Abstract
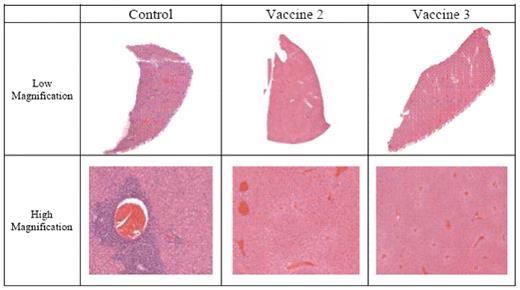
Diffuse Large B Cell Lymphoma (DLBCL) is an aggressive form of Non-Hodgkin’s Lymphoma (NHL) accounting for 40% of NHL in the United States. Current treatment options are ineffective in eliminating refractory lymphoma, which is responsible for patient relapse. Therefore, a carefully developed immunotherapeutic strategy to specifically target refractory lymphoma might be useful in improving therapy. In this study, anti-lymphoma immune outcome was evaluated in a syngeneic murine model using the aggressive and highly liver metastatic RAW117-H10 lymphoma cell line that expresses the murine leukemia virus protein gp70. Anti-lymphoma immunity was generated by immunizing Balb/c mice with two peptide-based vaccines in which a T cell epitope from gp70 (SSWDFITV) was covalently attached to the N-termini of two response-selective molecular adjuvants derived from complement component C5a: YSFKPMPLaR (EP54) and YSFKDMP(MeL)aR (EP67). In both vaccines, a protease-sensitive, double-Arg linker sequence (RR) was placed between the epitope and the EP54/EP67 molecular adjuvants: SSWDFITV-RR-YSFKPMPLaR (Vaccine 2) and SSWDFITV-RR-YSFKDMP(MeL)aR (Vaccine 3). To determine the protective effect of the EP54/EP67-containing vaccines against lymphoma growth, separate groups of Balb/c mice were administered 200 μg of Vaccine 2 or Vaccine 3 dissolved in water via intraperitoneal (100 μg) and intradermal (100 μg) injections. Following 4 weekly immunizations, the mice were intravenously transplanted with 5 × 103 RAW117-H10 cells to ascertain the protective benefit of the vaccines. The results showed Vaccines 2 and 3 providing complete protective benefit, with pre-vaccinated mice surviving greater than 235 days, whereas all control, unvaccinated animals died within 18 days of tumor inoculation. In addition to survival analysis, histopathological analysis was performed using liver sections from mice upon death, or after sacrifice following 60 days of survival, to evaluate tumor burden in the mice (See Figure 1). No tumor growth was found in livers taken from mice pre-vaccinated with Vaccines 2 and 3. Furthermore, to elucidate the mechanisms of vaccine-mediated protection against lymphoma, the frequency and functions of the immune effector cells from blood and spleens were analyzed at 72 hours following the second and fourth vaccinations using flow cytometry, T and B cell-specific mitogen proliferation assays, and lymphoma-specific cytotoxicity assays in vitro. The results of these analyses showed a significant increase in the frequency (p< 0.01) of helper and cytotoxic T cells, proliferation of both T and B cells (p< 0.05), and significant lymphoma-specific cytotoxicity (p< 0.001) compared to non-vaccinated mice. Therefore, these results demonstrate a pronounced protective effect of Vaccines 2 and 3 against lymphoma growth in mice. These results lay the foundation for further studies in testing the therapeutic efficacy of Vaccines 2 and 3 in mice pre-inoculated with lymphoma, as well as in initiating studies employing human lymphomas. In so doing, the principles underlying these molecular adjuvant-containing vaccines could eventually be translated from the mouse model to a clinical setting.
Hematoxylin and Eosin staining of liver sections taken from control and vaccinated mice inoculated with RAW117-H10 lymphoma.
Hematoxylin and Eosin staining of liver sections taken from control and vaccinated mice inoculated with RAW117-H10 lymphoma.
Disclosures: No relevant conflicts of interest to declare.
(This work was supported by the Lymphoma Research Foundation, New York, NY).
Author notes
Corresponding author