Abstract
Acquired and congenital aplastic anemias recently have been linked molecularly and pathophysiologically by abnormal telomere maintenance. Telomeres are repeated nucleotide sequences that cap the ends of chromosomes and protect them from damage. Telomeres are eroded with cell division, but in hematopoietic stem cells, maintenance of their length is mediated by telomerase. Accelerated telomere shortening is virtually universal in dyskeratosis congenita, caused by mutations in genes encoding components of telomerase or telomere-binding protein (TERT, TERC, DKC1, NOP10, or TINF2). About one-third of patients with acquired aplastic anemia also have short telomeres, which in some cases associate with TERT or TERC mutations. These mutations cause low telomerase activity, accelerated telomere shortening, and diminished proliferative capacity of hematopoietic progenitors. As in other genetic diseases, additional environmental, genetic, and epigenetic modifiers must contribute to telomere erosion and ultimately to disease phenotype. Short telomeres also may cause genomic instability and malignant progression in these marrow failure syndromes. Identification of short telomeres has potential clinical implications: it may be useful in dyskeratosis congenita diagnosis, in suggesting mutations in patients with acquired aplastic anemia, and for selection of suitable hematopoietic stem cell family donors for transplantation in telomerase-deficient patients.
Introduction
Historically regarded as pathophysiologically distinct entities, acquired and constitutional aplastic anemias recently have been linked by a common pathogenic feature—telomere shortening in leukocytes. About a decade ago, telomeres were first measured to be short in about one-third of acquired aplastic anemia cases by investigators at St George's Hospital (London, United Kingdom), and patients with the shortest telomeres appeared to have a longer duration of disease and to be more likely to develop late and malignant clonal complications.1 We and others reported that granulocytes were mainly affected by telomere erosion and that patients with short telomeres were less likely to respond to immunosuppression.2,3 Telomere shortening also is common in congenital aplastic anemias, such as Fanconi anemia,4,5 dyskeratosis congenita,6,7 and Shwachman-Diamond syndrome.8
Initially, telomere shortening in marrow failure was thought to be simply a consequence of “stressed” hematopoiesis. However, the discoveries that (1) some pedigrees of X-linked dyskeratosis congenita were mutant in a gene called DKC1, made by Dokal et al at the Hammersmith Hospital (London, United Kingdom),9 and (2) that the DKC1 gene product, dyskerin, physically associated with the telomerase complex, made by Mitchell and Collins at the University of California at Berkeley,6 provided a crucial connection between a discrete genetic lesion and telomere shortening of hematopoietic cells. Subsequently, mutations in similar genes related to telomere maintenance were described in other forms of dyskeratosis congenita (Table 1). In important contrast to hematologic examples of genetic diseases like sickle cell anemia, thalassemia, and enzymopathies, heterozygous mutations in the genes of the telomere repair complex cause disease, and the onset of symptoms and signs early or late in life, the ultimate severity of clinical manifestations, and the specific organs affected must be powerfully influenced by as-yet incompletely defined added environmental, genetic, and epigenetic modifiers.
This review describes our current understanding of the relationship of dysfunctional telomeres and defective telomerase to bone marrow failure and its complications. The detailed pathophysiologies of both acquired and constitutional aplastic anemias have recently been reviewed10-14 and will not be discussed here; the reader also is referred to comprehensive basic science reviews of telomere biology and telomerase.15-18
Telomere biology
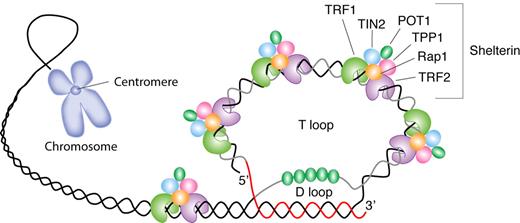
The telomere is an evolutionary solution to problems presented by chromosome linearity, essentially the need to distinguish between chromosomal termini and double-stranded DNA breaks (Figure 1). By adopting a uniform and repeated sequence of DNA for all of a species' chromosomes, multiple adaptive mechanisms are provided: (1) formation of a circular end (the t-loop); (2) a protective protein shield (shelterin); (3) a specialized enzymatic mechanism to add nucleotides to the chromosomes after each cell division and maintain telomere length (telomerase); and (4) substitution for coding sequences of important genes at the vulnerable ends of the chromosome.
Schematic representation of telomere structure. Telomeres are at the extremities of chromosome DNA. The telomeric 3′ end terminates as a single-stranded, G-rich overhang able to form the t-loop, in which the overhang invades the telomeric double helix, remodeling the DNA into a circle. Telomeres are capped by at least 6 proteins (TRF1, TRF2, TPP1, POT1, TIN2, and Rap1), collectively known as shelterin, that physically shield the DNA.19 TRF1, TRF2, and TPP1 specifically recognize and bind to double-stranded TTAGGG repeats; POT1 binds to the single-stranded telomeric overhang19,20 ; TIN2 and Rap1 complete the shelterin complex. Shelterin allows discrimination of telomeres from double-stranded DNA breaks; lack of shelterin allows telomeres to be identified as double-stranded DNA breaks and triggers DNA-damage pathways.19
Schematic representation of telomere structure. Telomeres are at the extremities of chromosome DNA. The telomeric 3′ end terminates as a single-stranded, G-rich overhang able to form the t-loop, in which the overhang invades the telomeric double helix, remodeling the DNA into a circle. Telomeres are capped by at least 6 proteins (TRF1, TRF2, TPP1, POT1, TIN2, and Rap1), collectively known as shelterin, that physically shield the DNA.19 TRF1, TRF2, and TPP1 specifically recognize and bind to double-stranded TTAGGG repeats; POT1 binds to the single-stranded telomeric overhang19,20 ; TIN2 and Rap1 complete the shelterin complex. Shelterin allows discrimination of telomeres from double-stranded DNA breaks; lack of shelterin allows telomeres to be identified as double-stranded DNA breaks and triggers DNA-damage pathways.19
Telomere structure
Double-stranded breaks that arise, for example, from DNA damage caused by irradiation, elicit the intranuclear DNA-damage machinery. In contrast, the telomere macromolecular complex caps the tips of chromosomes so that the free ends of the DNA molecule are not recognized by conventional DNA-repair mechanisms.16 In humans, telomeres consist of some thousands of TTAGGG tandem repeats (CCCTAA in the complementary strand21 ; Figure 1).
