By studying hematopoiesis in human FLs, 2 independent studies by Tunstall-Pedoe et al and Chou and colleagues have identified important clues to explain the high incidence of megakaryocytic leukemias in infants with DS.
Approximately 5% of children with Down syndrome (DS) are born with a unique transient clonal megakariyo-erythroblastic proliferation disorder often called transient myeloproliferative disorder (TMD). Spontaneous recovery usually occurs within up to several months. However, about one-fifth of these patients will develop full-blown acute leukemia with biphenotypic megakaryocytic-erythroid features (AMKL) during their first 4 years of life. Somatic mutations in the transcription factor GATA1 are present in most patients with DS-TMD and AMKL.1-3 These mutations invariably result in the expression of a shorter isoform, GATA1s. The expression of this isoform is thought to enhance the proliferation and reduce the differentiation of fetal liver (FL) megakaryoblastic progenitors.4 How cT21 synergizes with mutated GATA1 in initiation of this common and generally reversible congenital clonal hematopoietic proliferation syndrome has been a mystery.
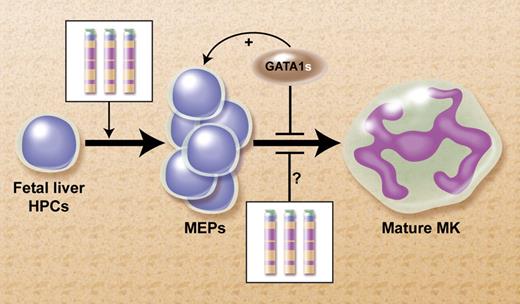
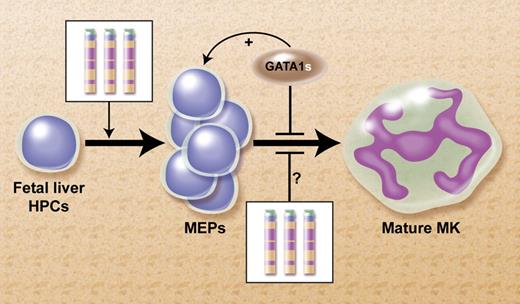
We have previously proposed a model to explain this collaboration between cT21 and GATA1s, as shown in the figure.5 We suggested that increased expression of several human chromosome 21 (Hsa21) genes in FL hematopoietic progenitor cells (HPCs) that normally regulate megakaryopoiesis (eg, ERG, ETS-2, RUNX1, BACH-1, and others) tilts the balance of fetal hematopoeisis toward the megakaryocytic lineage. Similar to a traffic accident during morning rush hour, an acquired mutation in GATA1 blocks further differentiation and induces a clonal “pile-up” of megakaryocytic precursors manifested at birth as TMD. This model predicts the presence of markedly enhanced megakaryopoiesis preceding the occurrence of GATA1s mutation in human livers from DS fetuses.
Proposed model for the mechanism for the leukemogenic cooperation between constitutional trisomy 21 (cT21) and GATA1s mutations in the megakaryoblastic leukemias of DS.5 Increased expression of genes from cT21 in fetal liver HPCs tilt the normal hematopoiesis toward increased production of megakaryocytic erythroid progenitors (MEPs). It's also possible that cT21 blocks terminal megakaryocytic (MK) differentiation. The somatically-acquired GATA1s mutation further blocks MK differentiation and enhances the proliferation of the MEPs. Professional illustration by Alice Y. Chen.
Proposed model for the mechanism for the leukemogenic cooperation between constitutional trisomy 21 (cT21) and GATA1s mutations in the megakaryoblastic leukemias of DS.5 Increased expression of genes from cT21 in fetal liver HPCs tilt the normal hematopoiesis toward increased production of megakaryocytic erythroid progenitors (MEPs). It's also possible that cT21 blocks terminal megakaryocytic (MK) differentiation. The somatically-acquired GATA1s mutation further blocks MK differentiation and enhances the proliferation of the MEPs. Professional illustration by Alice Y. Chen.
The 2 new studies by Tunstall-Pedoe et al and Chou and colleagues confirm that prediction. They compared second-trimester FL hematopoiesis in DS without GATA1 mutations to gestation-matched healthy controls. Strikingly, more than half of the HPCs in DS FLs were megakaryocyte-erythroid progenitors, 3 times more than in disomic FLs. In addition to the developmental shift toward the megakaryocytic-erythroid lineage, there was marked increase in proliferation and self-renewal of these progenitors.
Tunstall-Pedoe et al have also found that all these abnormalities were absent in bone marrows (BMs) of DS fetuses (although no BMs from older fetuses were analyzed). These observations are consistent with the clinical syndrome of TMD, which often involves the liver but not the bone marrow. The specific association with FL hematopoiesis could have been explained by either unique properties of the DS FL microenvironment or by a cell autonomous effect of cT21 on FL HPCs. Repopulation experiments performed by Chou et al in immunodeficient mice with hematopoietic cells from DS and control FLs suggest that the tilt toward the megakaryoblastic-erythroid lineage is a cell autonomous property endowed to the FL HPC by cT21. Since Chou et al have not studied BM cells, the specific association with FL progenitors needs additional confirmation. However, together these studies are intriguing because of previous research in a knock-in mouse model that demonstrated that the GATA1s isoform enhanced proliferation of early FL HPCs while having no effect on postnatal BM cells.4 It is therefore tempting to speculate that cT21 causes an increased proliferation and megakaryocytic-erythroid differentiation of a unique FL HPC that is sensitive to the GATA1s mutation.
The fascinating observations of human FL hematopoiesis in DS raise at least 2 interesting questions: The first is what are the characteristics of FL HPCs that are sensitive to cT21 and the GATA1s mutation? Indeed little is known on what distinguishes hematopoietic development in FL compared with the fetal BM. The second is which Hsa21 gene or genes contribute to the remarkable effects of cT21 on FL hematopoiesis? As multiple genes are expressed from trisomic chromosomes,6 it is possible that cooperation among several Hsa21's is responsible for cT21 effects, as has also been suggested for explanation of other phenotypes of DS.7
Similarly to the leukemias of DS, sporadic childhood leukemias are initiated in utero. Somatic structural or numerical chromosomal aberrations acquired in fetal hematopoietic progenitors initiate the growth of a preleukemic clone.8 Thus, the relevance of studies, such as the 2 reported by Tunstall-Pedoe and Chou and their colleagues, extends beyond DS. They are important for the general understanding of how normal fetal hematopoietic development is diverted toward leukemia.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■