Abstract
This analysis assessed the efficacy and safety of lenalidomide + dexamethasone in patients with relapsed or refractory multiple myeloma (MM) previously treated with thalidomide. Of 704 patients, 39% were thalidomide exposed. Thalidomide-exposed patients had more prior lines of therapy and longer duration of myeloma than thalidomide-naive patients. Lenalidomide + dexamethasone led to higher overall response rate (ORR), longer time to progression (TTP), and progression-free survival (PFS) versus placebo + dexamethasone despite prior thalidomide exposure. Among lenalidomide + dexamethasone-treated patients, ORR was higher in thalidomide-naive versus thalidomide-exposed patients (P = .04), with longer median TTP (P = .04) and PFS (P = .02). Likewise for dexamethasone alone-treated patients (P = .03 for ORR, P = .03 for TTP, P = .06 for PFS). Prior thalidomide did not affect survival in lenalidomide + dexamethasone-treated patients (36.1 vs 33.3 months, P > .05). Thalidomide-naive and thalidomide-exposed patients had similar toxicities. Lenalidomide + dexamethasone resulted in higher rates of venous thromboembolism, myelosuppression, and infections versus placebo + dexamethasone, independent of prior thalidomide exposure. Lenalido-mide + dexamethasone was superior to placebo + dexamethasone, independent of prior thalidomide exposure. Although prior thalidomide may have contributed to inferior TTP and PFS compared with thalidomide-naive patients, these parameters remained superior compared with placebo + dexamethasone; similar benefits compared with placebo + dexamethasone were not evident for thalidomide-exposed patients in terms of overall survival. Studies were registered at http://www.clinicaltrials.gov under NCT00056160 and NCT00424047.
Introduction
Despite advances in the treatment of multiple myeloma (MM), a disease characterized by the accumulation of clonal plasma cells in the bone marrow, the disease remains incurable. With the advent of novel therapies, the median survival of relapsed patients has been improved from about 1 to 2 years after relapse.1 It is estimated that about 20 000 people (11 000 men and 9 000 women) will be diagnosed with MM, and 11 000 will die because of the disease in the United States in 2008.2 Patients relapsing after 2000 had a median survival of 24 months, which was a clear improvement compared with those relapsing before 2000, indicating the benefit of new treatment options.3 Nevertheless, novel agents and their rational combinations are needed. In the mid-1990s, a new class of immunomodulatory drugs was designed and synthesized using the structural backbone of thalidomide as the template. The intention was to create analogs with enhanced efficacy and reduced toxicity relative to their parent compound.4,5 Lenalidomide (Revlimid; Celgene, Summit, NJ) is an oral derivative of thalidomide that has proven activity against MM in preclinical and clinical studies.6-10 Whereas the immunomodulatory effects and in vivo antitumor activity of lenalidomide are similar to thalidomide, improved potency (evidenced by a greater ability to stimulate T-cell proliferation, interleukin-2 and interferon-γ production, and to inhibit tumor cell growth) and reduced toxicity (reduced somnolence, constipation, and peripheral neuropathy; no evidence of teratogenicity or mutagenesis in preclinical models) favor lenalidomide.4,11,12 However, it must be noted that myelosuppression, not commonly observed with thalidomide, is often observed with lenalidomide.4,12 Recently, lenalidomide plus dexamethasone was shown to be also highly active in newly diagnosed MM, leading to durable responses and a low progression rate and mortality.13 In 2 prospective, randomized, double-blind, placebo-controlled phase 3 clinical trials (MM-009 and MM-010), it was shown that lenalidomide plus dexamethasone induced significantly higher rates of overall response (OR) and complete response (CR), as well as longer time to progression (TTP), and overall survival (OS), compared with placebo plus dexamethasone in patients with relapsed or refractory MM.14,15
Since lenalidomide is a derivative of thalidomide, there has been concern about possible resistance to lenalidomide in patients who had relapsed after, or who were refractory to, treatment with thalidomide. Preliminary data from early phase 1 and 2 trials suggested that lenalidomide alone and combined with dexamethasone produced a response in patients who had received prior thalidomide.9,10 The present prospective subgroup analysis of data pooled from the MM-009 and MM-010 phase 3 clinical trials assessed the efficacy of lenalidomide plus dexamethasone in patients with prior thalidomide exposure.