The end-replication problem
To initiate DNA synthesis, all DNA polymerases require a 3′-OH end, which can be provided by either RNA or DNA primers. With DNA replication, the removal of the RNA primer results in a terminal gap and a 3′ single-stranded overhang.22 In the body of the chromosomes, primer gaps are filled with DNA added by the adjacent Okazaki fragment, except at the ends of telomeres where an adjacent Okazaki fragment is absent. The “end-replication problem,”23,24 a shorter lagging daughter DNA strand, has the consequence that telomere length diminishes with each cell division. Additional factors, such as oxidative damage, further contribute to telomere erosion.25
When telomeres become critically short, protective responses are engaged: short telomeres recruit double-stranded DNA break markers, such as phosphorylated histone H2AX and DNA-damage checkpoint factors,26 and activate p53 through ATM, up-regulating the cell-cycle inhibitor p21 and blocking cell cycle in G1,27 ultimately producing cell proliferation arrest and apoptosis. In the absence of telomeres and the cellular protective responses, erosion of the chromosomal ends eventually would cause genomic instability and gene attrition.
Telomerase complex
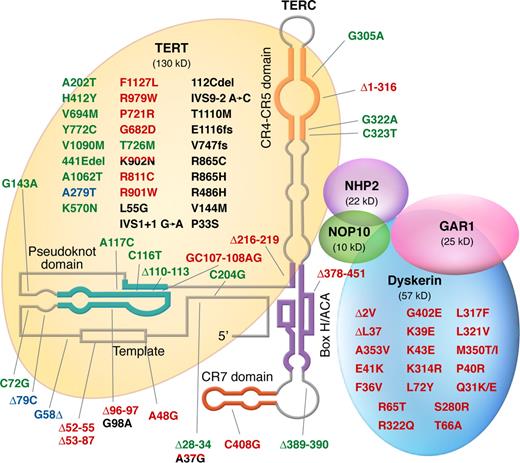
To counter telomere erosion, cells with a high replicative capacity, such as hematopoietic stem cells, express a specialized reverse transcriptase known as telomerase, which extends telomeres by catalyzing the addition of TTAGGG nucleotide repeats to the 3′ terminus of a chromosome's DNA (Figure 2A)29 ; the lagging strand is then duplicated by DNA polymerase. The catalytic portion of telomerase is composed of 2 molecules of each component: the enzyme, telomerase reverse transcriptase or TERT; an RNA template, encoded by TERC; and dyskerin, encoded by DKC1.30 TERT belongs to a family of reverse transcriptases specialized in elongating telomeres. (Figure 2B).15,18 In humans, the RNA template TERC spans 451 nucleotides, including an 11-nucleotide–long template, and contains several conserved regions essential for its stability (Figure 3C). TERC binds to other proteins: dyskerin, GAR, NHP2, and NOP10.
Structure and function of the telomerase complex. (A) TERT enzymatically adds TTAGGG nucleotide repeats to the 3′ end of telomere's leading strand using TERC as a template. Other proteins (dyskerin, NOP10, NHP2, and GAR) also bind to TERC and stabilize the complex. (B) Linear structure of TERT, which is highly conserved among eukaryotes and consists of the central reverse transcriptase (RT) motifs (1, 2, A, B, C, D, and E), a large N-terminal region, and a short C-terminal region, all necessary for telomerase enzymatic function. The N-terminal region comprises a telomerase-essential N-terminal domain (TEN), the CP, and the QFP domains, required for RNA interaction, and a telomerase-specific T motif. The C-terminal region contains 4 conserved domains (E-I to E-IV). (C) Secondary structure of human TERC, which contains 7 conserved regions (CRs), a pseudoknot important for interaction with TERT (CR2/CR3), and a template used by TERT for telomere elongation (CR1). TERC also encloses a small nucleolar H/ACA motif; box H/ACA refers to a tail region of small nucleolar RNAs (snoRNAs) carrying a conserved H motif (AnAnnA) and consensus ACA triplet positioned 3 nucleotides before the 3′ end of the RNA that characterize a major snoRNA family involved in pseudouridylation of pre-rRNAs.28 TERC binds to other proteins, such as dyskerin, GAR, NHP2, and NOP10, through box H/ACA.
