Abstract
Initial molecular events leading to natural killer lymphocyte (NK) and dendritic cell (DC) interactions are largely unknown. Here, the role of CX3CL1 (fractalkine), a chemokine expressed on mature dendritic cells (mDCs) has been investigated. We show that CX3CL1 promotes NK activation by mDCs. After blocking of CX3CL1 by antibody, no activation occurred but major histocompatibility complex (MHC) class I neutralization restored DC-mediated NK activation, suggesting an interaction between CX3CL1 signaling and the functioning of inhibitory KIR. Then the YTS NK cell line, in which the inhibitory receptor KIR2DL1 had been introduced, was used. The presence of KIR2DL1 did not decrease YTS activation by HLA-Cw4 DC when CX3CL1 was functional. In contrast, CX3CL1 neutralization led to killer cell immunoglobulin-like receptor (KIR) phosphorylation and SHP-1 recruitment in YTSKIR2DL1 cultured with HLA-Cw4 mDCs. Moreover, CX3CL1 neutralization promoted dispersion of lipid rafts and the formation of a multiprotein complex required for cytoskeletal rearrangements in YTS NK cells. These findings point to a pivotal role of CX3CL1 in the activation of resting NK cells by mature DCs.
Introduction
Natural killer (NK) cell activation is initiated when NK cells form tight contacts with target cells.1-4 However, this activation can be abrogated by interactions between major histocompatibility complex (MHC) class I molecules and inhibitory killer cell immunoglobulin-like receptors (KIRs). SHP-1 is then recruited to the KIR intracytoplasmic domain and a dominant inhibition of cytoskeletal and lipid raft polarization occurs, protecting MHC class I–positive targets from autologous NK cell–mediated cytotoxicity.5-11
We have previously described another type of synapse (DCNK-IS) that regulates DC-mediated NK activation.12 This dynamic network in which NK interacts with surrounding DC was also described in vivo during Leishmania major infection.13 The DCNK-IS has been shown to enable optimal NK activation by IL-15 and to display a spatial organization distinct from the classic target/NK synapses.12,14,15 However, the initial molecular events governing mature DC (mDC) and NK interactions remain to be identified. In particular, the mechanisms allowing autologous resting NK activation by mDCs in the presence of KIR on NK cells and self-MHC proteins on DCs, have not been studied.
Methods
Mice
C57BL/6 mice were obtained from Janvier (Le Genest St Isle, France), and maintained in the IFR133 animal facility according to the guidelines of the Animal Ethics Committee. C57BL/6 CX3CR1-knockout mice16 were provided by Bernhard Ryffel (GEM2358, CNRS, Orleans, France).
Dendritic cell differentiation
Human DCs were generated from adherence-selected monocytes in AIMV complete medium containing 1000 IU/mL of both rhuGM-CSF and rhuIL-4 (Peprotech, Neuilly-sur-seine, Saint-Quentin, France). DCs were exposed to lipopolysaccharide (LPS; 1 μg/mL, Sigma-Aldrich, France), characterized and used at day 6, as previously described.12 Mouse bone marrow–derived DCs (BMDCs) were differentiated as previously described.12
Purification of NK cells
NK cells were negatively selected from Ficoll-separated peripheral blood mononuclear cells (PBMCs) using the Miltenyi NK-cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany) prior to coculture with mDCs. The purity of NK cells was assessed by flow cytometry (anti-CD56 [B159] and anti-CD3 [UCHT1] mAb [BD PharMingen, San Diego, CA]) and ranged from 90% to 98%. In some experiments, NK cells were additionally selected according to KIR2DL1 (clone 143211; R&D Systems, Abingdon, United Kingdom) expression using fluorescence-activated cell sorting.
Slide preparation and confocal microscopy
mDCs (5 ×104) and NK cells (105) were mixed and spread onto poly-L- lysine–coated slides (Sigma, France) for 25 minutes at 37°C. Cells were fixed and permeabilized with 0.2% sodium dodecyl sulfate. After 20 minutes of blocking in 20% fetal bovine serum and washing, cells were stained with the appropriate mAbs. Stacks of confocal images were collected with an Olympus FV1000 laser scanning confocal microscope.
