Abstract
Background: In multiple myeloma (MM), a complete response (CR) is defined by the European Group for Blood and Bone Marrow Transplantation (EBMT) and the International Myeloma Working Group (IMWG) Uniform Response Criteria as absence of monoclonal protein in serum and urine by immunofixation (IFE), and a bone marrow (BM) aspirate/biopsy containing <5% plasma cells (PC). This definition of CR is widely adopted in clinical trials, but there is a high degree of non-compliance with the BM requirement. As a result, differences in CR rates across trials can occur not just as a result of treatment efficacy, but also on how rigorously the BM requirement for CR is implemented. Moreover, requiring BM examinations merely to confirm CR is cumbersome in clinical practice. The goal of this study was to determine the added value of BM examinations in patients who can otherwise be considered to be in CR by virtue of a negative IFE in the serum and urine.
Methods: For the purpose of the study, we included only patients with MM who had measurable monoclonal (M) protein levels at baseline (defined as >1 gm/dL in the serum and/or >200 mg/24 hour in the urine) on protein electrophoresis; patients with non-secretory and oligo-secretory MM were excluded. We then identified patients who since 1995 had a negative IFE in the serum and urine and a BM aspirate/biopsy, all done at the same time (within 30 days of each other). Baseline demographics and clinical characteristics; date of diagnosis, last follow up, and follow up status; serum and urine M protein levels at diagnosis; and results of serum and urine IFE, serum free light chain (FLC) ratio and BM aspirate/biopsy within 30 days of complete response were all collected from the existing databases.
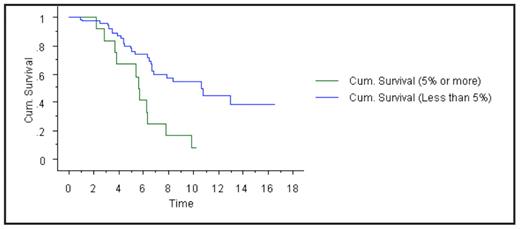
Results: Ninety-five patients met the criteria for the study, all of whom had measurable disease at baseline and subsequently achieved negative IFE in serum and urine (‘IF- CR’). Of these, only 83 patients (87%) met criteria for CR by the EBMT/IMWG criteria with less than 5% PC in the BM; 13% of patients had 5% or more PC despite the apparent IF-CR on serum and urine IFE. In fact, the BM showed 10% or more PC in 5% of IF-CR patients. We then assessed whether a normal serum FLC ratio in addition to negative serum and urine IFE would eliminate the need for a BM biopsy. The additional requirement of the serum FLC ratio to negative IFE was not fully adequate in eliminating false positive CR’s. Among 34 patients who had IF- CR plus a normal serum FLC ratio, only 88% of patients met CR definition with <5% BM PC; 12% had 5% or more PC, and one patient (3%) had 10% or more PC. We studied the survival (from time of diagnosis) of patients who had true CR’s (negative IFE and <5% PC) versus patients who had IF- CR’s but BM had 5% or more PC. Overall survival from diagnosis between the two groups was 10.7 years versus 5.6 years, respectively, P=0.001 (see Figure 1).
Conclusions: BM examination is essential to accurately estimate CR. Up to 13% of patients with negative IFE in serum and urine fail to meet the BM requirement. The addition of a normal serum FLC ratio to negative IFE did not significantly reduce the proportion of patients who fail to meet the BM requirement. Based on this study, we recommend that the CR definition in the EBMT and IMWG criteria should not be revised to eliminate the need for BM examination. Moreover, the consistent collection of BM samples is essential for future strategies to evaluate immunophenotypic CR and molecular CR in MM.
Kaplan-Meier curve showing survival benefit (in years) of patients in complete response with less than 5% bone marrow plasma cells.
Kaplan-Meier curve showing survival benefit (in years) of patients in complete response with less than 5% bone marrow plasma cells.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author