Abstract
Interleukin-6 (IL-6) plays pathologic roles in immune-inflammatory diseases such as rheumatoid arthritis (RA) and Castleman disease. By inhibiting IL-6 receptors (IL-6Rs), tocilizumab (a humanized anti–IL-6R antibody) ameliorates the symptoms of these diseases and normalizes acute-phase proteins, including C-reactive protein (CRP). We found that tocilizumab treatment increased serum levels of IL-6 and soluble IL-6R (sIL-6R). To investigate the pathologic significance of these increases, we analyzed the kinetics of serum IL-6 and sIL-6R and the proportion of sIL-6R saturated with tocilizumab after tocilizumab administration in patients with RA and Castleman disease and then compared the results with the CRP values. Serum IL-6 and sIL-6R markedly increased after tocilizumab administration in both RA and Castleman disease. As long as free tocilizumab was detectable, sIL-6R was saturated with tocilizumab and IL-6 signaling was completely inhibited. We concluded that it is likely that sIL-6R increased because its elimination half-life was prolonged by the formation of tocilizumab/sIL-6R immune complex, and that free serum IL-6 increased because IL-6R–mediated consumption of IL-6 was inhibited by the unavailability of tocilizumab-free IL-6R. We also concluded that the increased level of free IL-6 during tocilizumab treatment closely reflects the actual endogenous IL-6 production and true disease activity.
Introduction
Interleukin-6 (IL-6) is a multifunction cytokine that has a wide range of biological activities in various target cells and regulates immune responses, acute phase reactions, hematopoiesis, and bone metabolism.1 IL-6 signaling is mediated by a unique IL-6 receptor system consisting of 2 functional membrane proteins: an 80-kDa ligand-binding chain (known as IL-6 receptor [IL-6R], IL-6R α-chain, or CD126)2 and a 130-kDa non–ligand-binding signal-transducing chain (known as glycoprotein 130 [gp130], IL-6R β-chain, or CD130).3 In cells with sufficient membrane-bound IL-6R, IL-6 binds to these receptors, the IL-6/IL-6R complex induces homodimerization of the gp130 molecule, and a high-affinity functional receptor complex of IL-6, IL-6R, and gp130 is formed.4 In cells that do not express sufficient cell-surface IL-6R, IL-6 signal transduction starts with the binding of IL-6 to the free soluble form of IL-6R (sIL-6R), which lacks the membrane and intracytoplasmic portion of the 80-kDa membrane-bound IL-6R molecule.3,4 Thus, either membrane-bound or soluble IL-6R can mediate IL-6 signal into cells, as long as they express gp130. Considerable amounts of sIL-6R are observed in serum and body fluids,5,6 and sIL-6R may play physiologic roles as well as its pathologic role in immune-inflammatory and malignant diseases.7
Because IL-6 plays important physiologic roles, deregulated overproduction of IL-6 causes various pathologic conditions, including autoimmune, inflammatory, and lymphoproliferative disorders. It has been shown that IL-6 is involved in immune-inflammatory diseases such as rheumatoid arthritis (RA), Castleman disease, juvenile idiopathic arthritis (JIA), and Crohn disease.8-11 Elevated serum IL-6 has been observed in patients with these diseases and the IL-6 levels correlate with disease activity,5,10,12-14 although there are differences in IL-6 levels among the diseases.
Tocilizumab is a humanized anti-human IL-6R antibody engineered by grafting the complementarily determining regions of a mouse anti–human IL-6R antibody into human IgG1κ to create a human antibody with a human IL-6R binding site.15 Tocilizumab binds to the IL-6 binding site of human IL-6R and competitively inhibits IL-6 signaling. A series of clinical studies have shown that inhibition of IL-6 signaling by tocilizumab is therapeutically effective in RA, JIA, Castleman disease, and Crohn disease.16-20 In all of these diseases, tocilizumab ameliorates inflammatory manifestations and normalizes acute phase protein levels, including C-reactive protein (CRP), thus confirming the observation that IL-6 is essential for the production of CRP.
It was noticed that both serum IL-6 and serum sIL-6R increased in patients after administration of tocilizumab while the disease symptoms continued to be ameliorated, but the mechanisms of these increases and the pathologic significances of the increased levels remained obscure.
The objective of this study, therefore, was to elucidate the mechanisms and the pathologic significances of the increases in serum IL-6 and serum sIL-6R that occur when IL-6 signaling is inhibited by tocilizumab. To achieve this, we analyzed serum and blood samples collected in the previous clinical studies, and considered the results in combination with laboratory data already obtained in those studies.
