Abstract
Survival of patients with primary myelofibrosis (PMF) is highly variable, ranging from months to even decades. Although treatment of PMF remains mostly palliative, the increasing use of allogeneic hemopoietic stem cell transplantation and introduction of newer therapies has raised the need for a better prognostic stratification of these patients.
The initial features of 1,054 patients (638M/416F; median age 64 years, range 10–90) consecutively diagnosed with PMF in seven institutions from Europe and USA between 1980 and 2007 were evaluated for prognostic significance. Patients with the so-called “pre-fibrotic” form of PMF were not considered. The variables assessed were those previously shown to be of prognostic value in PMF, those clinically meaningful, and possible confounders (namely, diagnostic period and series of origin). The effect of the potential prognostic factors on survival was evaluated by the Cox proportional hazards regression and the proportional hazards assumption checked in every model by graphical methods and the Grambsch-Therneau test. A prognostic model was identified through a stepwise selection process, based on a z-test of the regression coefficients, and evaluated by calculating its discriminating power (measured by Harrell’s C concordance index), as compared to that of current PMF prognostic systems, and its positive predictive accuracy for actual survival longer (or shorter) than definite periods of time from diagnosis. The replicability of both the prognostic variables and the new prognostic score was tested by bootstrap re-sampling, in which 1,000 samples of the same size than the original series were built by random extraction with reposition.
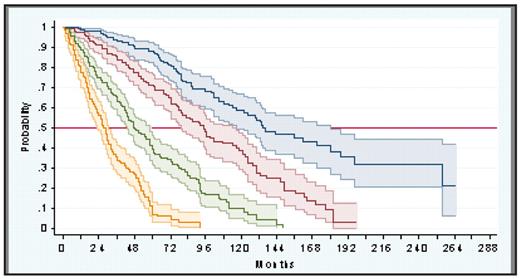
Median survival of the overall series was 69 months (95% CI: 61–76), with 49% of patients having died at the time of study. At multivariate analysis, five initial variables were associated with shorter survival: age > 65 years, constitutional symptoms (weight loss, fever, sweats), Hb < 10 g/dL, leukocyte count > 25×109/L, and blood blasts 1%. The hazard ratios of these variables did not differ substantially, ranging from 2.89 (95% CI: 2.46–3.61) for Hb < 10 g/dL to 1.80 (95% CI: 1.50–2.17) for blood blasts 1% and, therefore, one point was assigned to each one. Based on this, among the 1,001 patients of the series with all the necessary data available, four risk groups were identified with no overlapping in the 95% CI of their survival curves (figure): low risk (no factor, 22% of patients; median survival 135 months); intermediate risk-1 (one factor, 29% of patients; median survival 95 months); intermediate risk-2 (two factors; 28% of patients; median survival 48 months); and high risk (three factors, 21% of patients; median survival 27 months). The new prognostic score showed high predictive accuracy and replicability and had higher discriminating power than current risk-based PMF classifications. In the 409 patients with assessable metaphases, presence of cytogenetic abnormalities (observed in 30% of cases) was associated with shorter survival and contributed to prognosis, but only in patients in the intermediate-risk groups. No prognostic influence for either JAK2 V617F mutational status (n= 345) or blood CD34+ cell count (n= 150) was noted. This new prognostic scoring system substantially improves existing risk-based classifications of PMF and may help in treatment-decision making and in the design and analysis of newer therapies for the disease.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author