Abstract
We conducted a trial in 103 patients with newly diagnosed chronic phase chronic myeloid leukemia (CP-CML) using imatinib 600 mg/day, with dose escalation to 800 mg/day for suboptimal response. The estimated cumulative incidences of complete cytogenetic response (CCR) by 12 and 24 months were 88% and 90%, and major molecular responses (MMRs) were 47% and 73%. In patients who maintained a daily average of 600 mg of imatinib for the first 6 months (n = 60), MMR rates by 12 and 24 months were 55% and 77% compared with 32% and 53% in patients averaging less than 600 mg (P = .037 and .016, respectively). Dose escalation was indicated for 17 patients before 12 months for failure to achieve, or maintain, major cytogenetic response at 6 months or CCR at 9 months but was only possible in 8 patients (47%). Dose escalation was indicated for 73 patients after 12 months because their BCR-ABL level remained more than 0.01% (international scale) and was possible in 45 of 73 (62%). Superior responses achieved in patients able to tolerate imatinib at 600 mg suggests that early dose intensity may be critical to optimize response in CP-CML. The trial was registered at www.ANZCTR.org.au as #ACTRN12607000614493.
Introduction
Imatinib mesylate is the treatment of first choice for patients with chronic phase chronic myeloid leukemia (CP-CML).1-11 The International Randomized Study of Interferon versus STI-571 (IRIS) trial demonstrated the superiority of imatinib over the combination of interferon-α and cytosine arabinoside (Ara-C) in the initial treatment of CP-CML.12-14 The projected rate of complete cytogenetic response (CCR) achieved after 12 months of therapy was 69% in the imatinib arm compared with 7% on the interferon-α plus Ara-C arm. Major molecular response (MMR), defined as a reduction of at least 3 logs in the level of BCR-ABL relative to a standardized baseline, was achieved in 40% of patients receiving first line imatinib therapy.14 The imatinib dose used in the IRIS trial was 400 mg/day based on the early phase 2 data demonstrating good tolerance and good hematologic response at this dose in patients with chronic phase disease.4,15 However, the optimal dose in chronic phase with regard to cytogenetic and molecular response and long-term progression-free survival (PFS) has not been established. Evidence in patients with accelerated phase CML9 and late chronic phase,16 suggests that 400 mg may not be optimal in these settings. Dose increase from 400 mg to 800 mg has resulted in improved hematologic and cytogenetic responses in patients with imatinib failure or suboptimal response.17 An ongoing phase 2 trial at the M. D. Anderson Cancer Center (Houston, TX) where patients with newly diagnosed CP-CML received 800 mg/day has demonstrated superior early cytogenetic and molecular results compared with the IRIS trial results.18 With a median follow-up of 15 months, 90% achieved CCR and 28% had undetectable BCR-ABL by polymerase chain reaction (PCR). In an earlier cohort of patients at the M. D. Anderson Cancer Center who received 400 mg/day 74% achieved CCR at a similar time point (P = .01) and only 7% had undetectable BCR-ABL (P = .001). Another approach under assessment is the use of intermediate- or high-dose Ara-C combined with imatinib.19 In this study, a variety of imatinib doses were used, but most patients received 600 or 800 mg/day. CCR and MMR rates at 12 months were 63% and 46%, respectively.
