Abstract
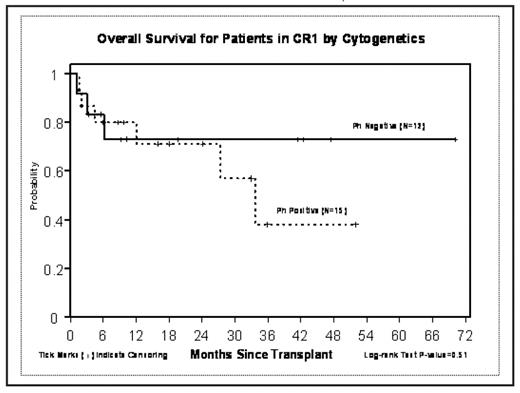
Although treatment outcomes for adult ALL remain stagnant at 35–40% 5 yr event free survival (EFS), allogeneic transplant particularly from unrelated donors has frequently been reserved for patient beyond first complete remission (CR1) with the exception of patients with a t(9;22) or Philadelphia (Ph+) cytogenetic abnormality. Between 4/1/2001 and 11/14/2007, 67 patients with ALL received URD-HCT; disease status at HCT was (CR1) in 27 pts (40%), CR2 in 20 pts (30%) and advanced disease (AD) which included induction failure, relapse or CR3 in 20 pts (30%). Age ranged from 2–58 yrs with median age of 29.5 yr. One third of the patients were Ph+. An additional 18 pts had high or very high risk cytogenetics abnormalities. The majority (≥75%) of Ph+ patients had received Gleevec prior to HCT. The conditioning regimen was FTBI 1320 Gy given as 11 fractions over four days followed by VP-16 60 mg/kg. Graft vs. host disease (GVHD) prophylaxis was tacrolimus (FK) and methotrexate (MTX) in 20 pts; sirolimus (Siro) was added to this regimen in 15 pts. Other GVHD regimens used were FK, MTX and Cellcept (12 pts) FK and siro (4 pts) and anti thymocyte globulin was added in 11 pts. Graft source was marrow in 12 pts and peripheral blood stem cells in 55 pts. Median time to ANC > 500 was 17 days (range 11–36d) and for platelets was 24 days (range 15–218d). Three patients died prior to day 18 of infectious complications and were considered unevaluable for engraftment. The incidence of acute GVHD Grade II-IV was 57% with Grade III-IV in 18%. Chronic GVHD occurred in 52% of 56 patients at risk. Treatment related mortality was 16% at 100 days and 28% at 1yr. At two years the event free survival (EFS) was 58% for CR1 pts vs 23% for CR2 and 17% for AD. Relapse rates were 18%, 24% and 40% respectively EFS was 62% at 2 yrs for Ph- pts and 50% for Ph+ in CR1 vs 19% for pt > CR1 who were Ph-and 40% for Ph+ (p=0.31). Remission status (CR1 vs > CR1) was a significant predictor of survival (p=0.01) and EFS (p=0.03) in multivariate analysis while age and Ph+ status had no impact on survival end points.
Conclusion: The preparative regimen of FTBI and VP16 offers 2 yr EFS rates of 50–60% in patients receiving URD-HCT in CR1, including patients with Ph+ and other high risk cytogenetic risk groups. These results are comparable to those reported with sibling donor transplants and provide a curative option for patients in CR1 lacking a family donor.
Disclosures: Snyder:Novartis and BMS: Honoraria, Speakers Bureau.
Author notes
Corresponding author