Cell labeling and antibodies
Phalloidin (Molecular Probes, Eugene, OR) and cholera toxin β subunit (CTX; Sigma-Aldrich, St Louis, MO) were used to detect polymerized F-actin and raft-associated GM1 gangliosides. NK cells were imaged using anti-KIR2DL1 (T-20; Santa Cruz Biotechnology, Santa Cruz, CA) and PT-100 (Cell Signaling Technology, Beverly, MA). Anti-CX3CL1 (51 637) and anti-CCL3 (93 342) were purchased from R&D Systems (Minneapolis, MN). Anti MHC class I (W6/32) were from Diaclone (Besançon, France). Cytoskeletal organization was studied using anti-FLAG, anti-myosin IIa, and anti-actin from Sigma-Aldrich. Secondary antibodies were purchased from Molecular Probes.
Reagents
Human IL-2 (1000 IU/mL) was obtained from Chiron (Amsterdam, The Netherlands). NK cells were treated with pertussis toxin (PTx; 100 ng/mL; Sigma-Aldrich) to block Gαi proteins. Enzyme-linked immunosorbent assay (ELISA) kits were from Diaclone. A single cell-based, fluorogenic cytotoxicity assay (CyToxiLux) was used to measure target cell death (OncoImmunin, Gaithersburg, MD).17
Statistical analysis
Results are expressed as the mean plus or minus the standard error of the mean (SEM). Group comparisons were performed using Student t test.
Results and discussion
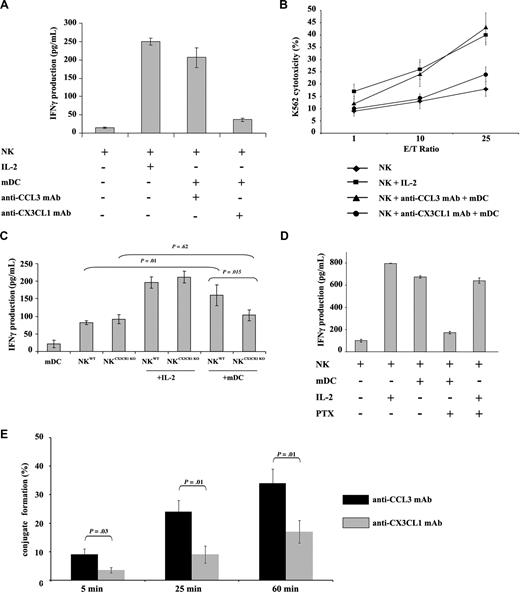
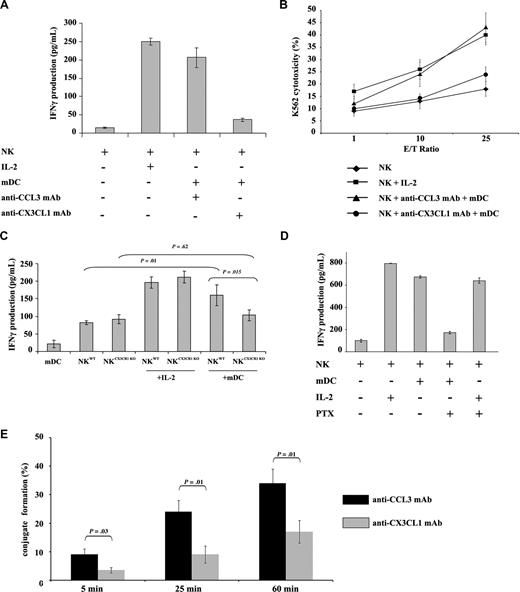
CX3CL1 is both a soluble and a membrane-anchored chemokine expressed by DCs and up-regulated upon maturation, while its receptor CX3CR1 is expressed on NK cells.18,19 To address the role of CX3CL1 in DC and NK-cell cross-talk, autologous resting human NK and mDCs were cultured 24 hours in the presence of CCL3-neutralizing mAb as control (5 μg/mL) or CX3CL1 neutralizing mAb (5 μg/mL). An alternative mode of NK-cell activation by IL-2 was included as a control. CX3CL1 but not CCL3 mAb prevented mDC-mediated NK IFN-γ production (Figure 1A) and cytotoxicity (Figure 1B).