The Australasian Leukaemia and Lymphoma Group (ALLG) has conducted a phase 2 trial (Therapeutic Intensification in DE-novo Leukaemia (TIDEL)) of higher-dose imatinib in patients with newly diagnosed CP-CML using imatinib 600 mg initially, increasing to 800 mg if specified response criteria were not met. All patients had monitoring of marrow for cytogenetics and blood for real-time quantitative PCR (RQ-PCR) of BCR-ABL mRNA levels every 3 months. This intensive monitoring was designed to facilitate rapid action in cases of suboptimal response or acquired resistance.20-34 If the prespecified response criteria were still not met after a further 3 months, patients received combination therapy with intermittent standard-dose Ara-C plus imatinib. The rationale for this approach was the expectation that many patients would achieve excellent responses on 600 mg/day and that the higher dose of 800 mg/day should be reserved for those patients with suboptimal response. The subsequent use of combination therapy was based on in vitro evidence of synergy between imatinib and Ara-C in CML cells.35,36
Methods
Study group
Eligibility criteria included age 16 to 75 years, CP-CML prior treatment with only hydroxyurea or anagrelide. Patients were eligible if they were within 8 months of diagnosis. Patients were required to have adequate performance status (ECOG 0-2), and serum creatinine, bilirubin, serum glutamic oxaloacetic transaminase, and serum glutamic pyruvic transaminase less than 1.5 times the institutional upper limits of normal. Patients with additional cytogenetic abnormalities in the Ph+ clone at diagnosis could be included. Exclusion criteria included uncontrolled medical disease, positive HIV serology, and women who were pregnant, breast feeding, or of child-bearing potential without a negative pregnancy test before study start. All patients had confirmed expression of the BCR-ABL transcript in their blood (either B2A2 or B3A2 transcript or both) before commencing imatinib. Patients provided written informed consent in accordance with the Declaration of Helsinki. The trial was reviewed and ethically approved at all participating centers. The trial was run by the ALLG, and data were collected and processed by the ALLG trial center. A total of 103 patients were enrolled between October 2002 and August 2003. Median age at registration was 50 years (range, 19-76 years). Baseline characteristics are summarized in Table 1. All patients have been followed to 24 months or until they came off study.
Treatment and dose modifications
Imatinib 600 mg/day orally was commenced in all patients. Dose interruptions were indicated for grade 3 or 4 nonhematologic toxicity as well as for platelet counts less than 30 × 109/L until the platelet count recovered to more than 75 × 109/L. Filgrastim (Amgen, Thousand Oaks, CA) was given if the neutrophil count was less than 0.5 × 109/L. The dose was 5 μg/kg per day or 300 μg/day subcutaneously, and the frequency was adjusted to keep the neutrophil count more than 1.0 × 109/L. This more aggressive approach to dose modification for hematologic toxicity was designed to maximize dose intensity and avoid dose interruptions unless there was a significant clinical risk of adverse events.
The criteria for increasing the imatinib dose from 600 to 800 mg/day were failure to achieve: (1) complete hematologic response (CHR) at 3 months, (2) major cytogenetic response (MCR) at 6 months, (3) CCR at 9 months, or (4) less than 0.01% BCR-ABL by RQ-PCR on the international scale (equivalent to more than 4 log reduction in BCR-ABL from the standardized baseline) at 12 months. If the dose could not be increased to 800 mg/day, it was maintained at the maximal tolerated dose. Patients could be escalated to 800 mg/day if their toxicities were no greater than grade 1 on lower doses. For dose-escalation criteria 1 to 3, if the response criterion was not achieved after a further 3 months of 800 mg/day, the patient was eligible for sequential combination therapy using cytarabine 100 mg/m2 per day for 7 days every 42 days up to a maximum of 4 cycles in combination with imatinib 800 mg. Patients who lost response were also eligible for dose escalation to 800 mg if already on 600 mg (or maximum tolerated dose). If they were taking 800 mg/day when loss of response occurred, they were eligible for combination therapy.
Primary end points
The primary end points for evaluation in the study were the proportions of patients who achieved (1) MMR and (2) MCR and CCR, over the 2-year study period in the entire group, and in cohorts who received dose escalation or combination therapy.
Definitions of loss of response
In this study, loss of response was defined as a loss of CHR (including progression to accelerated phase or blast crisis), loss of MCR, loss of CCR, or a 1-log increase in BCR-ABL to a BCR-ABL level more than 0.1% (international scale).
Monitoring
Blood counts and biochemistry were performed weekly for the first month and then every 4 weeks. Bone marrow morphology and cytogenetic studies were performed every 3 months. Patients were assessed for disease status, survival, adverse events, and mean daily dose (MDD) of imatinib achieved in 6-month intervals over the entire 24 months of the trial.
