Abstract
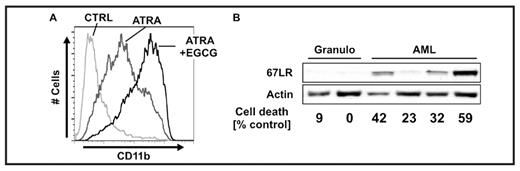
Acute promyelocytic leukemia (APL) patients are currently treated with all-trans retinoic acid (ATRA) successfully resolving the differentiation block. However, concurrent chemotherapy is still necessary in combination with ATRA and novel less toxic therapeutic approaches are a major demand. Epigallocatechin-3-gallate (EGCG), the main polyphenolic compound present in green tea, has been reported to have chemopreventive and chemotherapeutic effects in different neoplasms. EGCG inhibits cell growth in vitro and in vivo and induces apoptosis in cancer cells without adversely affecting normal cells. Also, EGCG has been found in earlier studies to effectively kill AML blasts, but it has never been applied in combination with ATRA. We have previously shown that expression of the death-associated protein kinase 2 (DAPK2) enhanced ATRA-induced neutrophil development, and recently it was shown that EGCG specifically killed multiple myeloma cells while inducing DAPK2. Therefore, we first tested whether EGCG treatment induces DAPK2 expression in myeloid leukemic blast cells and whether this will lead to cell death. EGCG treatment of HL60 and NB4 acute myeloid leukemic cells led to a dose-dependent increase of DAPK2 mRNA with a maximum of 5.5- and 3.9-fold, respectively. In parallel, DAPK2 protein was markedly upregulated in both cell lines accompanied by a 72% increase of cell death after 24h, as measured by reduction of tetrazolium salt (XTT assay), and by hallmarks of apoptosis: activation of caspase-3 and phosphatidylserine exposure on the plasma membrane surface. EGCG-induced cytotoxicity was reduced by 48% in HL60 and by 46% in NB4 cells upon stable short hairpin RNA-mediated silencing of DAPK2. Moreover, combined ATRA and EGCG treatment of HL60 cells resulted in cooperative DAPK2 induction and potentiated myeloid differentiation as measured by CD11b and CD15 expression using flow cytometry (Figure A) as well as by C/EBPε and G-CSFR mRNA levels using quantitative RT-PCR. Enhanced differentiation was significantly reversed by knocking down DAPK2. Moreover, EGCG toxicity of NB4 and HL60 cells correlated with 67 kDa laminin receptor (67LR) protein expression which is downregulated during ATRA treatment of HL60 and NB4 cells but not in the ATRA-resistant sublines HL60-R and NB4-R. In line, HL60-R and NB4-R cells are still susceptible to EGCGinduced cell killing, whereas pretreatment of parental HL60 and NB4 cells with ATRA, causing downregulation of 67LR, rendered these cells resistant to EGCG mediated killing. Likewise, susceptibility of primary AML (n=8) samples to EGCG treatment was closely associated with 67LR expression. Moreover, neutrophils and PBMCs from healthy donors did not express 67LR and hence were resistant to EGCG treatment (Figure B).
In summary, we found that DAPK2 is essential for EGCG-induced cell death in myeloid leukemic cells, and that a combination of ATRA and EGCG treatment significantly boosted neutrophil differentiation. This enhanced differentiation is most likely due to preferred EGCG-induced killing of ATRA-resistant leukemic cells expressing the EGCG receptor 67LR. We thus conclude that simultaneous ATRA and EGCG treatment might improve differentiation therapies for APL. In addition, other AML subtypes with high 67LR expression might also benefit from a combined chemotherapy and EGCG regimen.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author