Abstract
Introduction: Despite the availability of iron chelation therapy, accumulation of excess iron in the heart results in cardiomyopathy, congestive heart failure (CHF) and death in approximately 71% of transfused patients with β-thalassemia (β-thal) major. In preclinical and single-institution clinical studies, Exjade®(deferasirox, DFX) has demonstrated efficacy in decreasing cardiac iron. This ongoing study evaluates the effects of DFX on cardiac iron and left ventricular ejection fraction (LVEF) in patients (pts) with β-thal major in a prospective, single-arm, multi-center trial using cardiac MRI T2*. Here, we report preliminary results from patients who have completed 12 or 18 months of treatment.
Methods: Twenty-eight pts were enrolled at four US centers. DFX was administered at 30–40 mg/kg/day for 18 months. Entry criteria included MRI evidence of cardiac iron (T2* <20 ms) and normal LVEF (≥56%). Serum ferritin (SF) was assessed monthly and MRI assessments for liver iron concentration (LIC), cardiac T2* and LVEF were done every 6 months. Serum creatinine (SCr), biochemical and hematological status were also monitored. All results are reported as mean±SE (range) unless otherwise stated.
Results: At the time of analysis, 18 pts had 12-month evaluations and 12 pts had 18-month evaluations. Five pts discontinued (one non-compliance, two patient decisions, and two deaths). Both deaths were considered unrelated to DFX treatment; the first patient enrolled with markedly elevated baseline cardiac iron (T2*=1.8 ms) and died secondary to CHF. The second patient death was due to sepsis and multi-organ failure.
Baseline: All 18 evaluable pts (three male, 15 female; aged 10–44 years) received ≥150 lifetime transfusions. SF was 4324±912 ng/mL (395–16,249). Cardiac T2* was 9.6±0.97 ms (4.6–16.1), LIC was 18.7±3.8 mg Fe/g dry weight (dw; 3.6–62.3) and LVEF was 61.7±1.0%.
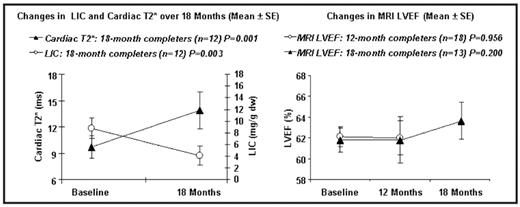
12-Month results: At 12 months, 7/18 pts were on 40 mg/kg/day. 12/18 pts (67%) had an increase in cardiac T2* with a mean difference of 2.2 ms (18%; P=0.025). 13/18 pts (72%) had a decrease in LIC with a mean difference of 2.4 mg Fe/g dw (25%; P=0.032). LVEF remained stable. SF fell by 583 ng/mL (n=18; 22%; P=0.147).
18-Month results: At 18 months, 3/12 pts were on 40 mg/kg/day. 10/12 pts (83%) had an increase in cardiac T2* with a mean difference of 4.1 ms (35%; P=0.001). 11/12 pts (92%) had a decrease in LIC with a mean reduction of 4.7 mg Fe/g dw (50%; P=0.003). Mean LVEF trended upward from 61.5 to 63.3% (n=13; P=0.2). SF fell by 1373 ng/mL (n=11; 46%, P=0.006).
Safety data from pts (n=25) treated with 30–40 mg DFX were in line with previous studies. The most common drug-related adverse events (AEs; eight pts; 32%) were gastrointestinal in nature. 1/25 patients experienced a suspected SAE (hospitalization due to abdominal pain and vomiting) but completed the study. One patient developed SCr >upper limit of normal (ULN). Two pts (8%) had abnormal transaminases (≥5×ULN) on ≥2 occasions but both had abnormal values at baseline.
Conclusions: DFX monotherapy significantly improved cardiac and liver iron after 12 and 18 months. Overall, doses from 30–40 mg/kg/day were well tolerated. Cardiac T2* improvement rates were 1.5–1.9% per month, which is comparable to other monotherapy trials. A trend towards improved LVEF was seen in patients completing 18 months of therapy; however, a larger, long-term study will be required to confirm whether DFX can significantly improve cardiac function in this population.
Disclosures: Wood:Apotex: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau. Thompson:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Paley:Novartis: Employment. Kang:Novartis: Employment. Giardina:Novartis: Research Funding, Speakers Bureau. Virkus:Novartis: Employment. Coates:Novartis: Honoraria, Research Funding, Speakers Bureau. Off Label Use: The use of deferasirox doses >30 mg/kg is considered off-label.
Author notes
Corresponding author