production of retroviral vector particles. This figure illustrates the concept of the strategy used to derive retroviral vector preparations free of replication competent virus. The diagram at the top shows the organization of a retroviral genome. Murine oncoretroviruses have coding sequences for matrix proteins (GAG), reverse transcriptase (POL), and envelope proteins (ENV). To reduce the risk of recombination and generation of replication competent particles, these coding sequences are separated on 2 or more expression cassettes before transfer into a tissue-culture cell line. The coding sequences for viral proteins are replaced by the coding sequences for the intended therapeutic protein in the vector. Various strategies are used for introducing the vector genome into the packaging cell, which initiates production of replication defective retroviral vector particles that can be used to introduce the therapeutic transgene into target cells.
Recombinant vector genomes were first created by replacing a portion of the viral protein coding sequences with cDNAs encoding a alternative gene product. The first vectors were assembled with genes that encoded a product that conferred resistance to a neomycin analog, G418, or human hypoxanthine phosphoribosyltransferase (HPRT). Infectious vector stocks were made and, although contaminated with replication competent viruses, were used to obtain evidence of functional gene transfer into primary mouse bone marrow cells,24 a variety of cell lines, or human cells deficient in HPRT from patients with Lesch-Nyhan syndrome.25 The next major advance involved definition and removal of the packaging signal in the retroviral vector genome. This allowed packaging cell lines to be generated which expressed the retroviral GAG, POL, and ENV structural proteins from mRNAs that had a diminished capacity to be packaged into vector particles.26,27 Ultimately, it proved necessary to separate the GAG/POL coding sequences from the envelope coding sequences in separate genomic elements (Figure 2) to prevent the formation of replication competent retrovirus by recombination during vector production.28,29
In 1984, David A. Williams, working in the laboratory of Richard C. Mulligan, reported the introduction of new genetic material into pluripotent hematopoietic stem cells of the mouse using a recombinant murine retroviral vector.30 The target for gene transfer was cells described by Till and McCulloch many years earlier that are capable of forming spleen colonies approximately 2 weeks after injection into lethally irradiated recipients.31 This advance was followed shortly thereafter by the report of the in vivo expression of a transferred gene in mice32 and evidence that genes could be transferred into murine stem cells with long-term repopulation potential.33
Other seminal advances, which provided the foundation for the field of gene therapy as we know it today, were the development of other viral vector systems. The first of these, based on adenovirus34 or adeno-associated virus,35,36 were described in 1984. Lentiviral vectors based on human immunodeficiency virus were described sometime later in 1996.37 Each of these vector systems have unique properties that make them potentially useful for specific gene therapy applications.38 Foamy virus vectors were introduced by David Russell and Dusty Miller in 199639 and continue to be developed as a promising system for stem cell–targeted gene transfer.
Creation of animal models of human diseases by transgenic and gene knockout technology has proved very valuable for the field of gene therapy. The first transgenic mouse strains having germ line transmission of the transgene were reported in 1981,40,41 and the gene knockout technology was developed in the late 1980s.42,43 The potential for this technology to create disease models was recognized early as reviewed by one of the pioneers in the field, Mario Capecchi.44 This technology required another decade to reach full fruition but its impact on the field has been very considerable. Such animal models provide unique opportunities to define the pathophysiology of specific disorders and the potential for disease correction by gene transfer.
Early clinical trials
An early advocate for the initiation of clinical trials, W. French Anderson, outlined the prospects for human gene therapy in 1984.45 The hemoglobin disorders, sickle cell anemia, and severe β-thalassemia were initially considered because of the high frequency of these disorders worldwide. Globin genes were the first to be molecularly cloned and many early discoveries and principles of molecular biology were based on the study of globin gene systems. However, molecular regulation of globin genes has proven to be extraordinarily complex and many additional years were required before vectors could be assembled that had the potential to achieve therapeutically meaningful levels of expression in maturing red cells. Metabolic disorders due to enzyme deficiencies seemed more amenable to gene therapy since these genes are ubiquously expressed and tight regulation is not likely to be necessary. The candidates that were identified were hypoxanthine-guanine phosphoribosyl transferase, which is deficient in Lesch-Nyhan syndrome, and 2 enzyme deficiencies that result in immunodeficiency, purine nucleoside phosphorylase and adenosine deaminase (ADA) deficiency.45 ADA deficiency seemed a particularly attractive target for early intervention since it could be cured by bone marrow transplantation by infusion of hematopoietic cells from histocompatible donors without myeloablation.46 Genetically normal donor lymphoid cells had a selective proliferative advantage and Anderson predicted that genetically corrected autologous cells would enjoy a similar proliferative advantage.45
Anderson developed collaborations with Richard O'Reilly at Memorial Sloan Kettering, an expert in bone marrow transplantation who had established a nonhuman primate bone marrow transplant model, and R. Michael Blease, an immunologist at the National Institutes of Health (NIH) with expertise in immunodeficiencies. Together with their many colleagues, they explored the ability to use retroviral vector–mediated gene transfer to introduce genes into repopulating stem cells in the nonhuman primate model.47 However, expression of the human ADA gene and a drug resistance marker also included in the vector were at very low levels and present for only a short period of time. Primate stem cells proved highly refractory to retroviral vector–mediated gene transfer and only after years of effort were transduction conditions improved to the point where successful clinical trials could be conducted as discussed below, in “Success: the immunodeficiencies.”
