Type and duration of anticoagulation is still matter of debate in cancer patients with acute Deep Vein Thrombosis (DVT) of the lower limbs. Residual Vein Thrombosis (RVT) has been proven to be effective for assessing the optimal duration of oral anticoagulants in non cancer patients (
Materials and Methods. Cancer patients with a first episode of DVT were treated with LMWH at therapeutic dosage for 1 month followed by dose reduction of 25% in the next 5 months. At this time, they were managed according to RVT findings: those with RVT were randomized to continue anticoagulants for 6 additional months (Group A1) or to stop (Group A2), while patients without RVT stopped LMWH (Group B). Outcomes were recurrent venous thromboembolism and/or major bleeding.
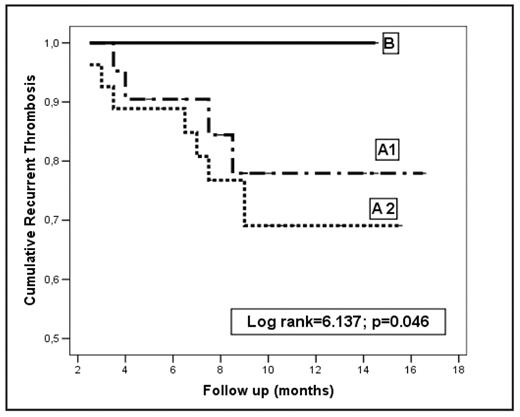
Results. Over a period of 18 months, 134 patients were evaluated across 12 centers in Italy; clinical characteristics and duration of follow-up are reported in the Table 1. RVT was detected in 92 (68.6%) patients; recurrent events occurred in 23.4% of those who discontinued and 15.5% of those who continued LMWH (Table 2 and Figure 1). The adjusted Hazard Ratio (HR) for age and sex (Group A2 vs A1) was 1.58 (95% confidence interval [CI], 0.85–2.93; P = .145). Of the 42 (31.3%) patients without RVT, one had a recurrence (2.3%) (Table 2 and Figure 1). The adjusted HR (B vs A1) was 4.54 (CI 2.3–6.66; P =.028). One major bleeding event occurred in each group of patients who stopped (Group A2 and B) and 2 in those who continued anticoagulation (Table 2). Overall, 31 (23.1%) patients died due to cancer progression after a median follow-up of 13.2 months after randomization.
Conclusions. The Cancer DACUS is the first study evaluating an individual marker for assessing duration of anticoagulation in active cancer population. This interim analysis shows that absence of RVT identifies a group of patients at low risk for recurrent thrombosis who can safely stop LMWH after 6 months.
Kaplan-Meyer curve for recurrent events among groups
Table 1. Baseline patients characteristics
| . | Group A 1 (n.45) . | Group A 2 (n.47) . | Group B (n.42) . | P value* . |
|---|---|---|---|---|
| *P value refers to chi-squared test unless specified. | ||||
| ^Time from randomization (6 months after the index Deep Vein Thrombosis) | ||||
| #Active cancer at the time of diagnosis | ||||
| Female sex (%) | 22 (48.8) | 23 (48.9) | 20 (47.6) | 0.999 |
| Age, mean + SD (y) | 58.2 ± 12.2 | 63.7 ± 11.1 | 57.8 ± 11.6 | 0.124 |
| Total duration of follow-up, (y)^ | 31.5 | 32.8 | 29.9 | 0.146 |
| Mean follow-up, (y)^ | 1.2 ± 0.56 | 1.1 ± 0.53 | 1.1 ± 0.60 | 0.156 |
| #Type of cancer: | ||||
| Gastrointestinal, n (%) | 16 (35.5) | 18 (38.3) | 16 (38) | 0.32 |
| Genitourinary, n (%) | 9 (20) 7 | 8 (17) | 7 (16.6) | 0.23 |
