Abstract
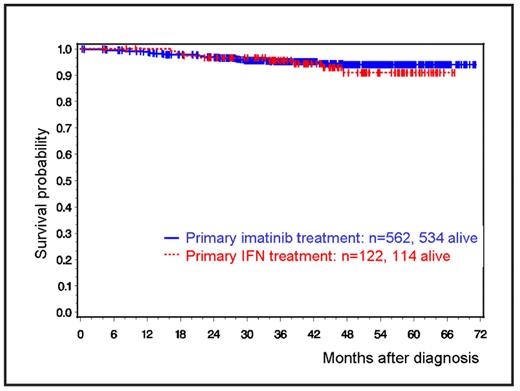
In spite of favorable response and survival results for the majority of CML patients on imatinib therapy, in a substantial minority imatinib fails or shows suboptimal responses. A treatment optimization study was therefore designed to compare in a randomized fashion standard imatinib vs. imatinib + interferon alpha (IFN) vs. imatinib + low dose araC vs. imatinib after IFN (for low- and intermediate-risk patients) or vs. imatinib 800 mg (for high-risk patients). Inclusion criteria were newly diagnosed BCR/ABL positive CML in chronic phase. In July 2005, randomization to the arms imatinib + araC and imatinib after IFN was discontinued and recruitment for imatinib 800 mg was expanded to low- and intermediate-risk patients. Primary goals are: rates of hematologic, cytogenetic and molecular remissions, duration of chronic phase, overall survival, adverse events and analysis of subsequent allografting. Since its activation in 7/2002, 1203 patients have been randomized. The current evaluation represents the first of three designed, statistically adjusted interim analyses of 710 patients randomized by the end of 2005 with a followup of at least 2 years. Analysis was according to intention to treat. 666 patients (545 with primary imatinib, 121 with primary IFN) were evaluable for hematologic, 621 for cytogenetic, and 631 for molecular responses. Median age was 53 years, 60% were male, median values were for Hb 12.5 g/dl, WBC 71.2/nl and platelets 384/nl, 35% had low, 53% intermediate and 12% high risk (Euro score). Median observation time was 3.5 years. Median duration of IFN pretreatment was <4 months. At 1 year, the cumulative incidence of complete hematologic remission (CHR) was 82.3% and 74.4%, of major cytogenetic remission (MCR) 65.6% and 40.6%, of complete cytogenetic remission (CCR) 52% and 19.7%, and of major molecular remission (MMR) 33.2% and 4.7% for primary imatinib and IFN therapies, respectively. At 3 years, the cumulative incidence of CHR was 96.4% and 93.8%, of MCR 89.5% and 89.1%, of CCR 85.2% and 78.5%, and of MMR 79% and 63% for primary imatinib and IFN therapies, respectively. 5-year-survival probability of all patients currently exceeds 90% (94% for imatinib-, 91% for IFN-based therapy, Figure 1). Event free survival after two years (no progression, no death, CCR within the first 18 months, no loss of CHR or MCR) was 80.3%. 36 patients died, 51 patients were transplanted in first chronic phase, and 80 patients progressed, 43 of which were switched to alternative treatments (16 to new drugs, 18 to transplantation, 9 received both). Type and severity of adverse events (AE) did not significantly differ from those reported previously. Hematologic AEs (leukopenia, thrombocytopenia) were most frequent in the imatinib 800 mg arm. Nonhematologic AEs (gastrointestinal) were most frequent in the combination arms and with imatinib 800 mg. In no case recruitment had to be changed due to superiority or inferiority of any arm. This applies also to the high dose imatinib arm where earlier response might translate into better survival. In conclusion, this first interim analysis shows favorable survival and long term response rates. Imatinib in combination with, or after, IFN or with low dose araC are feasible and equally safe treatment alternatives. More definite information will be provided by the next interim evaluation after recruitment has been terminated.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author