Still searching for an opportunity to use gene transfer in humans, Anderson teamed with Steven A. Rosenberg, an immunologist and surgeon who was developing tumor-infiltrating lymphocytes (TILs) for immunotherapy of patients with advanced melanoma. Integration of a retroviral vector genome provides a unique mark to a cell and its progeny, allowing retroviral marking to be used to study the distribution and survival of transduced cells following infusion. Retroviral vectors designed by Dusty Miller were the first to be used in clinical trials. Packaging cell lines were redesigned to avoid recombination and the potential for production of replication competent retroviruses.48 An extended packaging signal was included, which improved the titer.49,50 With French Anderson as the scientific leader, Genetic Therapy Inc (GTI) of Gaithersburg, Maryland, was founded with support from venture capitalists in 1987. Clinical grade vector was produced under GMP conditions by GTI. After extensive discussion and review, the Recombinant DNA Advisory Committee (RAC) recommended approval of the marking protocol on October 3, 1988, and it was approved by the NIH director on March 2, 1989. Five patients received gene-marked TILs and genetically modified cells could be detected in blood and tumor tissue. The investigators, in their publication in 1990, concluded that their studies had demonstrated the feasibility and safety of using retroviral gene transfer for human gene therapy.51
The Blaese, Rosenberg, and Anderson team quickly moved to open the first gene therapy trial for ADA deficiency. The challenge of bone marrow stem cell transduction proved daunting but the results from the initial TIL trial indicated that lymphocytes could be transduced in vitro. The laboratory of Claudio Bordignon at the Instituto Scientifico HS Raffaele, in Milan, Italy, using an immunodeficient mouse model in which human lymphocytes engrafted, provided evidence that retroviral-mediated gene transfer functionally corrected the ADA deficiency in lymphocytes from patients with ADA-SCID and that, in some cases, a progenitor was transduced which gave rise to a variety of clones having different TCR-beta gene rearrangements.52,53 The RAC recommended approval on July 31, 1990, and the study was approved by the NIH director on September 6, 1990. The first infusion of genetically modified lymphocytes was given about a week later on September 14.
The results of the Blaese-Rosenberg-Anderson trial proved to be tantalizingly ambiguous.8,54 The first patient treated with a series of infusions of autologous, genetically transduced lymphocytes exhibited expansion of lymphocyte populations with large numbers of genetically modified cells and measurable enzyme activity. However, the second patient, who had milder disease, had low numbers of genetically modified cells and low enzyme activity despite restoration of lymphocyte numbers to normal levels after the lymphocyte infusions. Both patients continued on enzyme replacement therapy with PEG-ADA. Approximately 20% of the first patient's lymphocytes were carrying and expressing the retroviral gene 10 years after the last cell infusion. The number of gene-marked cells was very low (less than 0.1%) without expression of the transgene in the second patient, who had developed persistent antibodies to lipoprotein present in the fetal calf serum in which the transduced lymphocytes had been cultured and to a viral envelope protein.54
A second clinical trial in ADA-SCID was done by Claudio Bordignon and his colleagues in Milan.55,56 Initially, they reported on 2 patients who received both transduced lymphocytes and transduced bone marrow cells in which the targets of gene transfer were primitive, repopulating cells. T lymphocytes derived from transduced peripheral blood cells were replaced by marrow-derived T cells in both patients with restoration of cellular and humoral immunity without PEG-ADA treatment. One of the 6 patients treated ultimately in this series received only transduced peripheral blood lymphocytes. Because of failure of reconstitution and persistent immunodeficiency associated with PEG-ADA–associated immune imbalance, the enzyme therapy was discontinued in this patient.56 Discontinuation of PEG-ADA was followed by progressive immune reconstitution and restoration of lymphocytes to normal numbers. Red blood cell deoxyribonucleotides remained increased, indicating that the gene-corrected lymphocytes failed to eliminate potentially toxic metabolites but were capable of immune reconstitution.
Another trial was performed by Don Kohn and his colleagues.57 Over a relatively short interval, 3 infants with ADA deficiency were born and the umbilical cord blood was collected and processed to isolate CD34+ cells. The patients were continued on PEG-ADA. Although marked cells were present in peripheral blood, their frequency remained very low initially. After longer follow-up, there was a progressive increase in genetically modified T cells in peripheral blood to levels of 1% to 10% compared with 0.01% to 0.1% in myeloid cells, demonstrating the predicted selective advantage of gene-corrected lymphocytes, but the patients remained immunodeficient with lymphocytopenia and dependent on PEG-ADA.58
These early trials stimulated a great deal of interest in clinical gene therapy and the development of many additional protocols. Some of the early marking studies, particularly those conducted by Malcolm Brenner and his colleagues at St Jude Children's Research Hospital, yielded interesting information in demonstrating that cells present in autologous bone marrow grafts in patients with neuroblastoma or acute myeloid leukemia could contribute to subsequent disease relapse.59-61 Marking studies continue to be useful in evaluating gene therapy approaches.62 A tantalizing result of the Brenner study was the suggestion that stem cells from children were more efficiently transduced than those from adults, a result that has yet to be definitively confirmed in animal models or subsequent clinical trials. Demonstration that selection of drug-resistant, genetically modified cells could be accomplished after transfer of a drug-resistant gene into mouse stem cells63 prompted the development of protocols to test this approach in the context of cancer chemotherapy.64 Despite some improvement in gene transfer efficiency, this approach was not successful in human clinical trials.65
Orkin-Motulsky report: back to basics