Methods
We evaluated data from 704 patients included in the MM-009 and MM-010 trials who had relapsed or refractory MM and were not resistant to dexamethasone. This is a secondary analysis of pooled data from 2 primary trials. Patients enrolled in these trials gave written informed consent in accordance with the Declaration of Helsinki, and an ethics committee at each study site (M. D. Anderson Cancer Center, University of Athens, Princess Margaret Hospital, University of Barcelona, Université de Purpan, Alfred Hospital, Mayo Clinic) approved the protocol. Patients were randomized to receive either oral lenalidomide (25 mg/day for 21 days, every 28-day cycle) plus dexamethasone (40 mg on days 1-4, 9-12, and 17-20 every 28-day cycle for 4 cycles, after the 4th cycle on days 1-4), or placebo plus an identical schedule of dexamethasone. Prophylactic anticoagulation was not recommended for the patients enrolled in the 2 trials. Patients who were dexamethasone resistant to more than 200 mg dexamethasone in a month were excluded from the 2 trials. We identified 274 patients (39%) who had received prior thalidomide treatment and 430 patients (61%) who had not been previously treated with this agent.
The present analysis is a posthoc analysis, performed without prespecified power calculation or adjustment for multiplicity, and is therefore considered exploratory in nature. For this analysis, patients with prior exposure to thalidomide were further categorized, according to their response to thalidomide, into the following 3 subgroups: (1) thalidomide-sensitive (T1) patients with best response of stable disease (SD) or better, who never progressed while on thalidomide; (2) thalidomide-relapsed (T2) patients with best response of SD or better, who progressed while on thalidomide; and (3) thalidomide-refractory (T3) patients who had progressed while on thalidomide and never responded to prior thalidomide treatment. Patients in T1 did not progress while on thalidomide treatment, but discontinued thalidomide treatment for other reasons, such as toxicity or stem cell transplantation. For the 41 patients from the thalidomide-exposed group not included in T1, T2, or T3, response to prior thalidomide was not evaluable or was unknown.
As previously reported,14,15 toxicity was graded according to the National Cancer Institute Common Toxicity Criteria version 2 (http://ctep.cancer.gov/reporting/ctcarchive.html). Response to treatment was assessed according to European Group for Blood and Marrow Transplantation criteria16 and the International Myeloma Working Group uniform response criteria,17 which define the following responses: CR: no M-protein detectable by immunofixation in the serum and urine, disappearance of any soft tissue plasmacytomas, and 5% or less plasma cells in the bone marrow; very good partial response (VGPR): 90% or more reduction in serum M-protein and urine M-protein level less than 100 mg per 24 hours; and partial response (PR): 50% or more reduction of serum M-protein and reduction in 24-hour urinary M-protein by 90% or more or less than 200 mg per 24 hours. Progressive disease was defined by any of the following: a 25% or more increase from baseline serum or urinary M-protein, which must also be an absolute increase of at least 500 mg/dL in serum or 200 mg per 24 hours in urine; new or increased size of bone lesions or plasmacytomas; or development of hypercalcemia (serum calcium > 2.875 mM [11.5 mg/dL]). TTP was measured from randomization to the date of the first assessment showing disease progression. Patients who died or discontinued the study without evidence of disease progression were censored at the last evaluation for assessment of TTP. Progression-free survival (PFS) was measured from randomization to the date of the first assessment showing disease progression or death during treatment, whichever occurred first. Patients who were alive and discontinued the study without evidence of disease progression were censored at the last evaluation for assessment of PFS.
OS was calculated as the time from randomization until death from any cause, or censored at the last follow-up visit. Follow-up data on OS were obtained up to January 2007, for a median follow-up duration of 31.3 months. Data on OR, TTP, and PFS were assessed up to unblinding, which occurred in June 2005 for study MM-009 and August 2005 for study MM-010, for a median follow-up duration of 17.5 months. Differences in OR rates between treatment groups were analyzed using continuity-corrected Pearson chi square tests. Time-to-event variables with censoring, including TTP, PFS, OS, and response duration, were estimated by Kaplan-Meier methods. Two-sided log-rank tests were used to compare survivorship functions between treatment groups for TTP, PFS, OS, and response duration.
Results
Patient characteristics
The prior thalidomide-exposed patients and thalidomide-naive patients were similar with regard to age; β2-microglobulin, hemoglobin, serum M-protein, and creatinine levels; and history of previous transplantation (Table 1). Patients previously treated with thalidomide had significantly more prior lines of therapy (P < .05) and a longer time since diagnosis (P < .05) compared with patients who were thalidomide naive (Table 1). The thalidomide-exposed patients were also more likely to have received prior dexamethasone (86%) compared with the thalidomide-naive group (62%) (P < .001). Although numbers were small, a nonsignificant trend was observed for prior bortezomib treatment (10% vs 7%). Within the lenalidomide plus dexamethasone treatment group, patients previously treated with thalidomide had a lower absolute neutrophil count (P < .05) compared with patients who were thalidomide naive (Table 1).
