Abstract
The eradication of minimal residual disease (MRD) in chronic lymphocytic leukemia (CLL) patients is associated with improved progression free and overall survival, when compared to patients with detectable disease following therapy. Unfortunately, the assays that allow for MRD detection, such as PCR analysis of patients specific immunoglobulin heavy chain rearrangements, or multiparametric multi-color flow cytometry are expensive, cumbersome, and require a high level of technical expertise. As such, MRD assessment in CLL is not routinely employed outside of specialized centers or clinical studies. Therefore, the development of new assays that can be employed uniformly for all CLL patients and easily implemented in general clinical practice would be of great benefit to the CLL community.
The expression pattern of the CLLU1 gene (CLL Upregulated gene 1) is extremely restricted: elevated levels of CLLU1 expression has only been detected in CLL patient samples, not in any other tissue or cell line. In the majority of untreated CLL patients followed in longitudinal analysis the CLLU1 expression levels were stable over time. Furthermore, in CLL patient samples analyzed for CLLU1 expression before treatment and after treatment response followed by relapse, the CLLU expression levels were similar. Hence, the CLLU1 status appears to be an intrinsic, constant parameter for the CLL clone.
We therefore hypothesized that CLLU1 expression levels will be very low or not detectable in patients who have experienced complete leukemia eradication, and likewise the persistence of elevated CLLU1 levels, should indicate persistent residual disease or relapsed leukemia.
We performed a retrospective analysis on cryopreserved specimens from patients who were in clinical remissions that underwent marrow biopsies for MRD evaluation by 4-color flow. RNA from peripheral blood mononuclear cell samples collected at the time of the marrow biopsy was extracted and analyzed by realtime RT-PCR using the comparative Ct method of relative quantification with b2-microglobulin as endogenous control and a pool of purified normal B-lymphocytes as calibrator. Seventeen patients underwent a total of 26 response evaluations with MRD assessed by 4 color flow in the marrow. MRD negativity by 4-color flow was based on a threshold of detection of 0.1% leukocytes expressing CD5, CD19, CD20, and CD79b. For patients found to have MRD in the marrow, the mean CLLU1 level was 34.7 (95% CI 5.03–64.38). For patients with undetectable disease in the marrow by 4-color flow, the mean CLLU1 level was 0.07 (95% CI 0.02–0.14). In 3 cases, all of which demonstrated <0.10% MRD by 4-color flow in both marrow and peripheral blood, CLLU1 levels were not detectable in the peripheral blood samples. Using a cutoff of 0.5, which represents the mean CLLU1 expression level of normal PBMCs (n=3) +2 standard deviations, the sensitivity for CLLU1 for detecting residual disease in the marrow was 73.3% with a specificity of 100% when compared to 4-color flow MRD assessment on marrow. A more conservative threshold of 1.0 was associated with a sensitivity of 60% while maintaining a specificity of 100%.
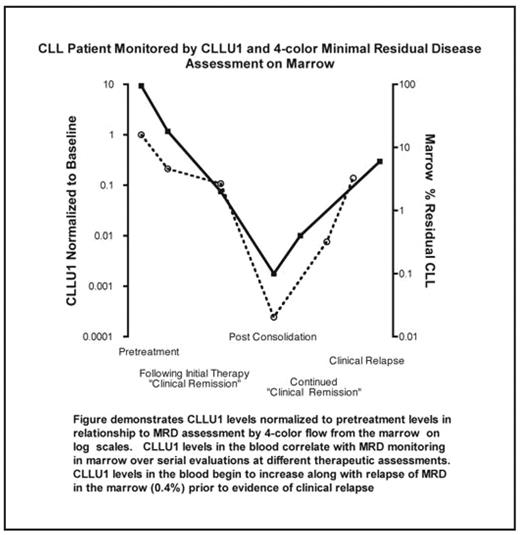
Six patients who achieved MRD negative remissions underwent serial surveillance by marrow biopsy for MRD determination while remaining in a clinical remission. CLLU1 levels were determined on peripheral blood specimens obtained at the same time of MRD determination in the marrow and were normalized to the individual’s CLLU1 pretreatment value (see figure). Sixteen paired evaluations revealed a strong correlation between CLLU1 gene levels and extent of leukemia in the marrow as assessed by 4-color flow (spearman’s rho=0.8613, p-value < 0.0001).
We have demonstrated a strong correlation between the invasive and technically challenging bone marrow evaluation of MRD and the quantification of CLLU1 levels in the blood. In addition, a one-time evaluation of CLLU1 levels in the blood is highly specific for detecting residual CLL with sensitivity that might preclude the need for marrow assessment of all patients. In summary, this novel molecular method for detecting MRD holds promise for serial monitoring of MRD, detection of early relapse, and obviating the need for marrow evaluation in some patients. The utility of CLLU1 levels in the blood as a monitoring strategy will be prospectively evaluated in a clinical study of consolidation therapy.
demonstrates CLLU1 levels normalized to pretreatement levels in relationship to MRD assessment by 4-color flow from the marrow on log scales. CLLU1 levels in the blood correlate with MRD monitoring in marrow over serial evaluations at different therapeutic assessments. CLLU1 levels in the blood begin to increase along with relapse of MRD in the marrow (0.4%) prior to evidence of clinical relapse.
demonstrates CLLU1 levels normalized to pretreatement levels in relationship to MRD assessment by 4-color flow from the marrow on log scales. CLLU1 levels in the blood correlate with MRD monitoring in marrow over serial evaluations at different therapeutic assessments. CLLU1 levels in the blood begin to increase along with relapse of MRD in the marrow (0.4%) prior to evidence of clinical relapse.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author