Structure and function of the telomerase complex. (A) TERT enzymatically adds TTAGGG nucleotide repeats to the 3′ end of telomere's leading strand using TERC as a template. Other proteins (dyskerin, NOP10, NHP2, and GAR) also bind to TERC and stabilize the complex. (B) Linear structure of TERT, which is highly conserved among eukaryotes and consists of the central reverse transcriptase (RT) motifs (1, 2, A, B, C, D, and E), a large N-terminal region, and a short C-terminal region, all necessary for telomerase enzymatic function. The N-terminal region comprises a telomerase-essential N-terminal domain (TEN), the CP, and the QFP domains, required for RNA interaction, and a telomerase-specific T motif. The C-terminal region contains 4 conserved domains (E-I to E-IV). (C) Secondary structure of human TERC, which contains 7 conserved regions (CRs), a pseudoknot important for interaction with TERT (CR2/CR3), and a template used by TERT for telomere elongation (CR1). TERC also encloses a small nucleolar H/ACA motif; box H/ACA refers to a tail region of small nucleolar RNAs (snoRNAs) carrying a conserved H motif (AnAnnA) and consensus ACA triplet positioned 3 nucleotides before the 3′ end of the RNA that characterize a major snoRNA family involved in pseudouridylation of pre-rRNAs.28 TERC binds to other proteins, such as dyskerin, GAR, NHP2, and NOP10, through box H/ACA.
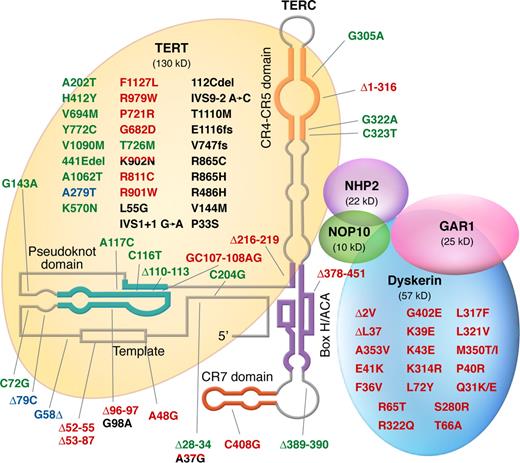
Mutations in telomerase complex genes and human disease. Mutations in green were described in patients with acquired aplastic anemia; mutations in red were described in patients with dyskeratosis congenita; mutations in black were described in patients with pulmonary fibrosis; and polymorphisms are represented in blue. Mutations found in more than one disease type are double-colored.
Mutations in telomerase complex genes and human disease. Mutations in green were described in patients with acquired aplastic anemia; mutations in red were described in patients with dyskeratosis congenita; mutations in black were described in patients with pulmonary fibrosis; and polymorphisms are represented in blue. Mutations found in more than one disease type are double-colored.
Telomerase regulation
Telomerase is expressed mainly in embryonic and adult stem cells, highly proliferative cells such as mature lymphocytes, and in cancer cells, but not in most mature cells.15 Telomerase is regulated by a wide variety of genes. TERT is repressed by retinoblastoma protein (Rb) and cyclin-dependent kinase inhibitor p21WAF1.31 Conversely, c-Myc activates TERT gene expression. The TERT promoter contains estrogen receptor elements and in reproductive tissues, estrogen and androgens have important roles in regulating telomerase expression and activity.32 TERT phosphorylation is another mechanism of telomerase activity regulation.33,34 In hematopoietic cells, tissue-specific modulators have not been characterized. However, we recently have discovered that both estrogen and androgens activate telomerase in hematopoietic cells, mediated by estrogen-estrogen receptor complex binding to estrogen response elements in the TERT promoter.35
Animal models
Telomeres of normal laboratory mice (Mus musculus) are much longer than in humans, despite a much shorter murine life span, and the mouse may not be the ideal organism in which to model human telomere biology. Nonetheless, murine models have been created to “knock out” TERT (Tert−/−) or the RNA component (Terc−/−).36,37 Other investigators have used the CAST/EiJ mouse strain, which has a shorter telomere length.38 A phenotype related to telomerase deficiency is initially absent in these knockouts, but appears and becomes more pronounced in successive generations. Homozygous mice are viable, but over a few generations, as telomeres shorten, they become infertile. Terc−/− mice also have splenic atrophy, a reduced lymphocyte proliferative capacity, and impaired hematopoietic function, although overt cytopenia is not observed.39 Later-generation knockout mice have reduced capacity to recover blood counts after challenge with 5-fluoracil.39 Intestinal atrophy, cardiac dysfunction, and limited proliferation of adult neural stem cells also are observed in telomerase-deficient mice.17 Fourth-generation Terc−/− murine cells show signs of chromosomal instability40 ; in addition to consistent telomere shortening and the appearance of chromosomes lacking telomere capping, there are aneuploidy and other chromosomal abnormalities, including end-to-end fusions,36 and an increased rate of tumor formation.39
Telomere shortening in human bone marrow failure
Dyskeratosis congenita
Dyskeratosis congenita is a rare constitutional bone marrow failure syndrome stereotypically characterized by mucocutaneous abnormalities (nail dystrophy, hyperpigmentation, and leukoplakia) and aplastic anemia in childhood. Patients with dyskeratosis are at increased risk for malignancies, pulmonary fibrosis, and liver cirrhosis.13 The genetic basis of dyskeratosis congenita was unclear until investigators Hammersmith Hospital, using conventional linkage analysis of large pedigrees reported to the Dyskeratosis Congenita Registry, found X-linked dyskeratosis congenita to associate with mutations in the DKC1 gene.9,41 The gene product dyskerin's activities were initially thought to be restricted to rRNA biosynthesis, pseudouridylation of rRNA precursors, and ribosomal assembly, based on the functions of the yeast ortholog Cbf5p (discussed in “Abnormal rRNA modification and dyskerin function”). However, box H/ACA, characteristic of mammalian TERC, and the ability of Cbf5p to associate with H/ACA snoRNAs suggested additional roles for dyskerin and implicated it as a component of telomerase.6 Indeed, dyskerin was found to physically associate with TERC, the RNA component of telomerase.6 Patients' leukocytes showed reduced telomerase activity,6 explaining the erosion of their telomeres.7 The discovery that autosomal dominant dyskeratosis is caused by heterozygous mutations or large deletions in TERC established dyskeratosis congenita as a disease of telomerase insufficiency.42 Homozygous mutations in TERT43 and in NOP10,44 a telomerase-associated protein, were described in some families with autosomal recessive dyskeratosis congenita. More recently, mutations in TINF2, which encodes TIN2, a shelterin protein that caps and protects the telomeres, have been identified in a third of patients with dyskeratosis congenita, further implicating abnormal telomere maintenance in the pathophysiology of the disease.45
