Abstract
Background: Outcome of patients with Multiple Myeloma (MM) is influenced by a variety of baseline prognostic factors, most importantly presence of chromosomal abnormalities. Nevertheless, these biologically determined risk-factors do not account for a significant subset of patients whose outcome varies greatly from what one expects based on the underlying prognostic characteristics. We have previously demonstrated that outcome in myeloma is dictated not only by baseline risk-factors but also response to therapy (R2, risk and response dependent outcome). We studied response duration following initial therapy as a prognostic factor for long-term overall survival in myeloma in three ECOG clinical trials that enrolled patients with newly diagnosed myeloma.
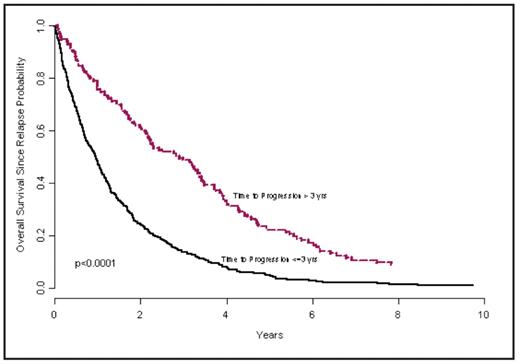
Methods: Data from three studies with long-term, mature follow up were analyzed: E9486, E5A93, and E1A00. The regimens used in these studies included Thalidomide/Dex, Dex, VBMCP, VBMCP + alpha-IFN, and VBMCP + Cyclophosphamide. Among progressed/relapsed patients, the association between time to progression and overall survival since relapse was explored using Cox proportional hazards model, in which time to progression was analyzed as a continuous variable. In addition, the association between response duration and overall survival since relapse was explored similarly among relapsed responders. Treatment effect and calendar time of diagnosis have been adjusted in the model. Also, to determine the best cut off point associated with the best differentiation of patients with short OS after relapse, an outcome-oriented method using log likelihood value was applied. Results: Among the 1136 patients enrolled in the three studies, 625 patients who have documented disease relapse have been included in the current study. Of these, 471 patients had responded to the treatment before relapse. Only 33 patients are alive as of Dec., 2007. For all 625 patients, the time to progression (continuous variable) was significantly associated with overall survival from the time of relapse/progression (P<0.0001), with hazard ratio of 0.77 (95%CI: 0.72~0.81) at time to progression increasing by 1 year (i.e., the later the progression/relapse, the lower the risk). The best cutoff point of the time to progression is 3 yrs (the 75th percentile). The hazard ratio of patients progressing within 3 yrs since randomization vs. after 3 yrs is 2.4 (95% CI: 1.9 ~ 2.9) and P-value<0.0001. The median OS from relapse was 0.96 years for the former vs. 2.43 years in the latter group (Figure). Among the 471 relapsed responders, the response duration (continuous variable) was also significantly associated with overall survival since relapse (P<0.0001), with hazard ratio of 0.76 (95%CI: 0.70~0.81) at response duration increasing by 1 year (i.e., the longer the response duration, the lower the risk). The effects seen with each regimen are detailed in the table. The best cutoff point of the response duration is 2 yrs (median). The hazard ratio of patients with response duration less than or equal to 2 yrs vs. more than 2 yrs is 2.1 (95% CI: 1.7 ~ 2.6) and P-value<0.0001.
Conclusions: Among patients treated with alkylator based therapy, the duration of response from the initial therapy is an important predictor of the subsequent outcome among patients with myeloma. Given the heterogeneity of the disease and imprecise predictive ability of the known baseline prognostic factors, the duration of response to initial therapy should be taken into account, to deliver risk and response-adapted therapy. The prognostic value of this feature across different clinical trials, points towards the role of underlying disease biology, both disease aggressiveness and ability to rapidly develop resistance to therapies as two important determinants of outcome.
Table 1: Hazard ratio of overall survival since relapse for patients progressed within 3 yrs since randomization vs. after 3 yrs in each treatment arm Treatment arm
| . | Treatment arm . | HR (95% CI) . | P-value . |
|---|---|---|---|
| TTP<=3yrs vs. >3 yrs | Thal/Dex | N/A | N/A |
| Dex | 0.4 (0.05, 3.9) | 0.46 | |
| VBMCP(E5A93+ E9486) | 1.9 (1.4, 2.7) | 0.0001 | |
| VBMCP+alpha2IFN (E5A93+ E9486) | 3.0 (2.2, 4.1) | <0.0001 | |
| VBMCP+CYCLOPHOSPHAMIDE | 2.3 (1.5, 3.6) | 0.0001 | |
| Response duration <=2 yrs vs. >2 yrs | Thal/Dex | 0.2 (0.003, 18.5) | 0.51 |
| Dex | 0.15 (0.02, 0.98) | 0.048 | |
| VBMCP(E5A93+ E9486) | 1.7 (1.2, 2.3) | 0.0009 | |
| VBMCP+alpha2IFN (E5A93+ E9486) | 3.0(2.1, 4.3) | <0.0001 | |
| VBMCP+CYCLOPHOSPHAMIDE | 2.1 (1.4, 3.3) | 0.006 |
| . | Treatment arm . | HR (95% CI) . | P-value . |
|---|---|---|---|
| TTP<=3yrs vs. >3 yrs | Thal/Dex | N/A | N/A |
| Dex | 0.4 (0.05, 3.9) | 0.46 | |
| VBMCP(E5A93+ E9486) | 1.9 (1.4, 2.7) | 0.0001 | |
| VBMCP+alpha2IFN (E5A93+ E9486) | 3.0 (2.2, 4.1) | <0.0001 | |
| VBMCP+CYCLOPHOSPHAMIDE | 2.3 (1.5, 3.6) | 0.0001 | |
| Response duration <=2 yrs vs. >2 yrs | Thal/Dex | 0.2 (0.003, 18.5) | 0.51 |
| Dex | 0.15 (0.02, 0.98) | 0.048 | |
| VBMCP(E5A93+ E9486) | 1.7 (1.2, 2.3) | 0.0009 | |
| VBMCP+alpha2IFN (E5A93+ E9486) | 3.0(2.1, 4.3) | <0.0001 | |
| VBMCP+CYCLOPHOSPHAMIDE | 2.1 (1.4, 3.3) | 0.006 |
Overall survival since relapse between patients progressed within 3 yrs since randomization vs. after 3 yrs.
Overall survival since relapse between patients progressed within 3 yrs since randomization vs. after 3 yrs.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author