At the time that this multidisciplinary panel was convened in 1995 by the NIH director, Harold Varmus, to review the field of gene therapy, 106 clinical protocols had been reviewed and approved by the RAC and 597 participants had been enrolled on these protocols. A quarter of the protocols were marking studies, one-half were focused on cancer treatment, and about 20% addressed inherited, single-gene disorders. The panel concluded that although often referred to as “clinical trials,” gene transfer protocols were really small scale clinical experiments.66 Only a few of these initial clinical studies were judged to be sufficiently well designed to address fundamental biologic questions. The panel also concluded that efficacy had not been established for any gene therapy intervention. Most exploratory studies, particularly those in patients with cancer, were judged to be focused on the feasibility and safety of gene transfer procedures and lacked adequate power to measure potential efficacy or to compare the intervention to conventional approaches for the same disease. At the time, the NIH was investing approximately $200 million a year in gene therapy research and it was estimated that commercial entities were investing even more. The panel recommended continued funding and challenged researchers in the field to focus on the basic biology of various vector systems, the defining of appropriate target cells in various tissues, the use of animal models to explore disease pathophysiology and its correction by gene transfer, and development of strategies to improve targeted and sustained gene expression in successfully transduced cells.66
Success: the immunodeficiencies
The panel to evaluate gene therapy noted that “confidence in current approaches to somatic gene therapy would rise if a genuine genetic deficiency in an animal model were unequivocally corrected.” Partial disappearance of lysosomal storage material was demonstrated earlier in the liver and spleen in a naturally occurring mouse model for human Mucopolysaccharidosis type VII but only partial myeloablation had been given, resulting in a low percentage of genetically modified cells and persistent phenotypic abnormalities.67 In 1998, restoration of lymphocyte function was reported in SCID mice secondary to Janus kinase 3 (Jak-3) deficiency by the laboratory of Brian Sorrentino.68 Their subsequent report documented virus-specific immunity after gene therapy in this model.69 X-SCID secondary to a deficiency of the common γ-chain of interleukin receptors accounts for many more cases of SCID than Jak-3 deficiency and hence it became the primary focus of clinical trials after it was also demonstrated in mouse models that immune function could be restored following retroviral vector–mediated gene transfer.70-72
Despite early success in murine models at achieving transduction of repopulating stem cells, transduction of stem cells in large animal models including nonhuman primates has proved challenging. A recent study suggests that baboon and rhesus stem cells divide only once every 23 to 36 weeks, whereas mouse stem cells divide once every 2.5 weeks.73 Thus, inherent biologic differences with respect to cell cycling may account, in part, for the differing efficiency in stem cell–targeted gene transfer. For more than a decade, the research group at NIH led by Cynthia Dunbar has used the rhesus macaque model to achieve improved transduction efficiency74 and Hans Peter Kiem and his collaborators at the Fred Hutchinson Cancer Center in Seattle have conducted similar studies in canine and baboon models.75 Emerging from their efforts and those of others as reviewed recently,74,75 are multiple strategies to improve gene transfer efficiency into primitive hematopoietic cells. These strategies include optimization of the cytokine combination as well as use of a fragment of fibronectin, retronectin, to colocalized vector particles and target cells during transduction as reported by the laboratory of David Williams.76 The use of alternative envelope proteins to pseudotype vector particles, including that derived from Gibbon ape leukemia virus (GALV),77 resulted in improved transduction efficiency of human hematopoietic cells.78 These improvements were first applied clinically by Harry Malech and coworkers in the context of attempted treatment of chronic granulomatous disease.79
Such modifications led to the first successful gene therapy trials in X-linked SCID and ADA-SCID. A recent review by Marina Cavazzana-Calvo and Alain Fischer,80 the collaborators who led the French trial, indicate that 17 of 20 enrolled X-SCID patients have been successfully treated in the French trial3,4 or the British trial led by Adrian Thrasher and Bobby Gaspar in London.5 For ADA deficiency, the immunodeficiency has been partially or fully corrected in over 10 patients enrolled in the trials led by Claudio Bordignon and Maria Grazia Roncarolo in Milan6 or Adrian Thrasher and Bobby Gaspar in London.81 Success in achieving genetic correction in SCID patients reflects the inherent selective advantage for gene-corrected lymphoid cells in the context of an absence or severe reduction in normal lymphopoiesis (Figure 3). The occurrence of leukemia in 4 of the children treated in the French trial and now one in the British trial (Adrian Thrasher, personal communication) secondary, at least in part to vector-mediated insertional mutagenesis,82 should not obscure the fact that more than 35 children with otherwise lethal diseases have been successfully treated for their immunodeficiencies. Two of the children who developed leukemia have been successfully treated and one is under active treatment at present. Gene therapy has proved less successful in older children with X-SCID83 although some improvement in 1 of 3 older children has been reported in a recent trial conducted in the intramural program of the NIH by Jennifer Puck and her colleagues.84
Gene correction in severe combined immunodeficiency. The desired targets for vector-mediated gene transfer include the most primitive hematopoietic cells. Evidence from clinical trials suggests that occasionally a multipotential progenitor is transduced but committed lymphoid progenitors may also be transduced and lead to gene-corrected lymphopoiesis. Once gene correction has been achieved, there is preferential amplification of gene-corrected cells in the lymphoid compartments.
Gene correction in severe combined immunodeficiency. The desired targets for vector-mediated gene transfer include the most primitive hematopoietic cells. Evidence from clinical trials suggests that occasionally a multipotential progenitor is transduced but committed lymphoid progenitors may also be transduced and lead to gene-corrected lymphopoiesis. Once gene correction has been achieved, there is preferential amplification of gene-corrected cells in the lymphoid compartments.
