Abstract
Introduction: As patients with thalassemia major survive into middle age and beyond, there is greater need to address endocrine complications such as diabetes mellitus. Pancreatic iron deposition is common in thalassemia major patients and correlates with cardiac iron deposition. However, unlike the heart, endocrine dysfunction may not be completely reversible once functional assays become abnormal. Thus it is imperative to characterize preclinical endocrine markers similar to MRI measurements of cardiac T2*. Our aim of this study was to compare pancreatic R2* (the reciprocal of T2*) measurements with the results of an oral glucose tolerance test (OGTT) to determine the relationship between pancreatic iron and pancreatic dysfunction.
Methods: We recruited 19 patients with thalassemia major to participate in a frequently sampled OGTT. Results of the OGTT were graded on NIH criteria: a 2 hour glucose level less than 140 was considered normal; between 140 and 200, impaired glucose tolerance; and greater than 200, diabetes mellitus. Acute Insulin Response (AIR) ws estimated from the OGTT measurements using the technique of Stumvall, et al. [1]. Insulin sensitivity was derived from the reciprocal of HOMA Insulin Resistance metric [2]. Pancreatic, cardiac, and hepatic iron burdens were estimated from MRI iron assessments using previously validated techniques. Where serial MRI measurements were available, we used linear interpolation to approximate the organ iron burden at the time of the OGTT. The remaining 7 patients had an MRI within 0.4 ± 0.3 years of the OGTT. The mean age was 21.9 ± 9.6 years; the mean BMI was 21.7 ± 2.3. All patients were on chronic transfusions and on standard iron chelation for their iron overload. The average pancreas R2* was 292.4 ± 259.4 Hz, cardiac R2* was 84.9 ± 64.0 Hz, and hepatic iron concentration (HIC) was 10.2 ± 10.1 mg/g dry weight. Pancreatic R2* correlated with cardiac R2* (r = 0.61, P = 0.006) in this population.
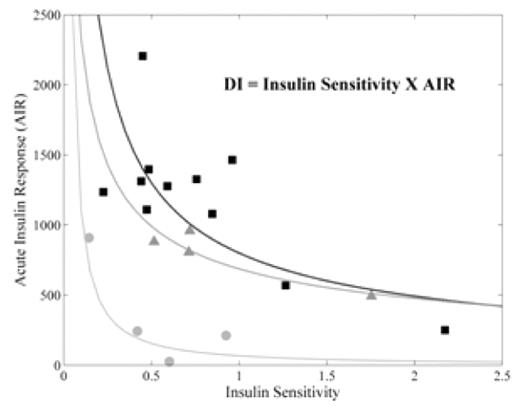
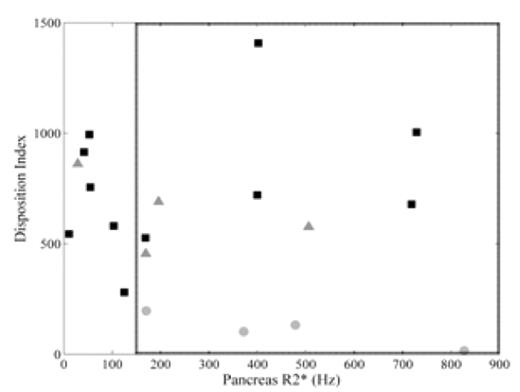
Results: Of the 19 patients enrolled, 4 had diabetes, 4 had impaired glucose tolerance, and the remaining 11 had normal glucose tolerance. Figure 1 shows the expected inverse relationship between AIR and insulin sensitivity for each patient. The product of these two variables is the disposition index (DI) and is used as a measure of beta-cell function. Corresponding log-log fits to the data are shown in the same color and represent isoclines of constant DI. The patients with diabetes mellitus (gray circles) lie on a DI curve closer to the origin than normal (black squares) and glucose intolerant patients (gray triangles); lower DI indicates decreased islet cell mass or function. The patients with impaired glucose tolerance lie on a curve at the lower limit of normal. Figure 2 shows the relationship between pancreatic R2* and DI. The dashed box encloses the upper two tertiles of pancreatic R2* (boundary at 150 Hz). In this group, 7/12 had either impaired glucose tolerance or diabetes mellitus. In contrast, only 1/7 with R2* < 150 Hz had impaired glucose tolerance (p=0.08 by Fisher’s exact test). Thus, 7/8 of the patients with impaired glucose tolerance or diabetes had moderate to severe pancreatic iron.
Discussion: These data confirm that pancreatic iron deposition and pancreatic dysfunction are common in patients with thalassemia major. However, the relationship between pancreatic iron and function is complicated because of other risk factors such as family history, age, body habitus, as well as the type and pattern of iron chelation therapy. Although most patients with pancreatic dysfunction had moderate to severe pancreatic iron, 5/12 patients with high pancreatic iron had normal OGTT results, not unlike the normal cardiac function observed in many patients with heavy cardiac iron burdens. Serial evaluation is necessary to see if these patients will develop glucose intolerance or diabetes prospectively. Despite the complicated relationship between pancreatic function and iron, increased pancreatic R2* appears to identify a higher risk population that would likely benefit from improved chelation and more frequent endocrine evaluation.
Disclosures: Wood:Novartis: Honoraria, Research Funding, Speakers Bureau; Apotex: Honoraria.
Acknowledgments: Cooley’s Anemia Foundation (Adult Translational Research Award), General Clinical Research Center at CHLA/USC (NIH #RR00043-43).
References:
Author notes
Corresponding author