Mechanisms of telomerase deficiency
TERC mutations in autosomal dominant dyskeratosis congenita disrupt telomerase function (Figure 3). In vitro, vectors containing wild-type TERC and TERT that are transfected into telomerase-deficient cell lines reconstitute telomerase activity; transfection of mutant TERC-containing vectors leads to absent or severely reduced telomerase activity. However, telomerase activity is only partially reduced in the patient's primary cells because the mutations are heterozygous. Telomerase activity almost always is impaired by haploinsufficiency; when both mutant and wild-type TERC vectors are cotransfected into a telomerase-deficient cell line, telomerase activity is only partly reduced, indicating that mutant TERC does not affect the wild-type molecule.46,47 For most nonstructural proteins, haploinsufficiency is insufficient to produce a disease phenotype, except in extreme circumstances, because the remaining wild-type allele produces sufficient protein, for example to catalyze enzymatic activity. However, the telomerase complex is tightly regulated and reduced TERC expression limits telomerase activity, unbalancing the equilibrium between telomere elongation and attrition to favor gradual telomere erosion.48 TERC mutations influence telomerase activity by disrupting base pairing in the TERC molecule, destabilizing the pseudoknot domain, and altering its secondary structure.46,49,50 None of the mutations so far described appears to affect telomerase complex assembly.
Disease anticipation
A new mechanism of disease anticipation has been demonstrated in large kindreds with autosomal dominant dyskeratosis congenita (with mutations in TERC)51 : the disease phenotype appears at younger ages in successive generations. Anticipation may be explained by simultaneous inheritance of the mutation and of chromosomes with short telomeres. Terc knockout mice show similar signs of hematopoietic proliferation deficiency as well as significant telomere shortening restricted to late generations.36,52
Abnormal rRNA modification and dyskerin function
Telomerase deficiency causing telomere shortening may not be the sole molecular mechanism responsible for the dyskeratosis congenita phenotype. That dyskerin's yeast ortholog Cbf5p is essential for pre-rRNA processing and participates in rRNA pseudouridylation suggested dyskeratosis as a ribosomal disease in early observations.53 In addition, products of genes mutated in other inherited marrow failure syndromes—Diamond-Blackfan anemia, Shwachman-Diamond syndrome, and cartilage-hair hypoplasia—are predicted to be involved in rRNA processing and biogenesis.54 In an animal model, hypomorphic Dkc1m mice showed defective rRNA pseudouridylation in early generations,55 and there was abnormal accumulation of immature rRNA species.55 Deficient translation dependent on internal ribosome entry site (IRES) also caused reduced expression of IRES-containing mRNAs, such as those for tumor suppressor p27 and antiapoptotic Bcl-xL and XIAP.56 In humans, against a role of abnormal ribosomal processing in the marrow failure of dyskeratosis congenita, cells of patients with X-linked DKC do not show abnormal rRNA processing or immature rRNA accumulation48 ; patients with either autosomal dominant or autosomal recessive dyskeratosis congenita, in which TERC, TERT, or NOP10 are mutated, are not predicted to have abnormal rRNA pseudouridylation, despite clinical features similar to X-linked dyskeratosis congenita.57 The role of abnormal ribosomal modification in the pathophysiology of dyskeratosis congenita deserves further elucidation, but at present evidence obtained from patient samples does not support a major role of this molecular pathway.54,58,59
Acquired aplastic anemia
An immune pathophysiology that targets hematopoietic stem cells has been inferred in most cases of acquired aplastic anemia from the hematologic response to immunosuppressive therapies; failure to respond to such treatments might be due to a nonimmune etiology or to properties of the hematopoietic compartment, a quantitatively severe loss of stem cell number or a qualitative abnormality of stem and progenitor cells.10 When some patients with acquired aplastic anemia were found to have accelerated leukocyte telomere attrition, the cause was initially presumed to be secondary, a physiologic response to stem cell “stress.”1-3 The discovery of the genetic basis of telomere erosion in dyskeratosis congenita made it plausible that some patients with acquired marrow failure also might have abnormal telomerase function as a basis for telomere shortening. In a first attempt to develop such an association, a TERC genetic variant, G58A, was reported in 12% of patients with acquired aplastic anemia,60 but G58A was soon shown to be a common polymorphism among African descendents that does not affect telomerase activity or telomere length.49 Subsequent studies demonstrated the presence of true pathologic mutations in TERC in a few patients with apparently acquired aplastic anemia who lacked the typical physical stigmata of dyskeratosis congenita and an obvious family history.47,61-63 These mutations were not observed in large numbers of healthy controls. The functional consequences of the genetic changes in TERC were reduced telomerase activity of patient primary cells, very short telomeres of leukocytes, and low telomerase activity in vitro.46 TERC mutations in either dyskeratosis congenita or acquired aplastic anemia are similar at the molecular level and produce the same effects on telomerase function, despite the diversity of clinical phenotypes (Table 2); an exception is large TERC gene deletions observed in dyskeratosis congenita families only.42 In cohort studies, the frequency of TERC mutations was low (approximately 4% of all patients with acquired aplastic anemia).62,64 Careful study of some families showed that healthy relatives of patients carrying TERC mutations also had short telomeres, some mild hematologic abnormalities (macrocytosis, mild anemia, thrombocytopenia, or granulocytopenia), reduced progenitor cells in peripheral blood, increased serum hematopoietic growth factors, and hypoplastic bone marrows.61 The potential clinical relevance of sometimes modest hematologic findings was dramatically manifested in hematopoietic stem cell graft failure in one proband, whose unknowingly affected sibling donor provided only very low numbers of CD34+ cells from both marrow and mobilized blood collected in multiple leukaphereses.61