RQ-PCR analysis
RQ-PCR measurement of BCR-ABL and BCR levels was performed before study start (baseline), every month for the first 3 months, and then every 3 months. The ratio of BCR-ABL over BCR was expressed as a percentage. Results were then adjusted according to published recommendations to the international scale, based on a locally derived conversion factor.37 Where cytogenetic results were not available, RQ-PCR measurement of BCR-ABL was used as a surrogate for CCR where BCR-ABL values less than 1% were assessed as achieving CCR. This was based on our previous study demonstrating the close correlation between these values.38
Kinase domain mutation screening
BCR-ABL kinase domain mutation analysis was performed using direct sequencing.39 Patients were tested for mutations on a significant rise in BCR-ABL that was not associated with a dose reduction or cessation or if there was clinical evidence of disease progression. A significant rise according to the measurement reliability of our RQ-PCR assay is greater than 2-fold.33 All patients who did not meet these criteria for mutation screening were tested for mutations at 6 months of imatinib therapy (n = 94), except when there was no sample available (n = 1) or BCR-ABL was undetectable (n = 2).
Statistical considerations
The rates of CCR and MMR by 12 and 24 months were calculated as the proportion of patients who achieved these responses during the 2-year study period. Ninety-five percent confidence intervals (CIs) for these rates were calculated using the Blyth-Still-Casella method. The durations of CCR and MMR, as well as overall survival (OS) and PFS were estimated using the Kaplan-Meier method. PFS was measured from the date of commencement on imatinib to the earliest of the dates of progression to accelerated phase, blastic phase, or death. The probability of achieving CCR or MMR during the study period was also estimated using the cumulative incidence function, the competing event being coming off protocol for any reason, other than completion of the protocol, before achievement of the response.
In exploratory analyses, the associations of several factors (Table 1) with the achievement of MMR at or before 12 months and at or before 24 months were studied using logistic regression analyses. The statistical significance of associations of these factors and, where appropriate, their underlying continuous variables, with the achievement of MMR was assessed with the Wald test in both unifactor and multifactor (forward selection) analyses.
Results
Cytogenetic response
Ninety-three of the 103 patients who commenced imatinib (90%; 95% CI, 83%-95%) achieved CCR at or before 24 months. The cumulative actuarial incidence of CCR on study was 80% at 6 months, 88% at 12 months, and 90% at 18 and 24 months. Kaplan-Meier estimates of the percentage of patients, who after achieving CCR, maintained CCR for 6 and 12 months are 96% and 93%, respectively. In 5 of the 10 patients who lost CCR, there was a period of dose interruption or dose modification that could explain the loss of response. Two of these 5 patients had dose interruptions (30 and 56 days) before loss of CCR, and 3 had dose modifications of imatinib to less than an MMD of 400 mg/day for at least 3 months before loss of CCR. Comparisons of cytogenetic response rates at 12 and 24 months with those of the IRIS study are summarized in Table 2.
MMR
Seventy of 103 patients who commenced imatinib (68%; 95% CI, 59%-76%) achieved an MMR at or before 24 months. The estimated cumulative incidence of MMR was 29% at 6 months, 47% at 12 months, 60% at 18 months, and 73% at 24 months. One of the 70 patients lost MMR while on the treatment protocol, after 350 days' duration. The Kaplan-Meier estimate of the percentage who remain in MMR 12 months after achieving MMR was 98%.
