Abstract
Background: A randomized Phase 3 trial was performed to compare the efficacy and safety of fludarabine/cyclophosphamide (Flu/Cy) combined with oblimersen (OBL; Genasense®; G3139) or administered alone in patients (pts) with relapsed or refractory CLL. The primary objective was to compare the proportion of pts who achieved complete response (CR, including nodular partial response) between treatment arms.
Methods: Pts (N = 241) were stratified according to response to prior fludarabine therapy (relapsed [sensitive] versus [v] refractory); number of prior regimens (1 to 2 v 3 or more), and duration of response to last therapy (> 6 months v ≤ 6 months) and randomly assigned (1:1) to treatment arms. Treatment included up to six 28-day cycles of OBL 3 mg/kg/d as a 7-day continuous IV infusion and Flu 25 mg/m2/d IV over 20 to 30 minutes followed by Cy 250 mg/m2/d IV over 30 to 60 minutes on days 5, 6, and 7, or the Flu/Cy regimen alone on days 1, 2, and 3. Response was determined according to National Cancer Institute-Sponsored Working Group guidelines (Cheson et al, J Clin Oncol, 1996), based on determinations of an independent hematopathologist and expert hematologist who were blinded to treatment. Univariate and multivariate analyses of protocol-specified prognostic factors for overall survival were performed. Five-year survival data were recently collected.
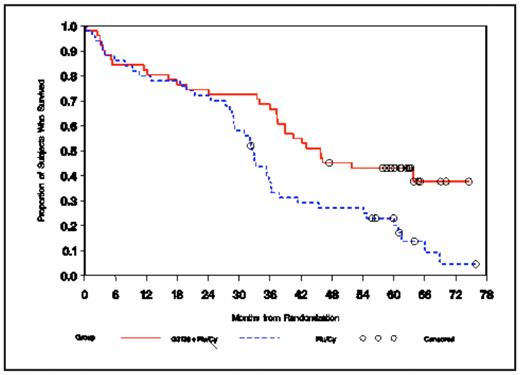
Results: The primary endpoint was met; the CR rate was 17% in the OBL + Flu/Cy arm and 7% in the Flu/Cy arm (p = 0.025). Only 7 subjects were lost to 5-year follow-up. Univariate analyses confirmed the prognostic value of the trial stratification factors for survival. In addition, baseline beta-2 microglobulin (< 4 mg/L v ≥ 4 mg/L) and baseline serum LDH (< ULN v ≥ ULN) were highly prognostic (p< 0.0001). Multivariate analyses showed that the number of prior regimens, age (< 65 years v ≥ 65 years), baseline beta-2 microglobulin (< 4 mg/L v ≥ 4 mg/L), and baseline serum LDH (< ULN v ≥ ULN) were prognostic for survival. Among all prognostic factors examined in multivariate analyses, a significant interaction with treatment was detected only for fludarabine sensitivity. Maximum benefit with OBL was observed in fludarabine-sensitive pts (OBL + Flu/Cy arm, 51 pts; Flu/Cy arm, 50 pts), ie, those who had a partial response or better for ≥ 6 months after their last prior fludarabine-containing therapy and then relapsed. The median age of fludarabine-sensitive pts was 64.0 years in both arms; 80% and 70% of these pts were men in the OBL + Flu/Cy arm and the Flu/Cy arm, respectively. Disease-related findings, prognostic factors, and prior treatments among fludarabine-sensitive pts were similar in the 2 treatment arms. The proportion of fludarabine-sensitive pts with CR was significantly greater in the OBL + Flu/Cy arm (25%) than in the Flu/Cy arm (6%; p = 0.016). With 5-year follow-up, there was a statistically significant survival benefit in the OBL + Flu/Cy arm among fludarabine-sensitive pts (hazard ratio = 0.50; p = 0.004; see figure).
Conclusions: In the setting of relapsed/refractory CLL in a randomized Phase III trial, the number of prior regimens, age, beta-2 microglobulin, and serum LDH were prognostic for survival based on multivariate analyses. Fludarabine sensitivity was predictive of the treatment effect of the OBL + Flu/Cy regimen, and a 50% reduction in the risk of death was observed among fludarabine-sensitive pts treated with OBL in addition to a standard Flu/Cy regimen.
Disclosures: O’Brien:Genta Inc.: Consultancy. Wu:Genta Inc.: Employment, Equity Ownership. Novick:Genta Inc.: Employment, Equity Ownership.
Author notes
Corresponding author