CX3CL1 is required for NK activation by mDCs. (A) Autologous resting human NK cells (105) and mDCs (104) were cultured in 96-well plates at 37°C in a humidified 5% CO2 incubator in AIMV medium for 24 hours. CX3CL1 or CCL3-blocking mAb were added to the cultures. NK-derived IFN-γ production was assessed in culture supernatants by ELISA. (B) After NK and mDC cocultures, NK cells were counted and cytotoxic functions were assessed against K562 target cells. Data represent means of triplicates plus or minus the standard error (SE). Five independent experiments were performed with similar results. (C) To confirm a role for CX3CR1 signaling in DC-mediated NK cell activation, murine CX3CR1-deficient and wild-type NK cells were cultured with syngeneic wild-type bone marrow–derived DCs for 24 hours. IFN-γ production was determined. Results of a representative experiment out of 3 are expressed as the mean of triplicate assays with error bars representing standard deviations from the mean. (D) Human PTx-treated NK cells were cultured with mDCs or IL-2 for 24 hours. Supernatants were harvested to assess IFN-γ production. The depicted data represent means of triplicates plus or minus SE of a representative experiment out of 5 performed. (E) The ability of mDCs to form tight conjugates with resting NK cells was studied in the presence or absence of anti-CX3CL1 mAb. mDCs were admixed with resting NK cells (at a 1:2 mDC/NK ratio) and analyzed by transmission light microscopy as described in “Methods.” More than 100 DCs per experiment were examined for their capacity to bind to resting NK cells. The percentages of DCs forming conjugates with NK cells are shown as a mean plus or minus SE of 3 independent experiments.
CX3CL1 is required for NK activation by mDCs. (A) Autologous resting human NK cells (105) and mDCs (104) were cultured in 96-well plates at 37°C in a humidified 5% CO2 incubator in AIMV medium for 24 hours. CX3CL1 or CCL3-blocking mAb were added to the cultures. NK-derived IFN-γ production was assessed in culture supernatants by ELISA. (B) After NK and mDC cocultures, NK cells were counted and cytotoxic functions were assessed against K562 target cells. Data represent means of triplicates plus or minus the standard error (SE). Five independent experiments were performed with similar results. (C) To confirm a role for CX3CR1 signaling in DC-mediated NK cell activation, murine CX3CR1-deficient and wild-type NK cells were cultured with syngeneic wild-type bone marrow–derived DCs for 24 hours. IFN-γ production was determined. Results of a representative experiment out of 3 are expressed as the mean of triplicate assays with error bars representing standard deviations from the mean. (D) Human PTx-treated NK cells were cultured with mDCs or IL-2 for 24 hours. Supernatants were harvested to assess IFN-γ production. The depicted data represent means of triplicates plus or minus SE of a representative experiment out of 5 performed. (E) The ability of mDCs to form tight conjugates with resting NK cells was studied in the presence or absence of anti-CX3CL1 mAb. mDCs were admixed with resting NK cells (at a 1:2 mDC/NK ratio) and analyzed by transmission light microscopy as described in “Methods.” More than 100 DCs per experiment were examined for their capacity to bind to resting NK cells. The percentages of DCs forming conjugates with NK cells are shown as a mean plus or minus SE of 3 independent experiments.
To confirm the role of CX3CL1 signaling, NK cells from wild-type and CX3CR1 knockout mice were cocultured with mature autologous BMDCs. While NK cells from CX3CR1-deficient and wild-type mice exposed to IL-2 produced equivalent levels of IFN-γ, the CX3CR1 knock-out abrogated the ability of NK cells to produce IFN-γ in response to mDCs (Figure 1C; P = .015).
CX3CR1 receptors in NK cells are coupled to Gαi–class G proteins.19 Human NK cells were treated with the Gαi–protein inhibitor PTx to further characterize the role of CX3CR1 signal transduction. PTx-pretreated NK cells produced IFN-γ in response to IL-2, but not when exposed to mDCs (Figure 1D).
Next, the role of CX3CL1 in DC/NK conjugate formation was studied. Indeed, 9% (± 2%), 24% (± 4%), and 34% (± 5%) of mDCs formed tight conjugates with a single resting NK cell at 5, 25, and 60 minutes, respectively, while only 3.5% (± 1%), 8.5% (± 3%), and 17% (± 4%) of mDCs did so after blocking CX3CL1 (Figure 1E). Altogether, the data shown in Figure 1 imply that CX3CL1 on DCs drives a molecular pathway that promotes NK-cell activation initiated through CX3CR1.