MDD achieved
The MDD achieved in 6-month time intervals up to 24 months is summarized in Table 3. Fifty-eight percent and 65% of patients were able to average 600 mg in the first and second 6-month intervals, respectively. Overall, 77% averaged more than 500 mg/day for the first 12 months. Just 6% of patients were unable to achieve an MDD of 400 mg in both the first and second 6-month intervals, and 32% of patients were unable to achieve an MDD of 500 mg in either or both of the first two 6-month intervals
Outcome according to actual dose received in the first and second 6 months
Patients who received full protocol dose over the first 6 months (MDD = 600 mg) had a significantly (P = .037) higher likelihood of achieving MMR by 12 months than patients with MDD less than 600 mg in the first 6 months (55% vs 32%). MDD in the first 6 months showed similar stratification of MMR rates at 24 months (77% vs 53%, P = .016). MMR was also achieved more rapidly (P = .013) in patients with an MDD of 600 mg in the first 6 months (Figure 1).
Probability of achieving MMR according to dose achieved in first 6 months (P = .013).
Probability of achieving MMR according to dose achieved in first 6 months (P = .013).
In the group sustaining an MDD of 600 mg throughout the first 12 months (n = 50), 82% achieved MMR by 24 months compared with 33% of patients averaging less than 600 mg in both the first and second 6 months (n = 18). An intermediate rate of MMR (70%) was achieved by 24 months in patients who had an MDD less than 600 mg in the first 6 months and 600 mg in the second 6 months (n = 20; Table 1).
Predictive value of early molecular response
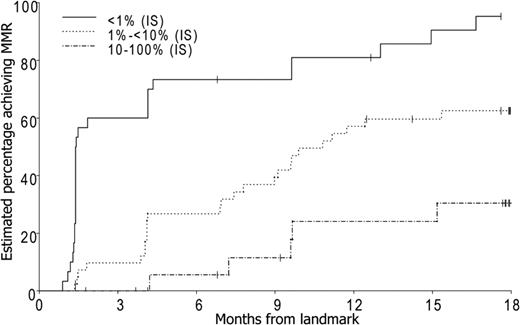
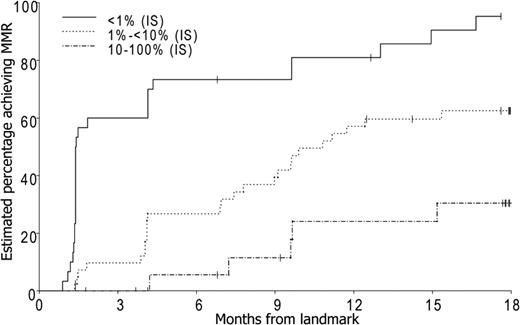
There was a 94% probability (95% CI, 78%-99%) of achieving MMR by 24 months for the 27 patients who achieved a BCR-ABL level less than 1% IS (> 2 log reduction) in BCR-ABL by 3 months. For the 43 patients who achieved a BCR-ABL level between 1% and 10% IS (1-2 log reduction) by 3 months, the probability of achieving MMR by 24 months was 62%. There was a 35% probability (95% CI, 18%-61%) of achieving MMR by 24 months in the 25 patients who had a BCR-ABL level more than 10% (< 1 log reduction in BCR-ABL) by 3 months (Figure 2).
Probability of achieving MMR according to molecular response at 3 months (P < .001).
Probability of achieving MMR according to molecular response at 3 months (P < .001).
Patients withdrawn from study
Twenty patients (19%) came off study by 24 months. Five withdrew for reasons that were not directly disease or treatment related: 3 of these patients died of unrelated causes and 2 were withdrawn when second malignancies were diagnosed. Two patients were lost to follow-up (1 in MMR at the time). The remaining 13 patients who came off study had treatment failure, 5 had primary imatinib resistance, and 8 acquired resistance (loss of CHR or MCR) with 3 of the 8 progressing to blast crisis.
Toxicity and dose modifications
The frequencies of grade 3 or 4 drug-related toxicities are listed in Table 4. Grade 3 or 4 toxicities were uncommon beyond the first 6 months. There were 10 reported episodes of grade 4 neutropenia on imatinib monotherapy. In 8 cases, filgrastim was used to support neutrophil recovery. Imatinib therapy was also concomitantly ceased in 5 (62.5%) of these episodes. Only one episode of grade 4 neutropenia resulted in hospitalization for febrile neutropenia. Grade 3 or 4 thrombocytopenia was observed in 11% of patients in the first 6 months, but only 4 serious adverse events related to bleeding were observed in the 2-year study, suggesting that this more aggressive approach to imatinib dosing in the presence of thrombocytopenia is clinically acceptable.
