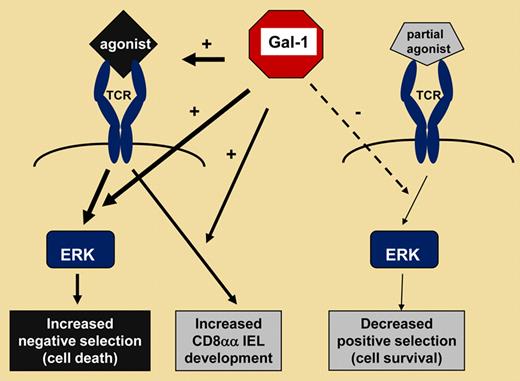
In this issue of Blood, Liu and colleagues demonstrate a novel mechanism for regulation of thymocyte-fate decisions by which a sugar-binding protein, galectin-1, enforces death while opposing survival of developing thymocytes.
The immune system's central tolerance is shaped in part in the thymus during T-cell development. Negative selection removes overtly autoreactive thymocytes, while positive selection promotes survival and development of self-tolerant T cells. Ligand binding to the T-cell receptor (TCR) controls both cell-fate decisions.1 High-affinity agonists are thought to induce thymocyte death during negative selection, or to promote the generation of regulatory T cells such as the CD8αα intestinal intraepithelial lymphocytes (IELs).1,2 On the other hand, partial agonists with low or moderate affinities trigger positive selection and thymocyte differentiation into T-helper or cytotoxic lineages.1 Liu and colleagues add a new flavor to this mechanism by demonstrating that a particular lectin, galectin-1 (gal-1), contributes to discriminating TCR-controlled fate decisions by promoting the association of the TCR and its ligand. This regulatory mechanism enforces agonist-driven signals leading to negative selection, while opposing partial agonist-induced thymocyte survival.
gal-1 is a modulator of thymocyte-fate decisions with opposing activities. By enforcing agonist ligand binding to the TCR, gal-1 promotes fast and transient activation of ERK and negative selection of autoreactive thymocytes, while permitting the generation of regulatory CD8αα IELs. In contrast, gal-1 expression antagonizes ERK signaling in thymocytes undergoing partial agonist-driven positive selection.
gal-1 is a modulator of thymocyte-fate decisions with opposing activities. By enforcing agonist ligand binding to the TCR, gal-1 promotes fast and transient activation of ERK and negative selection of autoreactive thymocytes, while permitting the generation of regulatory CD8αα IELs. In contrast, gal-1 expression antagonizes ERK signaling in thymocytes undergoing partial agonist-driven positive selection.
gal-1 is a member of a family of β-galactose binding proteins that, by cross-linking cell-surface receptors, influence fate decisions in a variety of cells and tissues.3,4 Relevant to T-cell ontogeny, thymic cortical epithelial cells that present antigens to thymocytes also express gal-1.3 This suggests a role for gal-1 in thymocyte-fate decisions. Liu and coauthors have investigated this question by using 2 distinct MHC class I–restricted, TCR-transgenic mouse models bearing a targeted deletion of gal-1. Ablating gal-1 markedly increased positive selection in these mouse models, while negative selection was decreased. Augmented positive selection correlated with a higher number of functional CD8 T cells in the spleens of gal-1−/− TCR-transgenic animals. Interestingly, Liu and colleagues found a higher degree of ERK activation in the post-selection thymocytes of gal-1−/− mice. This result might explain the augmented positive selection observed in the absence of gal-1. Indeed, the intracellular kinase ERK is required for transducing TCR-triggered thymocyte-fate decision signals, and its distinct kinetics of activation represent a specific signature for positive and negative selection.1 From their findings, the authors conclude that endogenous gal-1 opposes partial agonist-driven positive selection of conventional CD8 T cells by lowering the degree of ERK activation. It will be interesting to document, in the future, whether gal-1 modulates the kinetics of TCR partial agonist-driven ERK activation, as well as other TCR-linked signaling molecules such as the ERK-related kinase, JNK.
Nonetheless, Liu and colleagues demonstrate that by enhancing the association of agonist ligands to the TCR, gal-1 increases the magnitude of the fast and transient ERK activation signature that is characteristic for negative selection. Thus, by enforcing agonist-driven signal transduction, gal-1 promotes the removal of potentially autoreactive thymocytes from the repertoire. Interestingly, the authors found that gal-1 also promotes the selection of CD8αα IELs. These regulatory T cells preferentially migrate to the gut after positive selection on TCR agonists instead of partial agonists.2 Surprisingly, the authors observed a significant increase of CD8αα IELs in the absence of gal-1 and in the context of TCR partial agonists. This might reflect a means by which gal-1 can influence the control of selection of T cells bearing autoreactive potential. However, additional work needs to be done to determine whether CD8αα IELs can be selected by partial agonists, or whether these ligands simply promote better peripheral homing of CD8αα IELs in the absence of gal-1. Nonetheless, Liu and colleagues highlight a novel mechanism for gal-1 contribution to central tolerance via shaping the reservoir of CD8 T cells. This might lead to novel therapeutic strategies in which the addition, or specific blocking, of gal-1 could be used as a unique tool to fine-tune the development of T cells with maximal utility. In this regard, it is noteworthy that the administration of gal-1 successfully removed autoreactive T cells from a model of experimental multiple sclerosis.5
Conflict-of-interest disclosure: The author declares no competing financial interests. ■