Chronic granulomatous disease
An early clinical trial in patients with chronic granulomatous disease (CGD) resulted in prolonged production of gene-corrected granulocytes but the numbers were inadequate to correct the disease phenotype.79 More recently, 2 young adult patients with this disorder who received genetically modified cells after partial myeloablation had clearance of severe infection and clinical improvement associated with engraftment of genetically corrected, repopulating cells.84 In this study, a vector based on the spleen focus forming virus (SFFV) was used since its enhancer-promoter combination is particularly active in myeloid cells. Initially, approximately 15% to 20% of the granulocytes in these 2 patients were genetically corrected. Several months following the procedure, there was a substantial increase in the number of oxidase-positive cells. Molecular analysis demonstrated that this increase in the number of genetically corrected cells reflected clonal dominance engendered by insertional activation of one or more of the proto-oncogenes, MDS1-EVI1, PRDM16, or SETBP1.85 In one of the patients, more than 80% of the cells were derived from a single transduced primitive cell. Progressive silencing of the transgene in this clone and its replacement by a second clonal population with even lower transgene expression resulted in recurrence of the CGD phenotype in this patient.
Genotoxicity of retroviral integration
Because retroviral integration was thought to be random, the risk of insertional mutagenesis was initially judged to be very low.86 However, emerging evidence of the potential genotoxicity of retroviral integration in clinical trials coincided with substantial new evidence regarding the distribution of retroviral integration sites in target cells and the biologic impact of integration events as recently reviewed.87,88 The availability of the human genome sequence and the development of methodology to define DNA sequences at the site of retroviral integration have shown that integration is far from random. The murine γ retroviruses used in gene therapy trials to date favor the transcriptional start sites of actively transcribed genes in the target cell population,89 whereas lentiviral vectors that are being developed for future trials favor active genes with more uniform distribution within transcriptional units.90 Integration into or near genes involved in cell-cycle progression or cell survival has the potential to alter the biologic properties of cells which, in the context of engraftment, can result in preferential engraftment and expansion and even clonal dominance. The discovery of common integration sites (CISs), namely the nonrandom insertional clustering of integration sites into the same genetic locus in 2 or more cells in a population, is thought to reflect the biases toward integration into actively transcribed genes in the target cells and the potential of an integrated vector genome in that locus to alter the subsequent biologic properties of each target cell. A striking example of the occurrence of CIS is the discovery of 14 integration events into the MDS1-EVI1 locus in DNA from peripheral blood granulocytes and T lymphocytes of rhesus macaques after reconstitution with genetically modified cells.91 Such clustering is highly unlikely statistically. The occurrence of CISs is not necessarily followed by clonal expansion in that all of the animals continue to have polyclonal hematopoiesis with contributions from many clones present among the genetically modified, peripheral blood cells.
Retroviral integration site analysis has recently been reported in detail for successfully treated patients in the trials for X-SCID and the ADA trial in Italy.92-94 The distribution of integration sites favoring transcriptional start sites and CpG islands and the predilection for actively transcribed genes were similar to that reported for cell lines and animal models. CISs were defined in all 3 sets of patients but were most striking in patients in the French trial for X-SCID with 5 insertions within LMO2, 8 within ZNF217, and 9 within or near CCND2, a gene involved in cell-cycle progression. CCND2 and LMO2 also emerged as CISs in the patients in the Italian ADA trial. Although CISs were mapped in 5 patients in the British trial, all occurred only twice with no insertions found near or within LMO2. There was no evidence for clonal expansion in any of the patients at the time they were studied and, significantly, there were no clues from this integration site analysis that would have allowed identification of the patients who subsequently developed clonal expansion and leukemia.82
To date, 4 cases of leukemia have occurred in the French trial among 10 patients. The lack of occurrence of leukemia in the British trial for several years led to much speculation about the possible role of small differences in transduction conditions but recently a case of leukemia has occurred in one of the British patients (Adrian Thrasher, personal communication). Of potential relevance to the occurrence of leukemia after gene therapy for X-SCID is the demonstration of an expanded population of primitive cells in bone marrow of mice with X-SCID due to common γ chain deficiency.95 Apparently this expanded population is arrested in developmental progression because of the defects in interleukin signaling and may be rescued by retroviral vector–mediated gene transfer. Perhaps such cells may be susceptible to mutagenic events leading to cell transformation. While X-SCID patients may be uniquely sensitive to the adverse effects of insertional mutagenesis, sufficient evidence has accumulated in various experimental systems that indicate that clonal dominance and the risk of malignant transformation is general to the use of integrating retroviral vectors.87,88 A particularly striking example is one rhesus macaque studied by Cynthia Dunbar and her colleagues, which was transplanted with retroviral vector modified, primitive hematopoietic cells. Early during reconstitution this animal exhibited striking dominance of a clone with vector integration into an antiapoptotic gene. This clone went quiescent and re-emerged some 6 years later as a myeloid malignancy.96