Methods
Participants and tocilizumab treatment methods
The serum and blood samples and laboratory data analyzed in this study were from the following people: (1) 20 healthy adult volunteers who received tocilizumab in a phase 1 study (N.N. and T.K., unpublished data, November 1998); (2) 15 patients with RA who were treated with tocilizumab in a phase 1/2 study in RA;16 (3) 18 patients with RA who were treated with tocilizumab in a clinical pharmacology study in RA (K.T., T. Tsuru, M. Suzaki, T. Amamoto, H. Nakashima, S. Higuchi, T.K., and N.N., unpublished data, August 2008); and (4) 28 patients with Castleman disease who were treated with tocilizumab in a phase 2 study in Castleman disease.19 All of these clinical studies were approved by the Japanese Ministry of Health, Labor and Welfare and also by the independent ethics committees of the respective medical institutions. Written informed consent was obtained from the patients before enrollment in accordance with the Declaration of Helsinki.
The designs of these studies were as follows: (1) phase 1 study—a randomized, single-blind, placebo-controlled, intersubject dose-escalation study in which healthy adult men received 0.15, 0.5, 1, or 2 mg/kg tocilizumab intravenously over 2 hours (n = 5/cohort), and safety, tolerability and pharmacokinetics were evaluated; (2) phase 1/2 study in RA16 —an open-label trial in which 15 patients with active RA received 2, 4, or 8 mg/kg tocilizumab intravenously over 2 hours every 2 weeks for 6 weeks (n = 5/cohort), and safety, tolerability, pharmacokinetics, and efficacy at each dosage level were assessed on days 0, 14, 28, and 42. CRP and erythrocyte sedimentation rate (ESR) were measured as markers of IL-6R inhibition; (3) clinical pharmacology study in RA—an open-label single-dose study in which 18 patients with baseline CRP of at least 1.5 mg/dL received 8 mg/kg tocilizumab intravenously over 1 hour, and drug-disease interaction was assessed; (4) phase 2 study in Castleman disease19 —an open-label study in which 28 patients with active Castleman disease received 8 mg/kg tocilizumab intravenously over 1 hour every 2 weeks for 16 weeks (8 times in total), and improvement in disease activity was assessed based on biochemical markers such as CRP, hemoglobin, and serum albumin, and on general fatigue (visual analog scale).
Tocilizumab is a humanized anti–human IL-6R monoclonal antibody of the IgG1κ subtype, and was supplied by Chugai Pharmaceutical (Tokyo, Japan).
Determination of free tocilizumab in serum
Serum-free tocilizumab was determined by enzyme-linked immunosorbent assay (ELISA). In brief, 100 μL human sIL-6R (SR-344, 1 μg/mL; Chugai Pharmaceutical) was added to the wells of an immunoplate precoated with mouse anti–human IL-6R antibody (MT-18; Chugai Pharmaceutical), and the plate was incubated at room temperature for 2 hours. After washing, 100 μL of 1000-fold diluted serum specimen was applied to the plate, and the plate was incubated for another 2 hours to bind the free tocilizumab to the plate. After washing, the bound tocilizumab was measured using biotin-labeled goat anti–human IgG antibody, followed by development with avidin-labeled alkaline phosphatase and p-nitrophenyl phosphate substrate. The colorimetric reaction was quantified by measuring the absorbance at 405 nm (and at 490 nm as reference) using a microplate reader. The concentration of free tocilizumab in the specimen was calculated from a calibration curve prepared from the absorbance of calibration standard solutions. The lower detection limit for free serum tocilizumab was 1 μg/mL. All serum samples were stored below −20°C, and the assay was applied within 4 weeks after drawing.
Determination of IL-6 in serum
Serum IL-6 was determined by chemiluminescent enzyme immunoassay (CLEIA)21 using a Human IL-6 CLEIA Fujirebio (Fujirebio, Tokyo, Japan), which detects IL-6 whether it is free or bound to sIL-6R. In brief, a mixture of 20 μL of serum sample and the alkaline phosphatase–conjugated mouse anti–human IL-6 monoclonal antibody included in the kit was incubated at 37°C for 10 minutes and then added to particles covalently linked to another murine anti–human IL-6 monoclonal antibody that recognizes an epitope different from that recognized by the original antibody. After incubation for 10 minutes at 37°C, the particles were separated magnetically and washed in buffer. Subsequently, an enhanced luminol/peroxide substrate solution containing 3-(2′-spiroadamantane)-4-methoxy-4-(3′-phosphoryloxy)phenyl-1,2-dioxetane disodium salt was added at 37°C, and after 5 minutes the chemiluminescence was measured using a photon counter (Lumipulse 1200′ Fujirebio). The lower detection limit for serum IL-6 was 0.1 pg/mL. All serum samples were stored below −20°C, and the assay was applied within 4 weeks after drawing.
