Abstract
Introduction: Immunotherapy with innately competent natural killer (NK) cells can potentially synergize with chemotherapy by eradicating chemo-refractory residual disease. We recently reported that killer immunoglobulin-like receptor (KIR)-ligand mismatched NK cells from haplo-identical donors are cytotoxic to primary myeloma (MM) cells in vitro. In a pilot trial complete remission was achieved in 50% of patients with advanced MM. Although the initial clinical results were encouraging virtually all patients relapsed, suggesting that the number of allo-reactive NK cells transfused was too low to eradicate all MM cells taking into account that MM patients may harbor a tumor burden as large as 109–10 MM cells. This provided the rationale for expanding and activating NK cells from healthy donors with irradiated K562 cells transfected with 4-1BBL and membrane-bound IL15 (K562 transfectants), as described by Imai et al (
Methods: PBMNC from 6 healthy donors were co-cultured for 14 days with irradiated K562 cell transfectants. The cultures were then analyzed for fold expansion, gene expression using the Affymetrix U133Plus2.0 microarray platform, immunophenotyping and cytotoxicity.
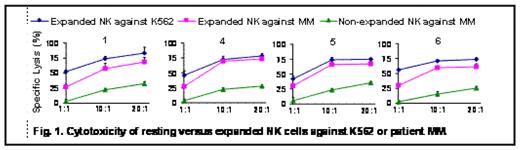
Results: There was an average of 166-fold (range 54–267) expansion of NK cells in the 12 experiments from 6 healthy donors studied after 14 days of co-culture with K562 transfectants without expansion of T-cells. Comparison of gene expression profiles of purified, non-expanded and expanded NK cells of healthy donors showed up-regulation of the expression of activation molecules (e.g. NKG2D, NCRs, DNAM-1), death receptors, granzymes and adhesion molecules on activated/expanded NK cells all consistent with increased proclivity to MM killing. Up regulation of activating receptors was confirmed by flow cytometry. In contrast to non-expanded NK cells, inhibitory KIR receptors were not up regulated on activated NK cells. Using the same number of NK cells, the median lysis of primary MM by expanded NK cells was 62% (range: 49%–70%, n=6) at a 10:1 E:T ratio compared to non-expanded NK cells (median: 23%; range: 15%–34%, p<0.001) (Fig 1). There was no killing of patient normal cells. Blocking experiments demonstrated that antibodies to NKG2D, NCRs, DNAM-1, CD27 and CD69 could partially abrogate NK cell-mediated lysis of MM cells. We also observed that after stimulation with modified K562 cells, the non-alloreactive NK cell population, which under non-activated conditions is inhibited by HLA-C on target cells, could be recruited to kill MM cell lines thus further increasing the number of NK cells with anti-MM activity.
Conclusion: K562 transfectants stimulated vigorous expansion of NK cells without expanding T lymphocytes. The expanded/activated NK cells were highly active and killed patient MM cells better than non-expanded NK cells without significant lysis of normal cells. Optimal activation of NK cells can overcome inhibitory signals via KIR and other inhibitory receptors and induce killing of MM by previously inhibited NK cells
Cytotoxicity of resting versus expanded N K cells against K562 or patient MM.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author