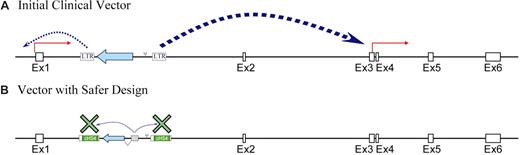
Fortunately there is much that can be done to reduce the risk of genotoxicity secondary to retroviral insertion.87,88 Several experimental systems have been developed and applied to evaluate vector design.97 The γ retroviral vectors used in clinical trials to date have a conventional design with 2 intact long-terminal repeats (LTRs), each of which has powerful enhancer and promoter elements. However, self-inactivating (SIN) vectors can be used in which a deletion in the 3′ LTR is copied over during viral reverse transcription to the 5′ LTR in the transduced cell, resulting in an integrated vector genome with 2 deleted LTRs. Evidence is already beginning to accumulate that such SIN vectors with an internal cellular promoter are less likely to activate gene expression near their integration sites.87,97 In addition, boundary elements such as the insulator from the chicken beta globin cluster, which has both enhancer blocking and chromatin boundary forming activity,98 can be added to the 3′ LTR during vector assembly leading so that the transgene is flanked by insulator elements following vector integration.99 Current efforts to develop vectors for stem cell–targeted gene therapy applications are focused on lentiviral vectors developed from human immunodeficiency virus (HIV).100 Lentiviral vectors, in contrast to murine γ retroviral vectors, are able to transverse the nuclear membrane without mitosis and thus are more efficient at transducing quiescent stem cells. Recent experiments in my own lab have shown that recreation of the insertion into the LMO2 gene with a γ retroviral vector having 2 LTRs activates the LMO2 gene in lymphoid cells but a lentiviral vector with SIN LTRs containing insulator elements with an internal cellular promoter does not significantly increase LMO2 expression (Figure 4).101
Design of safer retroviral vectors. (A) A strategy has been developed for recreating an insertion in the LMO2 gene that occurred in one of the patients who developed leukemia in the X-SCID trial.79 The experimental system is a human lymphoid cell line (Jurkat) in which the LMO2 gene is not expressed.100 When a conventional oncoretroviral vector having 2 intact long-terminal repeats (LTRs) is present in the intron of LMO2, its 2 promoters are strongly activated, leading to accumulation of LMO2 mRNA and protein. (B) Modification of the vector design to use an internal cellular promoter results in a weaker activation signal, which can be blocked by a chromatin insulator thereby preventing LMO2 activation.
Design of safer retroviral vectors. (A) A strategy has been developed for recreating an insertion in the LMO2 gene that occurred in one of the patients who developed leukemia in the X-SCID trial.79 The experimental system is a human lymphoid cell line (Jurkat) in which the LMO2 gene is not expressed.100 When a conventional oncoretroviral vector having 2 intact long-terminal repeats (LTRs) is present in the intron of LMO2, its 2 promoters are strongly activated, leading to accumulation of LMO2 mRNA and protein. (B) Modification of the vector design to use an internal cellular promoter results in a weaker activation signal, which can be blocked by a chromatin insulator thereby preventing LMO2 activation.
Suicide gene therapy for graft-versus-host disease
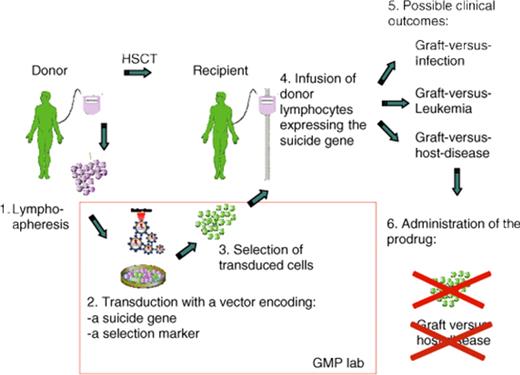
Another successful application of gene therapy has been the transfer of a suicide gene into donor lymphocytes for the purposes of controlling allo-reactivity in the context of allogeneic hematopoietic stem cell transplantation. The basic concept is to insert a gene that renders the target susceptible to drug-induced cell death. This approach, pioneered by Claudio Bordignon in Milan and recently reviewed by his group,102 represents the widest clinical application of T cell–targeted gene therapy to date. More than 100 patients have been treated worldwide to date in several phase 1 and 2 studies. Plans are underway for a phase 3 study with industry support.102 This approach seeks to preserve the graft-versus-infection and graft-versus-leukemia potential of donor lymphocytes while providing a safety valve for abrogating graft-versus-host disease (GVHD) if it occurs and proves clinically problematic (Figure 5). The thymidine kinase gene from Herpes simplex virus, which conveys sensitivity to Ganciclovir, has been used in all clinical trials to date and is highly effective at abrogating a GVHD response.102 Continued experimental efforts have identified strategies to refine this approach for disease control.102 Since much of the expense associated with care of patients following allogeneic bone marrow transplantation relates to the treatment of GVHD, the suicide gene therapy approach may prove cost effective despite the considerable technical expertise required to transduce lymphocyte populations ex vivo and recover the genetically modified subset for infusion. The Bordignon group has also evaluated retroviral integration site distribution in transduced lymphocytes before and after infusion and concluded, that although integration may deregulate gene expression, it has no apparent consequences on the biology and function of transplanted T cells.103
Strategy for controlling graft-versus-host-disease (GVHD) by suicide gene therapy. The allogeneic donor serves as a source of lymphocytes that are genetically modified by introducing the suicide gene, which has the potential to convert a prodrug into an active drug. The vector genome includes a selectable marker gene that facilitates recovery of genetically modified cells. Following hematopoietic stem cell transplantation (HSCT), the recipient receives the genetically modified lymphocytes in an effort to accelerate restoration of lymphoid function. As shown on the right, this provides an opportunity for beneficial graft-versus-infection and graft-versus-leukemia affects. If GVHD occurs, administration of the prodrug results in ablation of allo-reactive lymphocytes and control of the disease. Reprinted from Bonini et al102 with permission.