Achievement of response milestones
Of the 103 registered patients, 98% achieved CHR by 3 months, 90% MCR by 6 months, 83% CCR by 9 months, and 18% were less than 0.01% by 12 months.
MCR was not achieved by 6 months in 11 patients, and an additional 2 patients lost MCR by 6 months. Dose escalation was only possible in 2 of these patients, primarily because of ongoing toxicity or subsequent study withdrawal (Table 5).
CCR was not achieved by 9 months in 19 patients and 2 lost CCR between 6 and 9 months. Three were dose escalated, 3 were previously escalated to 800 mg, and the remaining patients were not escalated because of intolerance or study withdrawal.
Eighty-five patients failed to achieve a BCR-ABL level less than 0.01% at or before 12 months. Forty-five were able to escalate dose to 800 mg/day, 5 patients had already escalated, 10 patients were on reduced dose because of toxicity, and the remaining patients were either off study or unable to increase for other reasons (Table 5).
Response to dose escalation before 12 months
Two patients at 6 months and 6 patients by 9 months underwent dose escalation to 800 mg/day. Three of the 8 patients who underwent dose escalation achieved CCR by 24 months and 2 also achieved MMR. In the remaining 5 cases, 2 proceeded to an allograft before a response could be assessed and 3 had no response to dose escalation.
Response to dose escalation after 12 months
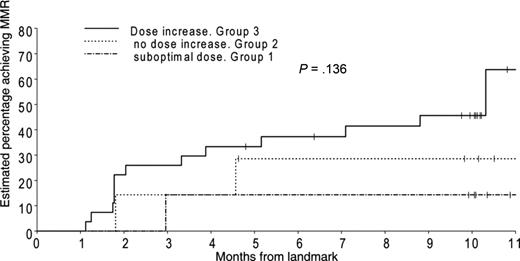
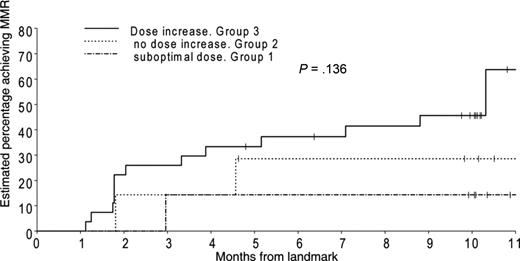
Eighty-five patients failed to achieve a BCR-ABL level less than 0.01% by 12 months. Seventy-three of these patients were still on study and eligible for dose increase to 800 mg/day after 12 months. Ten of these (group 1) were already on a reduced dose (< 600 mg) and were unable to dose escalate. A further 18 patients (group 2) were not able to dose increase but remained on 600 mg/day. The remaining 45 patients (group 3) increased imatinib dose to 800 mg/day. We analyzed the probability of achieving MMR after 12 months in the 3 groups, in those who had failed to achieve MMR by 12 months (Figure 3). MMR was achieved in 63% of patients who could dose escalate (group 3) compared with 30% in group 2 and 15% in group 1.
Probability of achieving MMR in those patients who had not achieved an MMR at 12 months. Patients were divided into 3 groups according to the dose they received in the second year. The first cohort on “suboptimal dose” had already had their dose modified and were on a maximal tolerated dose, which was less than 600 mg/day. The second group were unable to dose escalate but maintained 600 mg/day. The third group were able to increase to 800 mg/day.
Probability of achieving MMR in those patients who had not achieved an MMR at 12 months. Patients were divided into 3 groups according to the dose they received in the second year. The first cohort on “suboptimal dose” had already had their dose modified and were on a maximal tolerated dose, which was less than 600 mg/day. The second group were unable to dose escalate but maintained 600 mg/day. The third group were able to increase to 800 mg/day.