| Breast, n (%) | (15.5) 8 | 6 (12.7) | 6 (14.2) | 0.45 |
| Lung, n (%) | (17.7) 5 | 10 (21.2) | 10 (23.8) | 0.32 |
| Haematologic, n (%) | (11.1) | 5 (10.6) | 6 (14.2) | 0.67 |
| . | Group A 1 (n.45) . | Group A 2 (n.47) . | Group B (n.42) . | P value* . |
|---|---|---|---|---|
| *P value refers to chi-squared test unless specified. | ||||
| ^Time from randomization (6 months after the index Deep Vein Thrombosis) | ||||
| #Active cancer at the time of diagnosis | ||||
| Female sex (%) | 22 (48.8) | 23 (48.9) | 20 (47.6) | 0.999 |
| Age, mean + SD (y) | 58.2 ± 12.2 | 63.7 ± 11.1 | 57.8 ± 11.6 | 0.124 |
| Total duration of follow-up, (y)^ | 31.5 | 32.8 | 29.9 | 0.146 |
| Mean follow-up, (y)^ | 1.2 ± 0.56 | 1.1 ± 0.53 | 1.1 ± 0.60 | 0.156 |
| #Type of cancer: | ||||
| Gastrointestinal, n (%) | 16 (35.5) | 18 (38.3) | 16 (38) | 0.32 |
| Genitourinary, n (%) | 9 (20) 7 | 8 (17) | 7 (16.6) | 0.23 |
| Breast, n (%) | (15.5) 8 | 6 (12.7) | 6 (14.2) | 0.45 |
| Lung, n (%) | (17.7) 5 | 10 (21.2) | 10 (23.8) | 0.32 |
| Haematologic, n (%) | (11.1) | 5 (10.6) | 6 (14.2) | 0.67 |
Table 2. Study Outcomes
| Outcomes . | Group A 1 (n.45) . | Group A 2 (n.47) . | Group B (n.42) . | P value* . |
|---|---|---|---|---|
| * P value refers to chi-squared test unless specified. | ||||
| ** P value refers to Fisher exact test | ||||
| 0.021 | ||||
| 0.030 A1 vs B** | ||||
| Recurrences, n/total (%) | 7/45 (15.5) | 11/47(23.4) | 1/42 (2.3) | 0.010 A2 vs B** |
| 0.733 A1 vs A2** | ||||
| Recurrences, n/100 person-year (%) | 7/34.75 (20.1) | 11/40.33 (27.3) | 1/38.92 (2.5) | 0.008 |
| Type of recurrent VTE | ||||
| DVT | 5 | 8 | 1 | |
| DVT + PE | 2 | 2 | 0 | |
| Isolated PE | 0 | 1 | 0 | |
| Subtype Controlateral | 1 | 2 | 1 | |
| Major bleeding, n/total (%) | 2/45 (4.4) | 1/47(2.1) | 1/42(2.3) | 0.054** |
| Major bleeding n/100 person-yr(%) | 2/40.17 (4.9) | 1/46.83 (2.1) | 1/36.75 (2.7) | 0.390 |
| Outcomes . | Group A 1 (n.45) . | Group A 2 (n.47) . | Group B (n.42) . | P value* . |
|---|---|---|---|---|
| * P value refers to chi-squared test unless specified. | ||||
| ** P value refers to Fisher exact test | ||||
| 0.021 | ||||
| 0.030 A1 vs B** | ||||
| Recurrences, n/total (%) | 7/45 (15.5) | 11/47(23.4) | 1/42 (2.3) | 0.010 A2 vs B** |
| 0.733 A1 vs A2** | ||||
| Recurrences, n/100 person-year (%) | 7/34.75 (20.1) | 11/40.33 (27.3) | 1/38.92 (2.5) | 0.008 |
| Type of recurrent VTE | ||||
| DVT | 5 | 8 | 1 | |
| DVT + PE | 2 | 2 | 0 | |
| Isolated PE | 0 | 1 | 0 | |
| Subtype Controlateral | 1 | 2 | 1 | |
| Major bleeding, n/total (%) | 2/45 (4.4) | 1/47(2.1) | 1/42(2.3) | 0.054** |
| Major bleeding n/100 person-yr(%) | 2/40.17 (4.9) | 1/46.83 (2.1) | 1/36.75 (2.7) | 0.390 |
Disclosures: No relevant conflicts of interest to declare.