Strategy for controlling graft-versus-host-disease (GVHD) by suicide gene therapy. The allogeneic donor serves as a source of lymphocytes that are genetically modified by introducing the suicide gene, which has the potential to convert a prodrug into an active drug. The vector genome includes a selectable marker gene that facilitates recovery of genetically modified cells. Following hematopoietic stem cell transplantation (HSCT), the recipient receives the genetically modified lymphocytes in an effort to accelerate restoration of lymphoid function. As shown on the right, this provides an opportunity for beneficial graft-versus-infection and graft-versus-leukemia affects. If GVHD occurs, administration of the prodrug results in ablation of allo-reactive lymphocytes and control of the disease. Reprinted from Bonini et al102 with permission.
Viral infections
Adoptive transfer of T-cell clones to restore immunity to cytomegalovirus (CMV) in patients following bone marrow transplantation was first reported in 1992 by Stan Riddell and Phillip Greenberg and their coworkers at the Fred Hutchinson Cancer Center in Seattle.104 At the same time, Cleo Rooney, Helen Heslop, and Malcolm Brenner and their colleagues working at St Jude Children's Research Hospital in Memphis began efforts to use gene modified, virus-specific T lymphocytes to control Epstein-Barr virus (EBV)–related lymphoproliferation in immunocompromised patients.105 In these studies, EBV-infected donor lymphoid cells served as the antigenic stimulation for expansion of polyclonal T-cell populations, which were genetically modified by retroviral-mediated gene transfer to allow the marked cells to be tracked in vivo. Resolution of EBV reactivation, reflected by a decrease in the viral DNA, was documented following lymphocyte infusion and in the initial study, one patient exhibited resolution of an immunoblastic lymphoma.105
Over the years, the field of adoptive immunotherapy has evolved to encompass a broader array of infectious disorders as well and the use of genetically modified lymphoid cells for the treatment of cancer.106,107 A recent report described the use of polyclonal lymphoid cells expanded in vitro to successfully combat CMV, EBV, and adenoviral infection in patients after bone marrow transplantation.108 In these studies, gene transfer into peripheral blood mononuclear cells and into EBV-transformed donor lymphoid cells was accomplished with a recombinant adenoviral vector encoding the CMV protein, pp65. The donor lymphoid populations were initially stimulated with vector-transduced mononuclear cells to initiate expansion of lymphoid cells with adenoviral and CMV specificities. Subsequently, the cells were stimulated by irradiated donor EBV-transformed lymphoid cells also infected with the adenoviral vector to continue expansion of lymphoid cells with multiple viral specificities. Individual patients showed expansion in vivo of lymphoid populations with specificities for adenoviral, CMV, or EBV with resolution of viral infection as reflected by a decrease in viral load.108
The concept of “intracellular immunization,” a phrase coined by David Baltimore,109 arose when it was shown that expression of a truncated viral transactivator selectively impedes lytic infection by the corresponding virus.110 In his accompanying editorial, Baltimore pointed out how such strategies could be used to potentially combat HIV infection.109 Over the years, many different strategies have been proposed and tested to inhibit HIV infection as recently reviewed.111 Overall, the goal of this research is to provide an additional therapeutic option when drugs fail because of viral resistance or side effects and potentially, an adjuvant to drug therapy as patients continued to live longer with added risk for disease progression. The results of a small clinical trial which used a lentiviral vector expressing antisense sequences targeted to the HIV envelope protein has provided interesting clinical results that has prompted continued clinical trials.112 A recent commentary summarizes some of the other approaches that are either in, or proceeding toward, clinical trials.113 These approaches target the coding sequences for essential cellular proteins or viral sequences with inhibitory RNA molecules, express transdominant versions of HIV proteins, or use zinc finger nucleases with the goal of mutating endogenous CCR5 genes thereby creating resistance to HIV infection.113 The target cells for gene modification are either peripheral blood T cells or bone marrow stem cells with the potential for regenerating a virally resistant immune system. A vector containing 3 anti-HIV genes, a ribozyme targeting CCR5, an inhibitory RNA targeting rev, and TAR decoy to reduce tat activity has shown efficacy in macrophages and T cells114 and clinical trials using this vector for stem-cell transduction have been reviewed by the RAC.