Factors predictive of molecular response at 12 months
MMR was actually achieved by 45 patients (44%) at or before 12 months. In unifactor analyses, the Hasford score (P = .005), spleen size (P < .001), the percentage of Ph+ cells (P = .029), and pretreatment peripheral blood basophils (P = .005) were significantly associated with achievement of MMR. In multifactor analysis, spleen size (P < .001), pretreatment basophils (P = .004), and platelets (P = .020) were all significantly associated with the achievement of MMR by 12 months (Table 1).
The difference between the percentages of patients achieving MMR in the group of patients who had an MDD of more than or equal to 600 mg in each 6-month period (56%) and those who had an MDD of less than 600 mg in each 6-month period (22%) was significant (P = .028) and remained significant (P = .034) when spleen size, basophils, and platelets were fitted as continuous covariates in multifactor analysis (Table 1).
Factors predictive of molecular response at 24 months
Sixty-six patients (64%; 95% CI, 55%-73%) achieved MMR by 24 months. In unifactor analyses, the Sokal score (P = .044), Hasford score (P = .006), spleen size (P = .002), the percentage of Ph+ cells (P = .038), and the factor indexing MDD in the first two 6-month periods (P = .003) were significantly associated with achievement of MMR.
In multifactor analysis, spleen size (P < .001) and MDD groups (P = .004) were each significantly associated with the achievement of MMR. In patients with spleen size less than 5 cm below the left costal margin, 78% had achieved MMR by 24 months and in patients who had greater degrees of splenomegaly, 46% had achieved MMR by 24 months. There was a higher percentage of patients achieving MMR by 24 months in the group who had an MDD of more than or equal to 600 mg in both 6-month periods (82%) compared with those with an MDD of less than 600 mg in both 6-month periods (33%; P < .001). This remained significant when spleen size was also fitted as a continuous covariate in multifactor analyses (Table 1).
Data on Ara-C-imatinib combination
Ten patients were eligible for combination therapy, but only 3 received this. The remainder did not receive combination therapy because of patient refusal or noncompliance, Investigator discretion, withdrawal from the study to undergo an allogeneic transplantation, or imatinib intolerance. Two patients received 2 cycles of combination therapy and one patient received 3 cycles of a maximum of 4 cycles. There was no evidence of significant response in 2 of the 3 patients receiving combination therapy, whereas the third patient regained CCR and proceeded soon after to an allograft.
Acquired resistance and mutation screening
Overall, 18 patients lost response to imatinib. These were equally divided between loss of CHR (4 cases), loss of MCR (4 cases), loss of CCR (5 cases), and detection of a 1-log increase in BCR-ABL associated with loss of MMR (5 cases). In 9 of these 18 cases, dose interruption or reduction was closely associated with the loss of response, so that it was difficult to determine whether these cases could be truly classified as resistant. None of these 9 patients had kinase domain mutations. Six of the 9 patients who lost response while continuing full protocol dose imatinib had mutations detected by direct sequencing (67%). The actuarial probability of detecting a mutation by 24 months was 7% (95% CI, 2%-12%). The mutations first became detectable at a median of 6 months of imatinib therapy (range, 3-12 months). All 6 patients with mutations had significant increases in the BCR-ABL levels (median, 3.7-fold; range, 2.1- to 53-fold). Two of these 6 patients with mutations rapidly progressed to blast crisis at 3 and 6 months (both had P-loop mutations), and both subsequently died. Two additional patients lost CHR: one of these did not respond to an increased imatinib dose or sequential combined therapy and the other proceeded to allogeneic transplant after failure to respond to an increased dose. Two patients lost CCR and proceeded to allogeneic transplant.