Determination of sIL-6R in serum
Serum sIL-6R was determined by ELISA using a Quantikine Human IL-6sR kit (R&D Systems, Minneapolis, MN). We preliminarily examined and found that this assay kit could detect 3 forms of sIL-6R: sIL-6R free from IL-6 or tocilizumab, sIL-6R in a complex of IL-6/sIL-6R, and sIL-6R in an immune-complex of tocilizumab/sIL-6R (data not shown). In brief, sIL-6R in the sample was bound to a murine anti–IL-6R monoclonal antibody–coated microtiter plate and incubated with a horseradish peroxidase (HRP)–conjugated polyclonal anti–IL-6R antibody at room temperature for 2 hours. To determine the quantity of peroxidase bound, the plate was incubated with tetramethylbenzidine at room temperature for 20 minutes. The colorimetric reaction was quantified by measuring the absorbance at 450 nm (and at 540 nm as reference) using a microplate reader and the concentration of sIL-6R in the sample was calculated as described in “Determination of free tocilizumab in serum.” All serum samples were stored below −20°C, and applied the assay within 4 weeks after drawing.
Determination of the proportions of tocilizumab-bound and tocilizumab-free sIL-6R in serum
Tocilizumab-bound sIL-6R and tocilizumab-unbound sIL-6R in serum samples were separated by gel filtration chromatography in a Superdex 200HR 10/30 column (300 × 10 mm internal diameter [ID]; GE Healthcare Bio-Science AB, Uppsala, Sweden) at 4°C, and the amount of sIL-6R in each fraction was determined. The eluent was phosphate-buffered saline with 0.05% Tween 20, and the fraction volume was 0.5 mL.
Determination of IL-6 mRNA expression
In the clinical pharmacology study in RA (study 3), peripheral blood for measurement of IL-6 mRNA was drawn from 18 patients at 4 time points: 7 days and immediately before, and 14 and 35 days after administration of 8 mg/kg tocilizumab.
Total RNA was extracted from the blood samples using a PAXgene Blood RNA Kit (QIAGEN, Valencia, CA) and quantified spectrophotometrically by measuring absorbance at 260 nm. The purity and integrity of the RNA extracted from each PAXgene Blood RNA tube were estimated by the ratio of 28S to 18S ribosomal RNA using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA samples that showed severe degradation were excluded from further analysis. Expression of IL-6 mRNA was measured using a DNA microarray (Human oligo chip; Hitachi Software Engineering, Tokyo, Japan). After amino allylRNA (aRNA) was synthesized from 1 μg total RNA using the Amino Allyl Message Amo aRNA kit (Ambion, Austin, TX), the Cy3 and Cy5 dyes were then used to label RNA sequences. Human peripheral blood leukocyte total RNA purchased from BD Biosciences Clontech (San Jose, CA) was used as a universal control (reference RNA) and labeled with Cy3 in all the microarray experiments so that the ratio (Cy5/Cy3) means the relative expression level to the reference in the time course experiment series. The ratio (relative expression level) was plotted against the sampling time. All RNA samples were stored below −80°C until applied the assay. The DNA microarray dataset is available at the Gene Expression Omnibus (GEO) repository (http://www.ncbi.nlm.nih.gov/geo) under the accession number GSE12653.
Determination of CRP
CRP was determined in all clinical studies in which the serum was collected. CRP levels were measured at each clinical study site.
Results
Elevation of serum sIL-6R after administration of tocilizumab
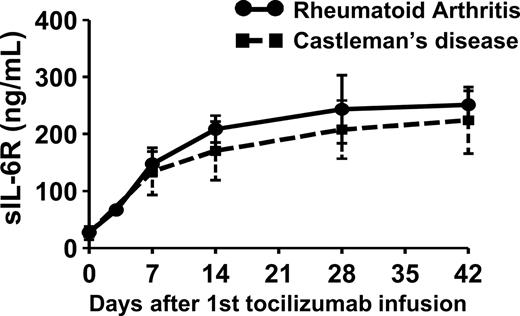
When patients with RA or Castleman disease were treated with 8 mg/kg tocilizumab once every 2 weeks, serum sIL-6R increased markedly in patients with a detectable serum tocilizumab concentration (1 μg/mL or more) and reached a steady state at day 42 of treatment (RA: 27.7 ± 4.4 ng/mL at baseline, 251.4 ± 24.7 ng/mL at day 42; Castleman disease: 26.4 ± 11.6 ng/mL at baseline, 224.2 ± 58.3 ng/mL at day 42; Figure 1). There was no significant difference between patients with RA and patients with Castleman disease with respect to either serum sIL-6R at baseline or serum sIL-6R at day 42. In the healthy individuals, increase in serum sIL-6R was transient because of single administration of tocilizumab (Figure 2A).