Malignant hematologic disorders
Several creative approaches that use gene transfer in the context of treating cancer, including lymphomas and leukemias, have been devised over the past 15 years. Recent reviews by experts in this field summarize progress.106,107,115-118 Among these approaches are the generation of T-cell populations in vitro with specificity for antigens expressed on tumor cells. For example, gene transfer is useful for generating the LMP2 protein of EBV, which is expressed on some human lymphomas, in antigen-presenting cells in vitro. Expansion of lymphoid populations with LMP2 specificity has yielded T cells with potent antitumor reactivity as reflected, in one recent study, by the response of 5 of 6 patients with active, relapsed lymphoma.119
Much effort has also focused on the development of effective tumor vaccines by expressing genes that enhance the immune response at the vaccine injection site by releasing cytokines, costimulatory molecules or gene products which block inhibitory signals from tumor cells.115-117 These strategies have relied on the transduction of primary autologous tumor cells, the use of allogeneic tumor cells which provide paracrine stimulation when mixed with the patient's primary tumor cells, or the use of other autologous cells after genetic modification. In the latter approach, 10 patients in a recent study (including 7 children with high-risk, acute myeloid, or lymphoblastic leukemia) received multiple injections of irradiated tumor cells mixed with autologous skin fibroblasts expressing IL-2 and CD40L.120 Immunization produced a substantial increase in frequency of T cells reactive against recipient-derived blasts. Eight patients remain disease free for up to 5 years after treatment.120
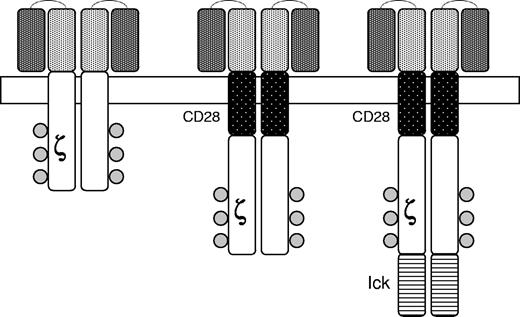
Another approach involves the creation of chimeric antigen receptors (CARs) in which a single chain antibody with specificity for an antigen expressed on human tumor cells is linked to an internal kinase domain which mediates cell activation when the antibody is engaged by the target antigen (Figure 6). For example, CARs targeting CD19 expressed on human B-cell malignancies have been developed and shown to eradicate tumors in a mouse model.121 Because of the need for costimulation to achieve T-cell activation in vivo, the cytoplasmic signaling domain of the CD28 receptor has also been added to the CAR (Figure 5). Analogous approaches are being used to derive primary natural killer cells for use in treating childhood leukemia.122
Chimeric antigen receptors. The external domain in the upper portion of the figure is composed of a single chain monoclonal antibody of the desired specificity. Signaling is achieved by an internal domain (ζ) taken from the T-cell receptor. Second generation CARs are modified to include portions of other signaling molecules designed to provide costimulatory signals upon engagement of the CAR by the target antigen. CD28 is a costimulatory molecule expressed on T-cells and lck is a protein kinase associated with the T-cell receptor. Reprinted from Rossig and Brenner106 with permission.
Chimeric antigen receptors. The external domain in the upper portion of the figure is composed of a single chain monoclonal antibody of the desired specificity. Signaling is achieved by an internal domain (ζ) taken from the T-cell receptor. Second generation CARs are modified to include portions of other signaling molecules designed to provide costimulatory signals upon engagement of the CAR by the target antigen. CD28 is a costimulatory molecule expressed on T-cells and lck is a protein kinase associated with the T-cell receptor. Reprinted from Rossig and Brenner106 with permission.
Hemophilia
There has been considerable interest in the development of gene therapy for hemophilia over the years involving the use of oncoretroviral, lentiviral, adenoviral, and adeno-associated viral vectors.123,124 These disorders have seemed particularly amenable to gene therapy approaches since small amounts of the clotting factor are sufficient to significantly correct the bleeding phenotype. Although less frequent than factor VIII (FVIII) deficiency, factor IX (FIX) deficiency has been the focus of much recent effort involving the use of adeno-associated viral vectors. The coding sequences for FIX are small and amenable to incorporation into an AAV vector despite the significant size constraint to the genome of such vectors. Others are exploring the use of lentiviral vectors for ex vivo transduction of hematopoietic cells125,126 or endothelial progenitors127 as a strategy for obtaining sustained expression of FVIII or FIX after reinfusion of the genetically modified cells.
After extensive studies in animal models, Mark Kay and Katherine High and their collaborators have conducted 2 clinical trials of AAV-mediated, FIX gene transfer. Both trials were performed with AAV vectors of capsid serotype 2. In the first trial, 8 adult men received vector injections at multiple sites.128 The trial had a traditional phase 1 design with increasing doses in successive cohorts of patients. Although transduction of myocytes was documented around the injection sites, therapeutic levels of FIX were not achieved.128 Extension of the study with higher doses of vector was not pursued because of the potential risk of evoking an immunologic response to FIX, a complication that has been observed with intramuscular injection of vector particles in animal models.129
The second clinical trial focused on liver targeted delivery of vector particles via injection into the hepatic artery.130 Therapeutic factor IX levels were initially detected in 1 of the 2 patients at the highest dose. This patient developed transient transaminitis thought to reflect activation of memory T cells specific for AAV capsid epitopes with subsequent destruction of transduced hepatocytes and loss of FIX production. The other patient receiving the highest dose of vector particles had detectable levels of antibody to AAV2 and did not exhibit FIX production. A third patient without AAV2 antibodies given a lower dose also developed transient transaminitis. A new study is underway that incorporates a course of immunosuppression beginning before and continuing several weeks after vector infusion in an effort to abrogate the memory T-cell response and achieve longer term production of FIX.
Work in animal models has established that administration of an alternative serotype can achieve functional levels of FIX production even in the presence of high titer antibodies to another AAV serotype. For example, AAV5 particles can be given effectively even in the presence of AAV8 antibodies.131 Over the past several years, Amit Nathwani at University College London and Andrew Davidoff at St Jude Children's Research Hospital have explored the use of alternative serotypes for FIX gene therapy. They and their collaborator, John Gray, have developed a novel vector that achieves hepatocyte-specific expression of FIX.131,132 The vector incorporates a self-complementary design in which both strands of the transgene coding sequences are present in the vector in a hair-pin configuration in a single DNA molecule. Expression from this genome begins within a day or 2 of injection. Over several weeks, monomeric vector genomes are assembled into high-molecular-weight concatamers which persist and express the transgene product for several years in rhesus macaques following a single injection of vector particles.131,132 Integration may occur and in one murine study was associated with insertional mutagenesis.133
Liver-targeted delivery via the hepatic artery or portal vein, to be effective, presumes substantial first pass clearance of the vector particles into transduced hepatocytes. There is little direct evidence for such high level, first pass clearance in large animal models and indeed the vector genome can be detected in plasma for several days after liver-targeted delivery.131,132 The biodistribution of vector particles for both serotype AAV8 and AAV5 is similar in rhesus macaques regardless of whether the vector particles are given via the mesenteric circulation or into a peripheral vein with the vast majority of the vector genome found in hepatocytes.132
Over the past 2 years, Dr High and her colleagues have accumulated evidence suggesting that, in contrast to antibodies, T-cell immunity to epitopes in AAV capsid proteins may be cross-reactive among serotypes134 although such serotype cross-reactivity has not been reported to date in murine135 or rhesus136 models. This concern has complicated the development of a clinical trial testing AAV8 vector particles encoding FIX but plans to initiate this trial with injection of vector particles into a peripheral vein are moving forward. Success in the development of AAV vectors for FIX gene therapy would undoubtedly further stimulate ongoing efforts to develop this vector system for gene therapy of a broad spectrum of metabolic and storage disorders.