As the number of patients with acquired aplastic anemia with short telomeres is much higher than those carrying TERC mutations, we also screened patients for mutations in TERT, the gene encoding the telomerase reverse transcriptase itself. Approximately 4% of patients with apparently acquired aplastic anemia had heterozygous TERT nonsynonymous mutations that disrupted telomerase activity by haploinsufficiency, causing short telomeres of leukocytes and a hematopoietic stem cell compartment of limited proliferative capacity.47,65 Several patients with TERT mutations also have a family history of blood dyscrasias, especially myelodysplastic syndrome evolving to acute myeloid leukemia, further suggesting a common genetic background for these disorders. As with TERC, apparently healthy relatives with TERT mutations had short telomeres and reduced hematopoietic function.47,65 The association between TERT mutations and aplastic anemia has been confirmed.47,66-68 Aplastic anemia–associated TERT mutations may occur in any domain of the enzyme; mutations disrupt telomerase's ability to elongate telomeres, but the tertiary structure of human TERT must be characterized before a comprehensive analysis of mutation effects on the complex structure is possible.
A few patients with marrow failure have genetic variants and specific haplotypes for genes coding for shelterin components (TERF1 and TERF2) that might contribute to disease.69 TRF1 and TRF2 bind directly to the telomere DNA strand, protecting it from damage as well as regulating telomere length, and mutations reducing their expression or impairing their binding to DNA result in telomere erosion.
Variability in phenotypic expression
Are telomerase complex mutations sufficient to cause disease? There is great variability in the phenotype caused by mutations in telomerase complex genes with clinical outcomes including isolated aplastic anemia, isolated pulmonary fibrosis or hepatic cirrhosis (see “Telomerase mutations and nonhematologic diseases”), or multiorgan dyskeratosis congenita. The same mutation either in TERC or TERT in a single pedigree can associate with aplastic anemia in one patient, and pulmonary fibrosis or hepatic cirrhosis in another, suggesting that other factors contribute to organ damage.47,66,70 As an example, telomerase-mutant patients with pulmonary fibrosis (see “Telomerase mutations and nonhematologic diseases”) often have a smoking history, suggesting this environmental insult as a trigger.
Conversely, many relatives of patients with aplastic anemia with the same telomerase mutation have low telomerase activity, short telomeres, a hypoplastic bone marrow, and reduced hematopoietic function, but they are clinically healthy and asymptomatic (see “Acquired aplastic anemia”).47,61,65 Although disease anticipation may play a role in determining manifestation, the hypoplastic bone marrow of the TERC- or TERT-mutant individuals appears capable of maintaining hematopoiesis under normal conditions, but may be hypothesized to be more susceptible to environmental injury, such as viral infections, drug exposure, or immune attack. Low telomerase function may result in a quantitative deficiency by reducing the number of hematopoietic stem cells able to maintain hematopoiesis, as well as a qualitative defect by impairing hematopoietic stem cell regeneration. We have observed higher serum interferon-γ and a limited T-cell receptor (TCR) Vβ usage in telomerase mutant patients—similar to the skewed T-cell population typically observed in patients with immune acquired aplastic anemia71 —consistent with oligoclonal T-cell expansion and immune destruction of marrow as pathophysiologic in these patients also.72 A telomerase mutation does not appear to be sufficient to determine aplastic anemia, but healthy relatives in which telomeres are short and/or a mutation need to be further observed in long-term prospective studies.
Other causes for telomere shortening in acquired aplastic anemia
As the number of patients with acquired aplastic anemia with short telomeres exceeds those with identified mutations, other genetic lesions or environmental factors also may contribute to accelerated telomere erosion. In addition to aging, some environmental factors are known to cause telomere attrition. Smoking73 and even psychological stress74 have correlated with leukocyte telomere shortening. Overdemanded or “stressed” hematopoiesis also can result in telomere erosion. Excessive telomere shortening has been observed in the first years after allogeneic bone marrow transplantation in comparison with donor leukocytes, consistent with increased stem and progenitor turnover to replenish the bone marrow.75 Chemotherapy for solid tumors also causes myelotoxicity, requiring increased hematopoietic regeneration to recover blood cell counts, and premature telomere shortening has been observed following multiple cycles of cytotoxic drug therapy.76 These observations are consistent with a contribution of “stress” hematopoiesis secondary to environmental factors to telomere shortening in aplastic anemia. However, the degree of telomere shortening produced in these clinical circumstances has been mild, less than 1 kb erosion in telomere lengths, as compared with the extreme attrition observed in individual mutations in telomerase deficiency (usually more than 3 kb).