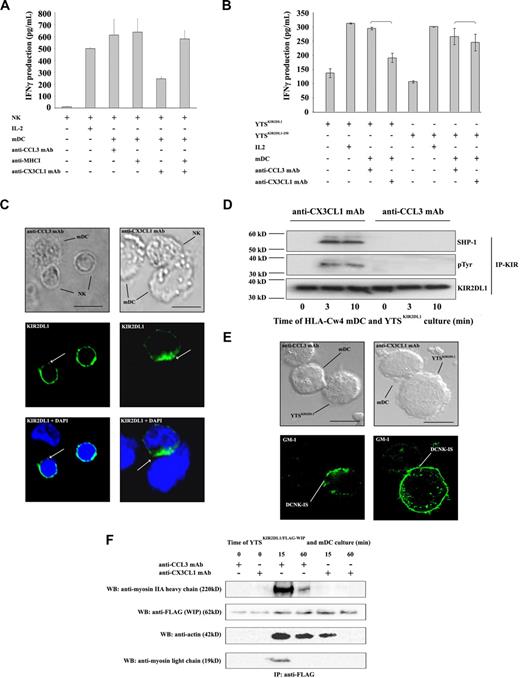
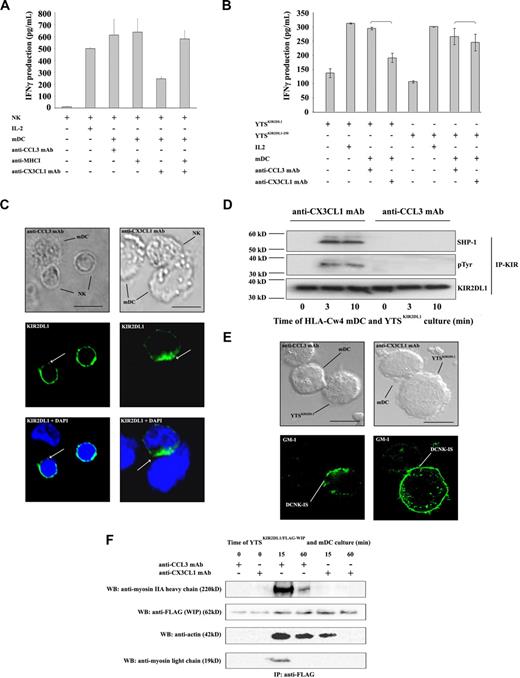
Receptor-ligand binding between MHC class I and inhibitory KIR leads to a negative signal that impairs activating synapse formation.10 Therefore, the MHC class I/KIR interaction according to CX3CL1 expression in DC/NK cultures was examined. After blocking of CX3CL1, activation by mDCs of human autologous resting NK cells was restored by anti-MHC class I mAb treatment (Figure 2A), suggesting a direct relation between CX3CL1 signaling and KIR function.
CX3CR1 signaling inhibits HLA-C–induced KIR phosphorylation and regulates DCNK-IS organization. (A) Autologous resting NK cells were exposed to mDCs (10:1 ratio) in the absence or presence of the indicated mAbs, during 24 hours. Anti-MHC class I mAb does not influence IFN-γ production in mDC and NK culture. In contrast, anti-MHC class I mAb prevents the effects of CX3CL1 neutralization. (B) HLA-Cw4 mDCs were cocultured for 24 hours in 96-well plates with the KIR-negative NK cell line YTS stably transfected with KIR2DL1 (YTSKIR2DL1) or with KIR2DL1(1-250) lacking the KIR intracytoplasmic domain (YTSKIR2DL1(1-250)). The levels of IFN-γ released in coculture supernatants were measured by ELISA. Data represent means of triplicates plus or minus SE. Five independent experiments were performed with similar results. (C) KIR2DL1 distribution in HLA-Cw4 mDC/autologous NK cocultures was studied by confocal imaging. HLA-Cw4 mDCs were admixed with autologous resting NK cells, spread onto a lysine-coated slide, and cultured 25 minutes at 37°C. After fixation, cells were permeabilized and stained with anti-KIR2DL1 mAb. Normaski (left panel) and fluorescence (right panel) images are shown. Fifty mDCs forming tight conjugates with KIR2DL1-expressing autologous NK cells were analyzed in each condition. (D) YTSKIR2DL1 were incubated with mDCs (ratio 2:1) during 0, 3, and 10 minutes at 37°C and immunoprecipitated with protein G beads coated with anti-KIR2DL1 mAb (T-20 clone; Santa Cruz). The immunoprecipitate was immunoblotted with anti-SHP-1 mAb (top panel), anti-phosphotyrosine mAb (middle panel), and with anti-KIR2DL1 mAb (clone 2F9, Abcam; bottom panel). (E) Lipid rafts containing ganglioside GM1 were stained using FITC-labeled cholera toxin and analyzed by confocal microscopy. Lipid rafts became clustered at the HLA-Cw4 mDC and YTSKIR2DL1 cell interface after conjugate formation. CX3CL1 neutralization prevented lipid raft clustering at the DCNK-IS. Representative pictures of 3 independent experiments are reported. Scale bar, 10 μm. (F) YTSKIR2DL1/WIP-FLAG were mixed with HLA-Cw4 mDCs for the indicated times at 37°C. Cell lysates were immunoprecipitated with anti-FLAG mAb. The formation of a multiprotein complex with or without CX3CL1 mAb blocking was studied as described.20 Immunoprecipitated proteins were visualized using anti-FLAG, anti-myosin IIA, and anti-actin.