Serum sIL-6R after tocilizumab infusion. Tocilizumab was administered to patients with RA (●; n = 5) or Castleman disease (■; n = 28) on days 0, 14, and 28 at a dose of 8 mg/kg. Points and error bars show geometric means plus or minus SDs.
Serum sIL-6R after tocilizumab infusion. Tocilizumab was administered to patients with RA (●; n = 5) or Castleman disease (■; n = 28) on days 0, 14, and 28 at a dose of 8 mg/kg. Points and error bars show geometric means plus or minus SDs.
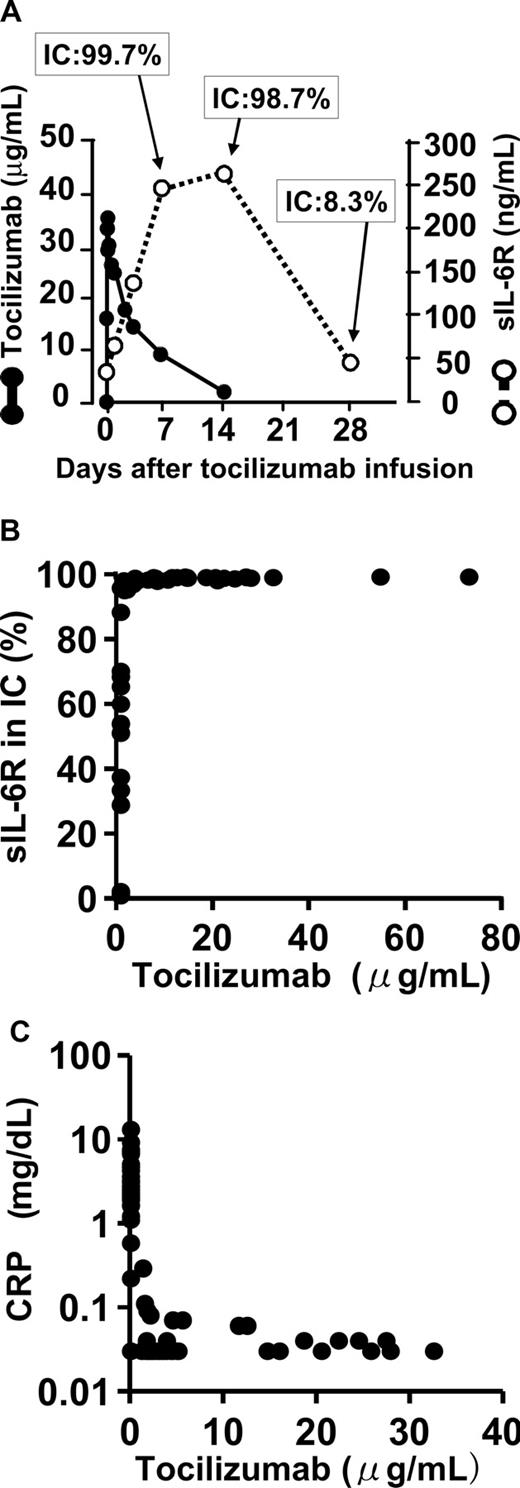
Relationships between free tocilizumab, sIL-6R, percentage of sIL-6R bound to tocilizumab, and CRP in serum. (A) Relationship between serum free tocilizumab (●), serum sIL-6R (○), and the percentage of sIL-6R bound to tocilizumab in an immune complex (IC). Serum sIL-6R (○) includes all sIL-6R: free, bound to tocilizumab, and bound to IL-6. Increased sIL-6R after tocilizumab infusion formed an immune complex with tocilizumab. Almost all sIL-6R was bound to tocilizumab while serum-free tocilizumab was detectable (1 μg/mL or more). This figure shows a representative data from the phase 1 study in healthy individuals. (B) Relationship between serum tocilizumab and percentage of sIL-6R bound to tocilizumab (phase 1/2 study in RA). More than 95% of sIL-6R was bound to tocilizumab while serum-free tocilizumab remained 1 μg/mL or more. (C) Relationship between serum tocilizumab and CRP. Serum CRP was normalized as long as the free tocilizumab concentration remained 1 μg/mL or more (phase 1/2 study in RA). The sensitivity of CRP assay in the present study was 0.03 mg/dL, and the normal range was less than 0.2 mg/dL.