Telomere shortening in other bone marrow failure syndromes
Fanconi anemia is a chromosome instability syndrome clinically characterized by bone marrow failure, developmental defects, physical anomalies, and an increased risk to cancer, and in vitro by chromosome instability, detected by increased chromosomal breakage in the presence of clastogenic agents.11 The disease is genetically determined by mutations in at least 13 different genes (FANC-A to FANC-N).11 Most Fanconi anemia gene products interact in a common pathway that recognizes and repairs damaged DNA; marrow failure is attributed to inadequate DNA repair and subsequent triggering of apoptosis and genomic instability, but the exact mechanism(s) remains unclear.12,59 Telomere shortening of leukocytes is also common in Fanconi anemia, and erosion of telomeres is prognostic, directly correlating with the severity of the marrow failure, the probability of developing frank aplastic anemia, the presence of organ malformation, and, inversely, with the age of onset for myelodysplasia and tumors.5 However, telomerase activity in hematopoietic cells of patients with Fanconi anemia is preserved, indicating that telomere erosion may be secondary to increased loss of telomeric nucleotides, the consequence of increased proliferation in response to pancytopenia. Alternatively, telomere loss could be the result of direct telomeric DNA damage followed by inadequate DNA repair; telomere damage in Fanconi anemia might be excessive due to increased production of reactive oxygen species,77 known to cause telomere erosion.78 Excess telomere breaks were observed in Fanconi anemia cells, correlating with extrachromosomic telomeres.79 Another mechanism of accelerated telomere loss may be analogous to that observed in Werner syndrome, which is caused by mutations in WRN, which encodes a helicase. At least 2 FA genes bear helicase domains (FANC-J and FANC-M).11 Werner syndrome cells show deletion of telomeres from a single sister chromatid, resulting in excessive telomere shortening that is not countered by telomerase. Deletions result from poor resolution of G-rich DNA, such as telomeres, in the absence of WRN helicase.80 Telomere shortening and deficient DNA repair are not necessarily accompanied by bone marrow failure, which is not observed in Werner syndrome, Bloom syndrome, ataxia-telangiectasia, or Nijmegen breakage syndrome. In these syndromes, however, telomere length has only been measured in patients' fibroblasts, not in leukocytes, and the extent of telomere erosion in their hematopoietic tissue is unknown. In addition, affected tissues in these disorders are mainly telomerase-negative, suggesting that tissues expressing telomerase can counterbalance telomeric loss and thus are spared from senescence.
Telomeres also are very short in leukocytes of patients with Shwachman-Diamond syndrome.8,81 Shwachman-Diamond syndrome is a rare autosomal recessive inherited bone marrow failure syndrome in which neutropenia is associated with exocrine pancreatic insufficiency, skeletal dysplasia, and predisposition to myelodysplasia and leukemia.14 More than 90% of Shwachman-Diamond cases are caused by biallelic mutations in SBDS, a gene which encodes a homonymous protein of unknown function in mammalian cells; again, the gene's yeast ortholog mediates translational activation of ribosomes.82 The pathogenesis of marrow failure in Shwachman-Diamond syndrome is obscure. In human cells, although SBDS coprecipitates with 28S rRNA, SBDS deficiency does not result in changes in the rRNA maturation profile.83 SBDS knockdown in murine hematopoietic progenitor cells compromises granulocytic differentiation in vitro and reduces the number of myeloid progenitors in vivo.84
Approximately 5% of patients with acquired aplastic anemia, all with very short telomeres, were heterozygous for an SBDS gene mutation associated with Shwachman-Diamond syndrome, in which telomeres are consistently short.81 These patients did not show pancreatic exocrine failure or skeletal abnormalities, but they had a poor prognosis and little response to immunosuppressive therapy. In patients with Shwachman-Diamond syndrome, telomere length correlates with SBDS protein expression, but no physical association between SBDS protein and the telomerase complex could be demonstrated.81 SBDS knockdown in HeLa cells results in increased production of reactive oxygen species and telomere shortening,85 suggesting telomeric DNA damage as the mechanism of telomeric erosion in this syndrome.
