Abstract
Glanzmann thrombasthenia (GT) is caused by genetic defects in the ITGA2B and ITGB3 genes that encode the proteins of the αIIbβ3 complex on the surface of the platelet. The αIIbβ3 complex on activated platelets acts a receptor principally for fibrinogen but can also bind fibronectin, vonWillebrand factor, vitronectin and CD40 ligand under conditions of high flow. The binding of these proteins results in the formation of protein bridges and aggregation of platelets. Patients with GT have deficiency of platelet surface αIIbβ3 resulting in insufficient platelet spreading and aggregation and resultant bleeding. Conventional platelet aggregation studies demonstrate aggregation with ristocetin but absence of aggregation with other platelet agonists including thrombin, collagen, arachadonic acid and epinephrine. Platelet immunophenotyping demonstrates absent (Type I) or diminished (Type II) surface expression of CD41 (Gp IIb) and CD61 (Gp IIIa). Bleeding phenotype in individual patients can be quite variable and is not well predicted by surface expression or platelet aggregation studies.
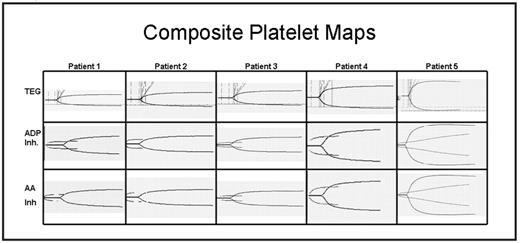
Thromboelastogram platelet mapping (TEG-PM) is a newer technology that employs antagonists of coagulation cascade meditated thrombosis in concert with platelet agonists to determine the level of platelet inhibition. Here we have examined the bleeding phenotype (Table I) of 5 GT patients, from three separate families, followed at our center in relation to conventional platelet aggregation studies, platelet immunophenotype and TEG-PM (Figure I). Three of the patients are from a single kindred and have had frequent severe bleeding episodes requiring treatment with activated FVII, transfused platelets and transfusion support for blood loss. The fourth patient has had an intermediate bleeding phenotype. The fifth patient has had only a single severe bleeding episode after nasal surgery and an otherwise mild bleeding phenotype including a caesarian section with a single platelet transfusion and no bleeding. The fifth patient displayed reduced CD41 and CD61 platelet expression (type II GT) while the other 4 patients have no CD41 and CD61 platelet expression (type I GT) by flow cytometry. While all 5 patients have similar, classic platelet aggregation studies the TEG-PM accurately predicted the bleeding phenotype. We believe TEG-PM may be a useful tool for the management of patients with GT.
Table I
| . | Patient I . | Patient II . | Patient III . | Patient IV . | Patient V . |
|---|---|---|---|---|---|
| Age | 4 years | 10 years | 20 years | 2 years | 29 years |
| Sex | Female | Female | Male | Female | Female |
| # of PRBC Transfusion | 0 | 2 | 0 | 3 | 0 |
| Hospitilizations | 2 | 4 | 7 | 4 | 1 |
| FVIIa days | 69 | 47 | 27 | 17 | 0 |
| Platelet transfusions | 9 | 3 | 13 | 0 | 1 |
| PFA Epi | >262 s | - | - | >196 s | 259 s |
| PFA ADP | 272 s | - | - | >210 s | 173 s |
| TEG K | - | - | - | 7.8 min | 2.2 min |
| TEG MA | 13.6 | 13.4 | 16.4 | 22.8 | 65.9 |
| Platelet Count | 83–439K | 91–364K | 76–449K | ||
| TEG ADP Inh | 89.3% | 100% | 83.2% | 100% | 6.8% |
| TEG AA Inh | 98.7% | 93.2% | 89.7% | 100% | 68.6% |
| . | Patient I . | Patient II . | Patient III . | Patient IV . | Patient V . |
|---|---|---|---|---|---|
| Age | 4 years | 10 years | 20 years | 2 years | 29 years |
| Sex | Female | Female | Male | Female | Female |
| # of PRBC Transfusion | 0 | 2 | 0 | 3 | 0 |
| Hospitilizations | 2 | 4 | 7 | 4 | 1 |
| FVIIa days | 69 | 47 | 27 | 17 | 0 |
| Platelet transfusions | 9 | 3 | 13 | 0 | 1 |
| PFA Epi | >262 s | - | - | >196 s | 259 s |
| PFA ADP | 272 s | - | - | >210 s | 173 s |
| TEG K | - | - | - | 7.8 min | 2.2 min |
| TEG MA | 13.6 | 13.4 | 16.4 | 22.8 | 65.9 |
| Platelet Count | 83–439K | 91–364K | 76–449K | ||
| TEG ADP Inh | 89.3% | 100% | 83.2% | 100% | 6.8% |
| TEG AA Inh | 98.7% | 93.2% | 89.7% | 100% | 68.6% |
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author