CX3CR1 signaling inhibits HLA-C–induced KIR phosphorylation and regulates DCNK-IS organization. (A) Autologous resting NK cells were exposed to mDCs (10:1 ratio) in the absence or presence of the indicated mAbs, during 24 hours. Anti-MHC class I mAb does not influence IFN-γ production in mDC and NK culture. In contrast, anti-MHC class I mAb prevents the effects of CX3CL1 neutralization. (B) HLA-Cw4 mDCs were cocultured for 24 hours in 96-well plates with the KIR-negative NK cell line YTS stably transfected with KIR2DL1 (YTSKIR2DL1) or with KIR2DL1(1-250) lacking the KIR intracytoplasmic domain (YTSKIR2DL1(1-250)). The levels of IFN-γ released in coculture supernatants were measured by ELISA. Data represent means of triplicates plus or minus SE. Five independent experiments were performed with similar results. (C) KIR2DL1 distribution in HLA-Cw4 mDC/autologous NK cocultures was studied by confocal imaging. HLA-Cw4 mDCs were admixed with autologous resting NK cells, spread onto a lysine-coated slide, and cultured 25 minutes at 37°C. After fixation, cells were permeabilized and stained with anti-KIR2DL1 mAb. Normaski (left panel) and fluorescence (right panel) images are shown. Fifty mDCs forming tight conjugates with KIR2DL1-expressing autologous NK cells were analyzed in each condition. (D) YTSKIR2DL1 were incubated with mDCs (ratio 2:1) during 0, 3, and 10 minutes at 37°C and immunoprecipitated with protein G beads coated with anti-KIR2DL1 mAb (T-20 clone; Santa Cruz). The immunoprecipitate was immunoblotted with anti-SHP-1 mAb (top panel), anti-phosphotyrosine mAb (middle panel), and with anti-KIR2DL1 mAb (clone 2F9, Abcam; bottom panel). (E) Lipid rafts containing ganglioside GM1 were stained using FITC-labeled cholera toxin and analyzed by confocal microscopy. Lipid rafts became clustered at the HLA-Cw4 mDC and YTSKIR2DL1 cell interface after conjugate formation. CX3CL1 neutralization prevented lipid raft clustering at the DCNK-IS. Representative pictures of 3 independent experiments are reported. Scale bar, 10 μm. (F) YTSKIR2DL1/WIP-FLAG were mixed with HLA-Cw4 mDCs for the indicated times at 37°C. Cell lysates were immunoprecipitated with anti-FLAG mAb. The formation of a multiprotein complex with or without CX3CL1 mAb blocking was studied as described.20 Immunoprecipitated proteins were visualized using anti-FLAG, anti-myosin IIA, and anti-actin.
One such receptor-ligand interaction could be specifically investigated using the KIR-negative NK cell line YTS and its transfectants expressing KIR2DL1 or the mutant KIR2DL1(1-250) lacking the cytoplasmic tail in coculture experiments with mDCs generated from HLA-Cw4 homozygous donors. Blocking of CX3CL1 on mDCs inhibited the ability of HLA-Cw4 mDCs to activate YTSKIR2DL1, but had no effect when YTSKIR2DL11-250 was used (Figure 2B).