Genetic instability in telomerase-deficient cells and the chromosome theory of cancer
In 1914, Theodor Boveri first postulated that genomic instability played a major role in the origin of cancer: “For unknown reasons, a ‘weakness’ may occur in specific chromosomes with respect to control of mitosis that at first remains latent and thus is transmitted to a large number for daughter cells…. [W]ith the beginning of senescence, perhaps this latent weakness becomes manifest in the failure of mitotic control in such a way that when cell division occurs, there is a possibility of generating daughter cells with recurrent abnormalities.”86, page 80
The role of chromosomal instability in oncogenesis is strongly inferred from aneuploidy, caused by aberrant chromosomal segregation during cell division, chromosomal translocation, and abnormal nucleotide sequences, all relevant for cancer formation and progression. Telomere shortening caused by cell division fits the “weakness” hypothesized by Boveri almost 100 years ago. We know now that dysfunctional and short telomeres cause genomic instability and may underlie the pathogenesis of malignant transformation.87,88 In vitro, damaged or short telomeres can be recognized as DNA double-stranded breaks, activate the DNA-repair machinery, and become the targets of nonhomologous end-joining or homology-directed repair.87,89 Homology-directed repair may recombine a telomere sequence from one chromosome with more centromeric sequences from another, producing inversions, translocations, and terminal deletions. Nonhomologous end-joining generates covalently bound chromosomes that are unable to properly segregate during mitosis, leading to breakage-fusion-bridge cycles, mediating amplification and deletion across the genome. Terc“knockout” mice have an increased incidence of cancer, including lymphoma, with successive generations: cancer incidence increases as telomeres shorten.39
In humans, there is some evidence linking telomere erosion to aneuploidy and malignant transformation. Telomere shortening appeared to correlate with genomic instability in ulcerative colitis.90 Telomere erosion also follows autologous hematopoietic stem cell transplantation, and telomere length is especially short in those patients who eventually develop posttransplantation myelodysplasia (T. C. Tarella, unpublished data, May 2007). Most recently, we observed constitutive telomerase mutations in approximately 8% of patients with acute myeloid leukemia; telomerase mutations were particularly associated with chromosomal translocations and aneuploidy in blast cells.91
Genomic instability resulting from dysfunctional telomeres offers a plausible explanation for the higher rate of cancer in patients with dyskeratosis congenita,11,13 and patients with acquired aplastic anemia also are at risk for myelodysplasia and leukemia.92,93 Almost always, these malignant clonal complications occur with loss or gain of chromosomes.94 Direct experimental in vitro data comes from bone marrow cell culture. In the presence of high doses of granulocyte colony-stimulating factor, cells from TERT- or TERC-mutant patients have increased chromosome end-to-end fusions and aneuploidy.95
Telomerase mutations and nonhematologic diseases
Skin abnormalities, pulmonary fibrosis, and hepatic cirrhosis are features of dyskeratosis congenita.57,96 Recently, genetic screening of families with idiopathic pulmonary fibrosis showed a strong association with telomerase mutations.70,97 In one study, among 73 probands from the Vanderbilt Familial Pulmonary Fibrosis Registry (Vanderbilt University School of Medicine, Nashville, TN), 6 (8%) were heterozygous for a telomerase gene mutation, which cosegregated in the families with disease phenotype. No family member had the classical cutaneous features of dyskeratosis congenita, but in one kindred with a TERC mutation, 4 relatives developed marrow failure while pulmonary fibrosis was diagnosed in 7; aplastic anemia and pulmonary fibrosis did not simultaneously occur in any individual. Another study identified 7 families with mutations in telomerase out of 46 families (a rate of 15%); among 44 patients with sporadic pulmonary fibrosis, only one tested positive for a TERT mutation.97 Affected patients had short telomeres of peripheral blood leukocytes and some also had anemia, neutropenia, or hepatic cirrhosis. Clinical histories of affected individuals suggested that active or passive smoking may have played a role in disease development and progression.97 Among the 7 families and the sporadic case of pulmonary fibrosis, smoke exposure was noted in 60% of affected individuals.97
Hepatic cirrhosis has been described in dyskeratosis congenita, and severe liver disease is not uncommon after hematopoietic stem cell transplantation for marrow failure in patients with this constitutional disease. In a large Mennonite kindred in which almost 60 individuals have been tested, a loss-of-function TERT mutation cosegregated not only with blood disorders but also with liver disease: hepatic fibrosis with inflammation was observed in 3 young to middle-aged women heterozygous for a TERT mutation.98
How telomerase deficiency causes pulmonary or hepatic fibrosis is unknown. Telomere shortening could predispose to exhaustion of bronchoalveolar stem cells and loss of alveolar cells; fibrosis would be secondary.70 Telomere shortening also has been associated with deficient liver regeneration in animals and accelerated development of liver cirrhosis in response to chronic liver injury.99 In addition, both environmental (smoking, toxin exposure, viral infections) and other genetic factors modulate the clinical manifestations of telomerase deficiency.
Clinical diagnosis and family screening
Telomere shortening observed in leukocytes is frequent in virtually all the bone marrow failure syndromes, acquired and constitutional. Nevertheless, a recent analysis of patients with dyskeratosis congenita and other marrow failure syndromes showed that measurement of telomere length of lymphocytes, but not of granulocytes, by flow–fluorescence in situ hybridization (FISH) distinguished dyskeratosis congenita from other marrow failure syndromes.100 Abnormally short telomeres of lymphocytes showed 91% sensitivity and specificity for the diagnosis of dyskeratosis congenita. Analysis of abnormal telomeres of granulocytes was more sensitive (96%) but less specific (74%). Unfortunately, telomere length by flow-FISH is not yet commercially available, nor is it approved for clinical testing in the United States.
Screening for mutations in telomerase genes is used in patients with dyskeratosis congenita and, once a mutation is identified, in their relatives. Patients with acquired aplastic anemia who do not respond adequately to immunosuppression or have a family history of hematologic disorders also may benefit from genetic testing. In contrast to telomere length measurement, genetic testing for DKC1, TERT, and TERC genes is commercially available.