Relationships between free tocilizumab, sIL-6R, percentage of sIL-6R bound to tocilizumab, and CRP in serum. (A) Relationship between serum free tocilizumab (●), serum sIL-6R (○), and the percentage of sIL-6R bound to tocilizumab in an immune complex (IC). Serum sIL-6R (○) includes all sIL-6R: free, bound to tocilizumab, and bound to IL-6. Increased sIL-6R after tocilizumab infusion formed an immune complex with tocilizumab. Almost all sIL-6R was bound to tocilizumab while serum-free tocilizumab was detectable (1 μg/mL or more). This figure shows a representative data from the phase 1 study in healthy individuals. (B) Relationship between serum tocilizumab and percentage of sIL-6R bound to tocilizumab (phase 1/2 study in RA). More than 95% of sIL-6R was bound to tocilizumab while serum-free tocilizumab remained 1 μg/mL or more. (C) Relationship between serum tocilizumab and CRP. Serum CRP was normalized as long as the free tocilizumab concentration remained 1 μg/mL or more (phase 1/2 study in RA). The sensitivity of CRP assay in the present study was 0.03 mg/dL, and the normal range was less than 0.2 mg/dL.
Relationship between free tocilizumab and the proportions of tocilizumab-free and tocilizumab-bound sIL-6R in serum
Total serum sIL-6R peaked 14 days after a single administration of 2 mg/kg tocilizumab to healthy individuals in the phase 1 study. Serum sIL-6R was tocilizumab-free before administration of tocilizumab, but after administration of tocilizumab, more than 95% of the sIL-6R molecules were bound in a sIL-6R/tocilizumab immune complex as long as the free tocilizumab concentration remained detectable in serum (at least 1 μg/mL). Representative data are shown in the Figure 2A. Similar data were obtained in the sIL-6R, which increased after repetitive administration of tocilizumab to patients with RA in the phase 1/2 study (Figure 2B). When free tocilizumab concentration fell below the detection limit in serum, the level of sIL-6R/tocilizumab complex decreased, and the proportion of free sIL-6R increased (Figure 2A,B).
Relationship between free tocilizumab and CRP level in serum
CRP is a representative acute-phase reactant, and change in CRP correlates with severity of inflammation. It has been shown that inflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor induce the production of CRP by hepatocytes, and that IL-6 is essential for the production of CRP.22
Tocilizumab normalized the CRP level in patients with RA in the phase 1/2 study16 as long as the free tocilizumab, which is capable of binding IL-6R and of inhibiting IL-6 actions, remained above 1 μg/mL in serum (Figure 2C). This shows that tocilizumab effectively inhibits IL-6 signaling when it is detectable in serum.
Serum IL-6 after administration of tocilizumab
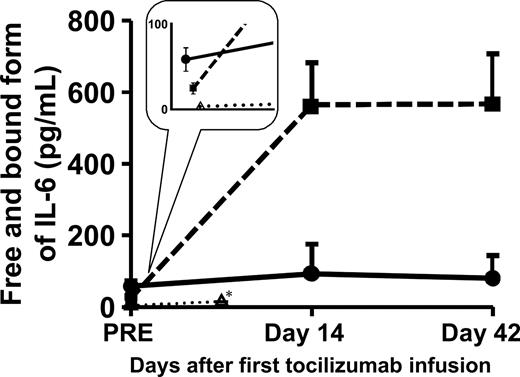
Serum IL-6 concentrations after a single dose of tocilizumab in healthy adult volunteers after the start of dosing (once every 2 weeks) in patients with RA, and in patients with Castleman disease were compared. At baseline, serum IL-6 was significantly higher in RA and Castleman disease than the normal range (< 4 pg/mL). Note that serum IL-6 was significantly higher in RA than in Castleman disease (58.4 ± 13.8 pg/mL vs 24.5 ± 6.5 pg/mL [geometric mean ± SE]; P < .05), yet serum CRP was significantly higher in Castleman disease than in RA (8.7 ± 5.0 mg/dL vs 5.4 ± 1.7 mg/dL; P < .05) and serum IgG was also higher in Castleman disease than in RA (5220 ± 1957 mg/dL vs 1516 ± 409 mg/dL; P < .05).