Of the 94 patients who were tested for mutations at 6 months who did not meet the other criteria for mutation screening (significant rise in BCR-ABL that was not associated with a dose reduction or cessation, or clinical evidence of disease progression), a mutation was detected in one patient. This patient subsequently had a significant rise in BCR-ABL
OS and PFS
Six patients have died by 24 months: 3 from unrelated causes, 2 after progression to blast phase, and 1 without prior progression, from complications after an allograft. Actuarial survival at 1 and 2 years was 98% and 94%, respectively. The actuarial PFS rates at 1 and 2 years were 95% and 93%, respectively.
Discussion
The importance of maintaining full-dose imatinib over the first 6 to 12 months is suggested by the impact of early dose intensity on molecular response. Possible confounding factors need to be considered here. Is modified dose associated with poorer response because patients with biologically unfavorable CML are more probable to experience toxicities requiring dose modifications? We speculated that peripheral blood cytopenias in the first few months, which commonly lead to dose interruption and dose reduction, might be a marker of unfavorable disease biology as it reflects a limited capacity for normal hematopoiesis to recover as the leukemic clone declines. Our data have not enabled us to completely exclude this possibility. Although thrombocytopenia in the first 6 months appeared to be associated with a reduced incidence of MMR by 24 months, this association was not significant in models that included MDD or both MDD and spleen size (data not shown). Importantly, MDD proved to be an independent predictor of response in multivariate analysis. Therefore, the most plausible explanation for these findings is that any modification of mean dose less than 600 mg/day in the first 12 months may be causally linked to an inferior molecular response. Pharmacokinetic studies were not included in this trial, but our findings would be consistent with recent studies showing a correlation between trough imatinib level and the probability of achieving CCR and MMR.40
The TIDEL study was designed to test the hypothesis that a higher dose of imatinib (600 mg/day) with earlier and more stringent criteria for dose escalation in cases of failure to achieve predetermined response targets would enable patients to achieve superior cytogenetic and molecular responses and PFS than the current standard imatinib dose of 400 mg/day. For patients with newly diagnosed CML, the optimal dose of imatinib and the best approach for management of patients with suboptimal disease response are not known.16-18,41-44 Promising results with higher doses of imatinib have raised questions about whether the dosing schedule of 400 mg/day selected for the IRIS study is the best approach. In addition, the IRIS trial did not allow dose escalation unless patients failed to achieve an MCR by 12 months or lost MCR. In the current study, we found that the cytogenetic response rates at 12 and 24 months were significantly better than response rates observed in the IRIS trial. Most striking was the comparison of CCR rates: 88% compared with 69% (P < .001) at 12 months and 90% compared with 80% at 24 months (P = .002). However, the rate of CCR at 12 months in the current trial was similar to that of the IRIS trial after 5 years,13 suggesting that there may not be an overall increase in the number of patients achieving this level of response, but that higher doses enable patients to achieve CCR more rapidly. Whether earlier achievement of CCR will result in a lower rate of treatment failure, and superior survival for patients on the TIDEL study may not be assessable for several years. At 2 years, PFS in this study was 93%, which is similar to that of the IRIS study at 2 years (95%).13
In terms of molecular response, the comparison is more difficult. Not all patients in the IRIS trial had molecular monitoring so that overall rates of MMR had to be estimated based on the rates within a subgroup of patients achieving CCR, who then underwent molecular evaluation. Based on this calculation, the rate of MMR in TIDEL and in IRIS at 12 months were similar, at 47% and 40%, respectively. However, by 24 months, the rate of MMR in this trial was 73% compared with 55% in the IRIS trial. It is too early to determine whether more patients are achieving an MMR on this more intensive imatinib therapy or whether they are just achieving MMR earlier.
In the M. D. Anderson Cancer Center phase 2 study of imatinib 800 mg/day, CCR and MMR were achieved by 95% and 60% of patients at 12 months, and OS at 2 years was 94%. These outcomes are markedly superior to their results for patients receiving imatinib 400 mg/day.18 It is not possible to compare rates of MMR in the TIDEL study and the M. D. Anderson Cancer Center high-dose study because of the different RQ-PCR methodologies used.37 Dose intensity actually achieved in the M. D. Anderson Cancer Center study was higher than in our study. Overall, 77% of patients in TIDEL were able to average an imatinib dose of 500 to 600 mg/day over the first 12 months, compared with an average of 600 to 800 mg/day in 82% of patients in the M. D. Anderson Cancer Center high-dose trial.
