Abstract
Vaso-occlusion in sickle cell disease is fundamentally biophysical in nature, involving a complex set of cellular interactions. Originally attributed solely to the entrapment of abnormally rigid sickle red cells (RBCs) in the microcirculation, this process is now known to involve the decreased deformability of white blood cells (WBCs) and increased endothelial adhesion to different cell types (sickle RBCs, reticulocytes, WBCs, platelets). These biophysical interactions, which are also mediated biochemically by a variety of soluble factors (coagulation proteins, inflammatory mediators, reactive oxygen species, free hemoglobin, etc.), then ultimately lead to microvascular obstruction. However, in vitro experimental approaches that measure the vaso-occlusive properties of sickle blood cells have been unable to separate the contributions of decreased cell deformability and cell-cell adhesion in a single assay. Historically, cell deformability is measured using techniques such as micropipette aspiration, micropore filtration, and ektacytometry, whereas adhesive interactions between the endothelium, blood cells, and soluble factors are assessed using endothelial-lined flow chamber assays. No existing technique effectively evaluates both cell deformability and cell adhesion simultaneously, which is required to comprehensively study sickle cell vaso-occlusion.
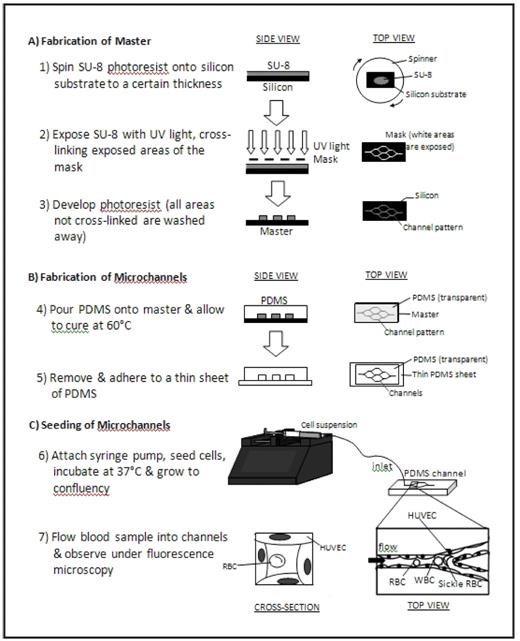
In the current study, we present an “endothelialized” microfluidic system that simultaneously integrates cell deformability and cell adhesion under physiologic microvascular flow conditions to investigate the underlying biophysical mechanisms of sickle cell vaso-occlusion. Briefly, a layer of human umbilical vein endothelial cells (HUVECs) was cultured along the inner walls of microfabricated microchannels made of the biocompatible and optically transparent polymer polydimethylsiloxane, or PDMS (Figure). Standard lab-on-chip photolithography techniques were utilized to design and create the microchannels, which geometrically emulate a branching microvasculature network with the smallest lumen size approximately 15 μm in diameter. Then, the inner lining of the microchannels were coated with fibronectin and seeded with HUVECs, which grew to a confluent monolayer within 4–5 days and encompassed the entire inner surface in all three dimensions.
Whole blood or cell suspensions can then be perfused into the system, and cells can be visualized under flow using standard immunofluorescence microscopy techniques, which allows for identification of specific cellular subpopulations. Pressure and flow rate can be tightly controlled, and cell transit time, time to cell aggregation or obstruction of the system can be recorded using automated image processing software. The effect of different cell types and biologic modifiers (i.e. cytokines, coagulation factors, etc) on in vitro vaso-occlusion can be systematically analyzed and quantified. Furthermore, adhesion-blocking antibodies and drugs that increase cell deformability can be used to quantify the vaso-occlusive effect of adhesion versus cell rigidity. As this system physically constrains cells three-dimensionally in lumens the size of the human microvasculature, physical cellular interactions that are likely to occur in vivo and the endothelial cell response to these processes can also be directly evaluated with live cell fluorescence, immunofluorescence or western blotting.
Overall, this system will provide a quantitative and controlled approach to investigate the underlying complex biophysical processes that govern sickle cell vaso-occlusion. In addition, this platform will also serve as a useful complimentary technology to in vivo experiments using sickle cell mouse models.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author