In healthy volunteers, serum IL-6 showed a significant increase at day 7 after tocilizumab administration (3.0 ± 0.6 pg/mL at baseline, 9.3 ± 1.0 pg/mL at day 7). In patients with RA, serum IL-6 showed a greater increase at day 14 and had not changed at day 42 (58.4 ± 13.8 pg/mL at baseline, 92.8 ± 82.4 pg/mL at day 14, 89.7 ± 63.7 pg/mL at day 42; n = 5). In patients with Castleman disease, serum IL-6 showed an even more significant increase at day 14, even though it was significantly lower in Castleman disease than in RA at baseline, and had not changed at day 42 (24.5 ± 6.5 pg/mL at baseline, 564.8 ± 127.6 pg/mL at day 14, 567.0 ± 141.0 pg/mL at day 42; n = 28; RA vs Castleman disease: P < .001 at day 14 and at day 42; Figure 3). These data clearly indicate that the degree of increase in serum IL-6 after IL-6R inhibition is not the same in RA and Castleman disease.
Change in serum IL-6 after administration of tocilizumab. Serum IL-6 increased after tocilizumab infusion and reached steady state at different levels in patients with RA (●; n = 5), patients with Castleman disease (■; n = 28), and healthy volunteers (▵; n = 4). Points and error bars show geometric means plus or minus SEs. *Samples from healthy volunteers were drawn on day 7.
Change in serum IL-6 after administration of tocilizumab. Serum IL-6 increased after tocilizumab infusion and reached steady state at different levels in patients with RA (●; n = 5), patients with Castleman disease (■; n = 28), and healthy volunteers (▵; n = 4). Points and error bars show geometric means plus or minus SEs. *Samples from healthy volunteers were drawn on day 7.
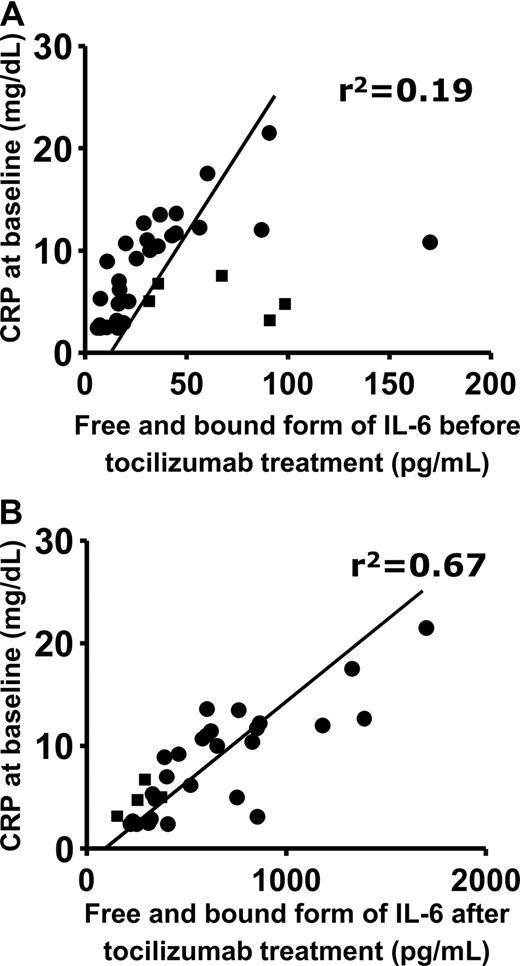
Furthermore, there observed much stronger correlation between the CRP at baseline and the serum IL-6 increasing after IL-6R blockade than that between the CRP at baseline and the serum IL-6 before IL-6R blockade in patients with RA and in Castleman disease (Figure 4).
Correlation between serum CRP at baseline and serum IL-6 at baseline or after tocilizumab administration. ■ and ● show patients with RA and patients with Castleman disease, respectively. The x-axis shows (A) serum IL-6 at baseline (r2 = .19) and (B) serum IL-6 after tocilizumab infusion (r2 = .67).
Correlation between serum CRP at baseline and serum IL-6 at baseline or after tocilizumab administration. ■ and ● show patients with RA and patients with Castleman disease, respectively. The x-axis shows (A) serum IL-6 at baseline (r2 = .19) and (B) serum IL-6 after tocilizumab infusion (r2 = .67).
IL-6 mRNA expression in peripheral blood cells before and after administration of tocilizumab
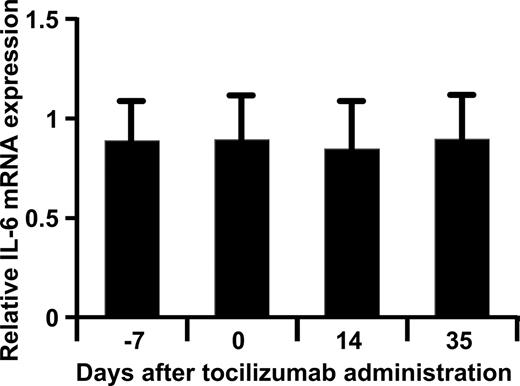
In order to know whether the IL-6R inhibition augmented the IL-6 production through the elimination of possible negative feedback by IL-6 on IL-6 production, changes in IL-6 mRNA expression after tocilizumab administration in peripheral blood cells were examined. However, there was no significant difference in IL-6 mRNA expression at these 4 sampling time points, so administration of tocilizumab did not increase the production of IL-6 (Figure 5).