Hemoglobin disorders
As noted earlier, sickle cell anemia and severe β-thalassemia were envisioned as the initial targets for gene therapy during the early days of the field. Efforts to develop suitable γ retroviral vectors for globin gene transfer were initiated in the mid 1980s and facilitated by the fact that the genomic globin genes were among the first to be molecularly cloned. Vectors that included an intact and functional promoter, the coding exons and the 2 introns were first assembled in the laboratory of Richard Mulligan.137 However, the globin gene in such vectors expressed very poorly in mice that had been reconstituted with transduced stem cells.
The next era of globin gene vector design began with the discovery of the locus control region (LCR) upstream from the human β-globin gene cluster.138 Studies in transgenic mice defined core elements of the LCR, reflected in chromatin as DNAseI hypersensitive sites,139 which enhanced globin gene expression when included in retroviral vectors. Unfortunately, it proved extremely difficult to incorporate these LCR core elements into γ retroviral vectors containing a globin gene.140,141 Ultimately, the instability of such vectors during production or upon attempted transfer into target cells precluded their further development.
A major breakthrough occurred in 2000 when it was demonstrated by Michele Sadelain and his colleagues that lentiviral vectors were capable of transferring an expression cassette with required regulatory elements necessary to achieve potentially therapeutic levels of globin expression in a murine model of β-thalassemia.142 Correction of the phenotype of murine β-thalassemia intermedia143,144 and sickle cell disease145 has been achieved. Correction of the thalassemia phenotype has also been demonstrated in patient cells, as reflected by the establishment of effective erythropoiesis in erythroid cultures.146 However, in a murine model of thalassemia major, only 1 of 6 animals demonstrated a relatively high level of hemoglobin; the other animals remained severely anemic with a thalassemia intermedia phenotype.147
Despite progress, there are still barriers to overcome before gene therapy can be applied for hemoglobin disorders. Studies in mouse models suggest that approximately 20% of the bone marrow cells must be genetically modified and that a level of globin production of at least 20% that of the endogenous β-globin genes must be achieved.148 Evidence is accumulating that insulator elements, first introduced into globin vectors by George Stamatoyannopoulos and his colleagues,149 will improve globin gene after retroviral vector–mediated gene transfer.150 The development of stem cell–targeted gene therapy would be greatly facilitated by the derivation of stable producer clones generating large numbers of vector particles. Currently, vector production is done by transient transfection of multiple plasmids into cultured cells, a laborious and tedious process. Although vector producer clones have been described,151,152 none have yet been reported for globin vectors. The availability of a stable clone would also facilitate the development of methodology for purifying vector particles and increasing their concentration, which is likely to improve stem cell transduction frequency.
Although some progress has been made in improving the efficiency of transduction of primitive hematopoietic cells,74,75 long-term repopulating stem cells remain relatively resistant to genetic modification. Individual integration sites have been found in both lymphoid and myeloid cells in patients with X-SCID92,93 and ADA-SCID,94 consistent with stem cell transduction, but most integration sites are only found in lymphoid populations, which is consistent with transduction of a common lymphoid progenitor (Figure 3). Modification of transduction conditions or the use of alternative envelope pseudotypes are some of the strategies that are being explored to improve the efficiency of multipotential stem cell–targeted gene transfer.
Summary
A brief historic review cannot, by its nature, be comprehensive. Rather, I have attempted to highlight significant accomplishments and in so doing have undoubtedly omitted many that have been equally important to the field. Some areas of research focused on diseases relevant to hematology (eg, lysosomal storage disorders have not been addressed and those interested are directed to a recent review on this topic.153 Overall, there has been continued progress during the nearly 3 decades since the development of recombinant DNA technology made gene therapy a potential reality. Despite occasional setbacks and rare but highly publicized adverse events,154 many early phase clinical trials have been safely conducted. Currently there are 143 active protocols that have been reviewed by the Recombinant DNA Advisory Committee and most are enrolling patients (http://www.gencris.od.nih.gov.). Thirty of these trials are focused on evaluating therapeutic interventions for blood disorders or viral infections in the context of bone marrow transplantation. Such trials continue to yield promising data on which to develop specific clinical applications for treatment of blood disorders. Given the high costs for medical care for these severe and often chronic conditions, the successful development of gene therapy also has the potential to be of significant economic benefit.
Acknowledgments
I thank Derek Persons and Brian Sorrentino for reading the manuscript and providing many constructive suggestions for improving it, Byoung Ryu for assistance in developing the figures, and Pat Streich for help in preparing the manuscript.
Authorship
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Arthur W. Nienhuis, St Jude Children's Research Hospital, 332 N Lauderdale St, Memphis, TN 38105; e-mail: arthur.nienhuis@stjude.org.