An important feature of the TIDEL study was the attempt to “rescue” patients identified as being at risk of suboptimal response or failure by applying a policy of close cytogenetic and molecular monitoring. This enabled high-risk patients to be identified early and potentially allowed them to benefit from dose escalation. However, our experience in the 17 patients who were eligible for a dose increase before 12 months of therapy demonstrated that such a “rescue” is neither universally feasible nor highly effective. Many of these patients were already on reduced doses of imatinib; and in 53% of eligible patients, it was not possible to dose increase. Even in the 8 patients who were able to dose increase at 6 or 9 months, it is not possible to conclude that dose escalation contributed to an improved response. Only 2 of these patients achieved MMR by 24 months.
We have previously shown that dose escalation at 12 months leads to improved molecular response in patients with low OCT-1 activity, but not in patients with high OCT-1 activity.45 However, when we take the whole cohort of patients who were dose escalated at or after 12 months, there is no significant difference in the probability of achieving MMR by 24 months when molecular responses in the cohort of patients who were dose escalated at 12 months are compared with the cohort who remained on 600 mg/day in the second year because of intolerance. It should be noted that a 4 log reduction has not been shown to be superior to a 3 log reduction in terms of the probability of PFS and is not recommended to be a trigger for dose escalation outside of clinical trials. The lack of benefit with higher dose in this setting is interesting given our findings that early dose intensity is so important. We speculate that each patient may have a different optimal dose that varies according to pharmacokinetic factors, including OCT-1 activity. Increasing dose up to this patient-specific optimal dose will lead to incremental improvements in response, but doses above this threshold would not provide further benefit, perhaps because target inhibition is already maximized. Based on these findings, we also speculate that the threshold dose may be approximately 600 mg/day for many CML patients.
In conclusion, maintaining imatinib dose at 600 mg/day in the first year of therapy for patients with newly diagnosed CML predicts for higher molecular response rates compared with lower doses. However, there is little evidence, in the setting of upfront higher-dose imatinib, that further dose escalation can rescue those patients with suboptimal disease response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Jane Matthews and Nancy Guzzo-Pernell, who were involved in the design and activation of this clinical trial; Dr Bereha Khodr, who assisted with data management; Drs Ken Bradstock, Naomi MacKinlay, Stephen Opat, Miles Prince, Paul Cannell, Gavin Cull, Richard Herrmann, Keith Fay, and John Moore, who registered patients on the trial; and all the patients who participated.
This work was supported in part by grants from Novartis Pharmaceuticals Australia and Amgen Australia. T.P.H. is a National Health and Medical Research Council Practitioner Fellow.
Authorship
Contribution: T.P.H., S.B., D.L.W., J.R., A.G., R.K., J.F.S., and K.L. designed the study, performed research, and wrote the manuscript; and K.T., C.A., A.S., J.M., J. Cooney, M.F.L., P.R., J. Catalano, M.H., R.F., A.K.M., K.F., S.D., H.J., D.J., C.U., and S.D. performed research and reviewed the manuscript.
Conflict-of-interest disclosure: K.L. was an employee of Novartis when this study was performed; T.P.H., S.B., and D.L.W. received research funding from Novartis; T.P.H., S.B., D.L.W., A.G., J.F.S., A.S., K.T., C.A., and D.J. serve on advisory boards for Novartis and receive honoraria from Novartis. All other authors declare no competing financial interests.
Correspondence: Timothy P. Hughes, Division of Haematology, Institute of Medical and Veterinary Science, Adelaide, South Australia, 5000 SA, Australia; e-mail: timothy.hughes@imvs.sa.gov.au.