Treatment implications
For most syndromes in which telomere shortening is known to be pathogenic, hematopoietic stem cell transplantation is the only potential cure. In dyskeratosis congenita, the success of this procedure is significantly affected by early transplantation-related fatal complications, especially pulmonary toxicity, veno-occlusive disease, and renal microangiopathy,96 probably all as a consequence of preexisting cryptic organ abnormalities related to the underlying molecular pathophysiology. As in Fanconi anemia, low-intensity transplantation conditioning regimens (fludarabine, low-dose cyclophosphamide, and antithymocyte globulin) have been advocated in order to reduce transplantation-related complications in dyskeratosis congenita.101
Modulation of telomerase activity may have a role in the treatment of telomere deficiency syndromes such as dyskeratosis congenita and telomerase-mutant acquired aplastic anemia. Clinical observations suggest that androgen therapy can induce improvements in peripheral blood counts, achieving transfusion independence in as many as 60% of patients.13 Androgens stimulate telomerase gene expression in hematopoietic cells, including CD34+ cells and lymphocytes, and this mechanism of action may explain their efficacy in hematologic disease.35 Androgen therapy may cause severe adverse events, such as hepatocarcinoma and peliosis hepatis, and liver function must be monitored. Immunosuppressive therapy seems to be relatively or entirely ineffective for dyskeratosis congenita.47,62,65,102
For patients with acquired aplastic anemia, hematopoietic stem cell transplantation and intensive immunosuppression with antithymocyte globulin and cyclosporine are the main therapies.10 Patients with acquired aplastic anemia and short telomeres appears to have poorer responses to immunosuppression.2,3,62,65 However, this relationship was retrospectively observed, and prospective studies of the prognostic value of telomere length are required before such measurements influence therapeutic decisions. Some patients with aplastic anemia with telomerase complex mutations respond hematologically to androgen treatment,47 as do patients with dyskeratosis congenita.
Conclusions
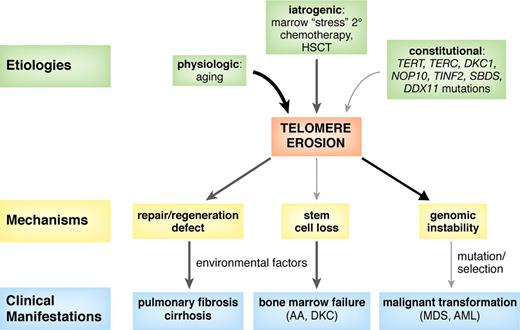
Although heterogeneous in its causes, telomere shortening is one common pathway underlying bone marrow failure in constitutional and acquired aplastic anemias (Figure 4). A family history of blood disease or hematologic manifestations has been a diagnostic criterion for constitutional aplastic anemias, but identification of telomerase complex mutations is broadening these definitions, making family histories of pulmonary fibrosis, liver cirrhosis, or premature hair graying indicators of an inherited marrow failure syndrome. Genetic mutations contribute to telomere shortening, reducing hematopoietic stem cell numbers as well as its proliferation ability, and causing a hypoplastic bone marrow. Environmental factors may disrupt the equilibrium in compensated hematopoiesis, inducing marrow failure.
Proposed model for the role of dysfunctional and short telomeres in the pathogenesis of human disease. Aging, bone marrow stress, and genetic factors, such as mutations in telomerase complex genes (TERT, TERC, DKC1, and NOP10), shelterin components (TINF2, TERF1, and TERF2), or in other genes (SBDS and DDX11) produce progressive telomere erosion. Excessive telomere shortening results in defective cell proliferation, senescence, apoptosis, and genomic instability. Environmental factors, such as viruses, drugs, smoking, or asbestos exposure, may contribute to telomere shortening as well as injury to an organ with limited regeneration capacity, thus triggering disease development (aplastic anemia, pulmonary fibrosis, and hepatic cirrhosis). Short telomeres also promote genomic instability, breakage-fusion-bridge cycles, and aneuploidy, which can lead to myelodysplasia (MDS) or leukemia (AML).
Proposed model for the role of dysfunctional and short telomeres in the pathogenesis of human disease. Aging, bone marrow stress, and genetic factors, such as mutations in telomerase complex genes (TERT, TERC, DKC1, and NOP10), shelterin components (TINF2, TERF1, and TERF2), or in other genes (SBDS and DDX11) produce progressive telomere erosion. Excessive telomere shortening results in defective cell proliferation, senescence, apoptosis, and genomic instability. Environmental factors, such as viruses, drugs, smoking, or asbestos exposure, may contribute to telomere shortening as well as injury to an organ with limited regeneration capacity, thus triggering disease development (aplastic anemia, pulmonary fibrosis, and hepatic cirrhosis). Short telomeres also promote genomic instability, breakage-fusion-bridge cycles, and aneuploidy, which can lead to myelodysplasia (MDS) or leukemia (AML).
Acknowledgments
The authors are grateful to Drs Kathy Collins of the University of California at Berkeley and Peter Lansdorp at the University of British Columbia in Vancouver for their careful reading of the manuscript and helpful comments.
This work was supported by the National Institutes of Health Intramural Research Program.
National Institutes of Health
Authorship
Contribution: R.T.C. and N.S.Y. equally contributed to this review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rodrigo T. Calado, 10 Center Dr, Bldg 10/CRC, Rm 3E-5140, Bethesda, MD 20892-1202; e-mail: calador@nhlbi.nih.gov.
References
Author notes
*R.T.C. and N.S.Y. contributed equally to this review.