Abstract
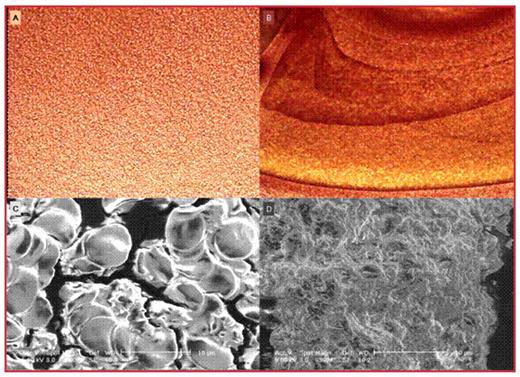
Ankaferd Blood Stopper (ABS) comprises a standardized mixture of the plants Thymus vulgaris, Glycyrrhiza glabra, Vitis vinifera, Alpinia officinarum and Urtica dioica. The basic mechanism of action for ABS is the formation of an encapsulated protein network that provides focal points for vital erythrocyte aggregation. ABS–induced protein network formation with blood cells particularly erythrocytes covers the primary and secondary haemostatic system without disturbing individual coagulation factors (Figure 1). The aim of this study is to perform functional proteomic analyses of ABS, which has been approved for the management of hemorrhages in Turkey (www.ankaferd.com). The protein samples for 2D gel electrophoresis were prepared: 10 ml of Ankaferd solution was precipitated with Trichloroacetic Acid Precipitation (TCA). 100 μl of 100% TCA. was added for each 1ml of sample. The total protein concentration was measured using the BCA protein assay Kit (Pierce, Rockford, USA). 2D Gel Electrophoresis of Proteins were performed. Gels were stained with SYPRO Ruby protein stain and images were acquired and analyzed using PDQuest software (Bio-Rad Laboratories, USA). Gel Digestion of Proteins: Selected spots from the gel were excised using a Proteome Works Spot Cutter (Bio-Rad Laboratories, USA) and transferred to a 96-well plate. The proteins were enzymatically digested and the tryptic peptides ZipTip (Millipore, France) purified. After ZipTip purification, the tryptic peptides were eluted from the ZipTip with 2 mg/ml cyano-4-hydroxycinnamic acid (CHCA) solution in 50% ACN/0.1% TriFlora acetic acid and spotted directly onto wax-coated matrix-assisted laser desorption/ionization (MALDI) target plates. Mass Spectrometry & Database Search: The tryptic peptides on the MALDI target plate were analyzed with MasLynx MALDI-time of flight mass spectrometer (Waters, UK). Mass spectra were recorded in the positive-ion mode. All spectra were acquiredwith external calibration of sub-P, anjiotensin, renine, ACTH and glu fib mix. PLGS (Waters, UK). Was optained with Swiss-Prot data base. With 50 ppm sensitivity. Proteins were evaluated by considering the number of matched tryptic peptides, the percentage coverage of the entire protein sequence, the apparent MW, and the pI of the protein.
Results: Proteins of plant origin in Ankaferd were NADP-dependent malic enzyme, Ribulose bisphosphate carboxylase large chain, Mturase K, ATP synthase subunit beta, ATP synthase subunit alpha, Chalcone-flavonone isomerase 1, Chalcone-flavonone isomerase 2, and Actin-depolymerizing factor. Furthermore, functional proteomics studies revealed that proteins resembling human peptides have been detected within Ankaferd including ATP synthase, mucin 16 (CD164 sialomucin-like 2 protein), coiled-coil domain containing 141 hypothetical protein LOC283638 isoform 1, hypothetical protein LOC283638 isoform 2, dynactin 5, Complex I intermediate-associated protein 30, mitochondrial, NADH dehydrogenase (Ubiquinone) 1 alpha subcomplex, TP synthase, H+ transporting, mitochondrial actin binding 1 isoform, LIM domain and actin binding 1 isoform a, LIM domain and actin binding 1 isoform b, Spectrin alpha non erythrocytic 1, Prolactin releasing hormone receptor, Utrophin, tet oncogene family member 2 isoform b, Protein phosphatase 1 regulatory subunit 12A, NIMA (never in mitosis gene a)-related kinas, ATP-binding cassette protein C12, Homo sapiens malic enzyme 1, Mitochondrial NADP(+)-dependent malic enzyme 3, ME2 protein, Nuclear factor 1 B-type, Abhydrolase domain-containing protein 12B, E3 SUMO-protein ligase PIAS2, Alpha-1,2-glucosyltransferase ALG10-A, Cofilin, non-muscle isoform, 18 kDa phosphoprotein, p18, Actin-depolymerizing factor, ADF, Twinfilin-1, Ankyrin repeat and FYVE domain-containing protein 1, Usherin Precursor, Urotensin II receptor. Those proteins represent a true basis for the upcoming Ankaferd studies focusing on its wound healing hemostatic, anti-infective, preservative biological actions.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author