Distribution of KIRs was then examined by confocal microscopy. As recently described,14 KIRs were present at 5 minutes at the synapse after conjugation in 74% plus or minus 3% and 70% plus or minus 6% of DC/NK conjugates exposed to either anti-CCL3 or anti-CX3CL1 mAb respectively (P = .29). After 25 minutes, KIR expression decreased from the interface of DCNK conjugates in the presence of control anti-CCL3mAb (Figure 2C left). By contrast, KIRs accumulated at the interface of 41% (± 8%) DC/NK conjugates in the presence of anti-CX3CL1 mAb (Figure 2C right) compared with 14% (± 5%) in the presence of anti-CCL3 mAb (Figure 2C left; P = .012).
To further assess the function of inhibitory KIRs during mDC and NK cell interactions, phosphorylation of KIR2DL1 was investigated. For this purpose, YTSKIR2DL1 cells were cultured for 0, 3, or 10 minutes at 37°C with HLA-Cw4 mDCs before KIR2DL1 immunoprecipitation. KIR2DL1 was not phosphorylated in HLA-Cw4 mDC/YTSKIR2DL1 coculture in presence of control anti-CCL3mAb. By contrast, CX3CL1 neutralization in the YTSKIR2DL1/mDC cocultures allowed KIR2DL1 phosphorylation and led to SHP1 recruitment (Figure 2D) and failure to activate NK cells (Figure 1A,B).
In addition, these experiments were reproduced using human peripheral NK cells sorted into either KIR2DL1+ or KIR2DL1− subsets and exposed to autologous HLA-Cw4 mDCs. Using the KIR2DL1 NK cells, CX3CL1 prevented the inhibitory signal resulting from KIR binding to MHC class I during the DC/NK interaction (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
KIR signaling induces GM1-enriched lipid raft exclusion from the synaptic region and prevents formation of a multiprotein complex, containing both Wiskott-Aldrich syndrome protein (WASp) and WASp-interacting protein (WIP) required for actin cytoskeleton remodeling at the synapse.7-10,20 During DCNK-IS formation, lipid rafts accumulated at the synapse in the presence of control anti-CCL3 mAb when CX3CL1 was functioning normally to inhibit KIR function. However, when CX3CL1 was neutralized by mAb and KIR function was restored, then GM-1–enriched lipid rafts were dispersed. CX3CL1 mAb prevented the accumulation of these rafts in DCNK-IS (Figure 2E). Finally, stably expressing FLAG-WIP/YTSKIR2DL1 was used to define the cytoskeletal complex in NK cells. Analysis of anti-FLAG immunoprecipitates revealed that YTSKIR2DL1 activation by HLA-Cw4 mDCs allowed recruitment of actin and myosin IIA to the WIP complex. In the presence of CX3CL1-neutralizing mAb, this complex could not be formed. CX3CL1 neutralization decreased actin binding to WIP and prevented myosin IIA recruitment (Figure 2F).
Thus, CX3CL1 is a pivotal molecule driving IS formation during DC/NK interactions. Besides their classic role in inducing cell migration, chemokine receptors have also been proposed to act as T-cell costimulators by prolonging the duration of T cell–antigen-presenting cell (APC) interaction and by avoiding premature splitting due to chemoattractant sources.21 In line with these observations, CX3CL1 signaling is shown here directly to allow IS organization during DC/NK interactions, despite the presence of an inhibitory KIR on NK cells and its cognate HLA-C ligand on DCs. This feature is essential in the physiologic context.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
J.R.P. and R.B. received a fellowship from the conseil regional de Franche Comté. This work was also supported by Inserm, the Ligue contre le cancer, comité du Doubs, and comité de Haute Marne and by National Institutes of Health (NIH) grant AI053330.
National Institutes of Health
Authorship
Contribution: C.B. designed the research and wrote the manuscript; C.B., J.R.P., and R.B. performed research and analyzed experiments; K.K., B.R., and A.C. provided vital reagents; P.S.R., L.Z., and X.P. provided critical suggestions to the study; and P.T. and J.L.S. contributed to the design and writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christophe Borg, Department of Medical Oncology and Cancer Immunotherapy, Inserm U645, Etablissement Français du Sang Bourgogue Franche Courté, 2 Bd Fleming, 25020 Besançou, France; e-mail: christophe.borg@efs.sante.fr.