Relative expression of IL-6 mRNA. The relative expression levels of IL-6 mRNA compared with the reference calculated by the logarithm base 2 of ratio (Cy5/Cy3) at each time point were plotted. Expression of IL-6 mRNA in peripheral blood cells from 18 patients with RA was measured using a DNA microarray before and after administration of tocilizumab. Bars and error bars show means plus SEs.
Relative expression of IL-6 mRNA. The relative expression levels of IL-6 mRNA compared with the reference calculated by the logarithm base 2 of ratio (Cy5/Cy3) at each time point were plotted. Expression of IL-6 mRNA in peripheral blood cells from 18 patients with RA was measured using a DNA microarray before and after administration of tocilizumab. Bars and error bars show means plus SEs.
Discussion
This study demonstrates that IL-6R inhibition with tocilizumab results in an increase in the levels of serum IL-6 and serum sIL-6R. The normalization of serum CRP, however, shows that IL-6 signaling was inhibited as long as free tocilizumab was detectable in serum. This matches the finding that a very high percentage of serum sIL-6R (> 95%) was bound to tocilizumab while free tocilizumab was detectable in serum. Since CRP is mainly produced by hepatocytes, which express cell-surface IL-6R, membrane-bound IL-6R would be also fully occupied by tocilizumab. CRP is therefore a useful surrogate marker for tocilizumab levels that are high enough to inhibit the effects of IL-6 in patients.
Since immune complex formation of antigen and antibody prolongs the elimination half-life of antigen in serum,23 the increase in serum sIL-6R seen after administration of tocilizumab is probably due to the formation of a sIL-6R/tocilizumab immune complex. This hypothesis is supported by the decrease in the C3, C4, and CH50 levels after tocilizumab administration that we observed in another study (data not shown) because complement factors are consumed during the elimination process of immune complexes.23
It is noteworthy that there was no difference between RA and Castleman disease with respect to serum sIL-6R either at baseline or after tocilizumab administration. This suggests that there is no difference in sIL-6R production between these 2 diseases.
For serum IL-6, on the other hand, the level after tocilizumab administration differed greatly between the diseases as well as between individual patients (Figures 3,4). This suggested to us that the degree of increase in serum IL-6 after inhibition of IL-6R by tocilizumab may reflect different levels of endogenous IL-6 production in these diseases and in individual patients.
The serum IL-6 level depends on the balance between IL-6 production and elimination, so tocilizumab could potentially have caused serum IL-6 to increase either by stimulating production or by inhibiting elimination. One possibility is that tocilizumab might stimulate the production of IL-6 if its blockade of IL-6 signaling inhibits a negative feedback effect of IL-6 on IL-6 production. This seems unlikely, however, because serum IL-6 did not continue to increase but remained steady between day 14 and day 42, and because there was no significant increase in IL-6 mRNA expression in peripheral blood cells after administration of tocilizumab. The relevance of the latter observation may be limited, however, by the fact that we did not examine IL-6 mRNA expression in cells of the affected joints of patients with RA or the affected lymph nodes of patients with Castleman disease, which are important sources of IL-6 in these diseases.
Another possible explanation for the increase in serum IL-6 after tocilizumab administration is that tocilizumab may inhibit the clearance of IL-6 from serum. There are 2 possible elimination pathways of IL-6 from serum: one is receptor-mediated clearance via the binding of IL-6 to IL-6R; the other is direct degradation of IL-6 protein. The main elimination pathway may be receptor-mediated clearance. If so, this would explain why free IL-6 accumulates in serum when IL-6R is occupied by tocilizumab. IL-6 levels reach a steady state when the IL-6 production rate matches the IL-6 degradation rate.
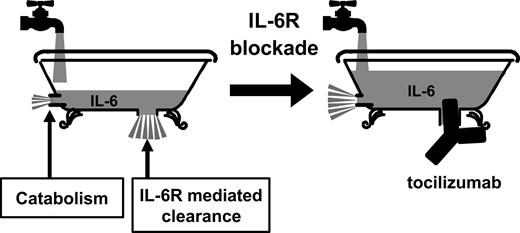
We would like to explain this mechanism of action with the help of the schematic bathtub model illustrated in Figure 6. In this model, endogenous IL-6 production is represented by the stream of water flowing from the faucet into the tub at a constant rate that depends on the level of true disease activity. Receptor-mediated IL-6 clearance is represented by water flowing out of the bathtub drain. Direct degradation of IL-6 is represented by minor water flowing out from the side small drain in this figure. When the bathtub drain is plugged, the water level will depend on the flow rate from the faucet. Likewise, when IL-6R is inhibited by tocilizumab, the serum IL-6 level will reflect the actual level of endogenous IL-6 production that correlates with the level of true disease activity while inflammatory symptoms are ameliorated by the inhibition of IL-6 signaling through IL-6R.
