Abstract
Lipid encapsulation of chemotherapeutic agents facilitates local delivery of highly concentrated antineoplastics to tumors while ensuring an adequate duration of drug exposure and reducing systemic toxicity. Using novel technology, we have encapsulated nanoparticulate (100 nM) arsenic trioxide (ATO) at high density in “nanobins,” biocompatible lipids and polymers that undergo a change in permeability when exposed to intracellular conditions, releasing drug cargo into the acidic tumor microenvironment (
J. Am. Chem. Soc. 2006, 128: 13348-9
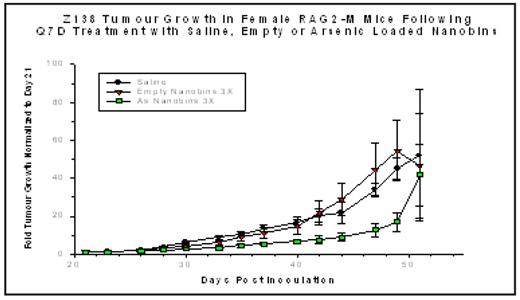
). At low concentrations, ATO is an effective agent in the treatment of acute promyelocytic leukemia, causing differentiation and turnover of PML. At higher but potentially toxic concentrations, ATO induces programmed cell death through the generation of reactive oxygen species and its effects on other proapoptotic signaling pathways. A preliminary dose-range finding study was completed comparing the toxicity profiles of free ATO and nanobin formulations of arsenic based on loading procedures using either cobalt (Co) or nickel (Ni). Female Balb/c mice were injected intraperitoneally with either a single dose of free or nanobin formulations of ATO at 20, 40, 60, 100 or 200 μmol/kg of elemental As. Mice were assessed daily for the first 5 days after injection and then every second day thereafter for a total study duration of 14 days and monitored for body weight and clinical signs. The maximum tolerated doses for both the Co- and Ni-ATO formulations were approximately 100 – 200 μmol/kg as a single intraperitoneal injection. Efficacy experiments for the Ni-ATO-nanobins were conducted in a murine (RAG2-M) xenograft model in which the mantle cell lymphoma cell line Z138C was injected subcutaneously. On day 21, when the average tumor volume was 50–100 mg, weekly injections of saline, empty nanobins, or the Ni-ATO nanobins at 60 μmol/kg were begun. Cohorts of mice received either two or three weekly injections. Mice bearing tumors of 1000 mm3 or greater were sacrificed. Results were normalized to the tumor size at the time that treatment was initiated (day 21) and are represented as fold-increase in tumor measured with calipers three times weekly. The results of the three weekly injections are illustrated below. Following two injections, the curves began to separate showing a significant inhibition of tumor growth that persists for two weeks beyond the last injection (p < 0.001). Two weekly injections also impacted tumor growth significantly (p < 0.01; data not shown). These results provide rationale for further preclinical investigation of this novel therapeutic.Disclosures: O’Halloran:Northwestern University: Patents & Royalties.
Author notes
Corresponding author
2008, The American Society of Hematology
2008