Schematic model of the mechanism by which serum IL-6 is increased when IL-6 receptor is blocked by tocilizumab. The bathtub model explains the elimination of IL-6 from serum before and after administration of tocilizumab. The rate of water flowing from the faucet into the tub (the IL-6 production rate) remains constant. Before tocilizumab administration, the rate of water flowing out of the bathtub (the elimination of receptor-bound IL-6 from serum and IL-6 catabolism) is also constant, so the water level (serum IL-6 level) remains constant. In the second diagram, the flow of IL-6 from the bathtub is greatly restricted by a “plug” (IL-6R–mediated elimination is inhibited by tocilizumab). The water level increases and then remains constant at a higher level (serum IL-6 increases to a new steady-state level when the IL-6 production rate matches the IL-6 degradation rate).
Schematic model of the mechanism by which serum IL-6 is increased when IL-6 receptor is blocked by tocilizumab. The bathtub model explains the elimination of IL-6 from serum before and after administration of tocilizumab. The rate of water flowing from the faucet into the tub (the IL-6 production rate) remains constant. Before tocilizumab administration, the rate of water flowing out of the bathtub (the elimination of receptor-bound IL-6 from serum and IL-6 catabolism) is also constant, so the water level (serum IL-6 level) remains constant. In the second diagram, the flow of IL-6 from the bathtub is greatly restricted by a “plug” (IL-6R–mediated elimination is inhibited by tocilizumab). The water level increases and then remains constant at a higher level (serum IL-6 increases to a new steady-state level when the IL-6 production rate matches the IL-6 degradation rate).
In practice, the correlation between CRP (an indicator of resultant inflammation) at baseline and serum IL-6 level after administration of tocilizumab was much closer than that between CRP at baseline and serum IL-6 level before tocilizumab administration. Furthermore, serum IL-6 was much higher in patients with Castleman disease than in patients with RA after tocilizumab administration, even though serum IL-6 in patients with Castleman disease was lower than that in patients with RA at baseline. The difference in increased IL-6 level between RA and Castleman disease after tocilizumab treatment closely reflected the difference between RA and Castleman disease in baseline inflammatory activity and in laboratory abnormalities such as increased CRP and IgG values, whereas the IL-6 levels before tocilizumab treatment did not. The fact that IL-6 was lower in Castleman disease than in RA at baseline indicates that the elimination of serum IL-6 is much faster in Castleman disease than in RA without tocilizumab treatment, and the fact that this faster elimination in Castleman disease was greatly slowed by tocilizumab suggests that it is receptor-mediated elimination.
We conclude that the serum IL-6 level during inhibition of IL-6R by tocilizumab represents the actual endogenous production of IL-6 and the true disease activity of patients with different diseases much better than the serum IL-6 level before tocilizumab treatment. If the causal factors of IL-6 overproduction are neutralized by adequate therapy (the faucet of the bathtub is closed), the serum IL-6 level will decrease by natural protein degradation. Decrease in serum IL-6 during tocilizumab treatment may therefore indicate disease remission and may allow us to safely discontinue tocilizumab treatment without the risk of an acute flare. This idea remains to be confirmed in future studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Prof T. Kishimoto for his valuable comments and the doctors of the MRA study groups in Japan for treating and evaluating their patients.
Authorship
Contribution: N.N. wrote the manuscript; N.N. and K.T. planned the study and wrote the study protocol; N.N., T.M., and H.N. treated and documented the patients; K.T., N.T., and T.K. organized and monitored the study; and K.T. performed the statistical evaluation.
Conflict-of-interest disclosure: K.T., N.T., and T.K. are employees of Chugai Pharmaceutical, whose product (tocilizumab) was studied in the present work. N.N. and T.M. received a consulting fee as medical advisers from Chugai Pharmaceutical. N.N. is on the scientific advisory board of Hoffmann-La Roche. H.N. declares no competing financial interests.
Correspondence: Norihiro Nishimoto, Laboratory of Immune Regulation, Graduate School of Frontier Biosciences, Osaka University, 1-3 Yamada-oka, Suita-City, Osaka 565-0871, Japan; e-mail: norihiro@fbs.osaka-u.ac.jp.