Outcomes
For patients treated with lenalidomide plus dexamethasone, the OR rate was higher in the thalidomide-naive than in the thalidomide-exposed group of patients (65% vs 54%, P = .04; Table 2), but response duration was not different (median of 16.2 months vs 13.4 months, P = .41; Table 2). The same difference in OR rate was seen for patients treated with dexamethasone alone (28% vs 14%, respectively; P = .003). In T2 and T3 subgroups, despite the small sample sizes and therefore limited statistical power, treatment with lenalidomide plus dexamethasone resulted in a significantly higher OR rate than dexamethasone alone (P < .05; Tables 2,3). Even the group categorized as being refractory to thalidomide (T3) benefited from lenalidomide plus dexamethasone treatment with a higher OR rate compared with dexamethasone alone (P = .042; Table 3).
Treatment with lenalidomide plus dexamethasone led to a longer duration of response than treatment with dexamethasone alone in thalidomide-naive patients and in those who had received prior thalidomide (P < .01 for both comparisons; Table 2). Duration of response was similar among patients in these thalidomide-relapsed (T2) and thalidomide-refractory (T3) subgroups treated with lenalidomide plus dexamethasone (T2 vs T3: P = .88),
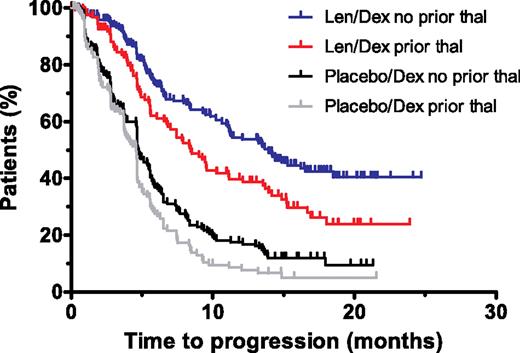
Lenalidomide plus dexamethasone was significantly more effective than dexamethasone alone in prolonging TTP and PFS in all subgroups (Tables 2,3; Figure 1). The group categorized as being refractory to thalidomide (T3) benefited from treatment with lenalidomide plus dexamethasone, with prolonged TTP and PFS compared with dexamethasone alone (P < .05; Table 3). For patients treated with lenalidomide plus dexamethasone, TTP and PFS were longer in the thalidomide-naive patients compared with the thalidomide-exposed patients (median of 13.9 vs 8.4 months, P = .004 for TTP; median of 13.2 months vs 8.4 months, P = .02 for PFS; Table 2). There were no differences in TTP or PFS between the thalidomide-relapsed and the thalidomide-refractory subgroups (Table 3). Results were similar for patients treated with dexamethasone alone (Table 2).
Kaplan-Meier plots of the time to progression in the lenalidomide plus dexamethasone and dexamethasone alone groups for patients with or without prior thalidomide exposure. The estimate of time to progression for the intent-to-treat population of the lenalidomide plus dexamethasone and dexamethasone alone groups. Len/Dex indicates lenalidomide plus dexamethasone; Dex, dexamethasone; and thal, thalidomide.
Kaplan-Meier plots of the time to progression in the lenalidomide plus dexamethasone and dexamethasone alone groups for patients with or without prior thalidomide exposure. The estimate of time to progression for the intent-to-treat population of the lenalidomide plus dexamethasone and dexamethasone alone groups. Len/Dex indicates lenalidomide plus dexamethasone; Dex, dexamethasone; and thal, thalidomide.
Per protocol, patients treated with dexamethasone alone could receive lenalidomide-based therapy following disease progression or unblinding of the study (164 patients crossed over). Nevertheless, OS was significantly longer for thalidomide-naive patients originally assigned lenalidomide plus dexamethasone than for those given dexamethasone alone (P = .04; Table 2). For thalidomide-exposed patients, there was a trend toward a longer OS in the originally assigned lenalidomide plus dexamethasone group, although it was not statistically significant (P = .23; Table 2), possibly due to the smaller sample sizes of the subgroups and the confounding factors in OS analysis including crossover and other treatment options after patients discontinued the original assigned treatment. For patients receiving lenalidomide plus dexamethasone, there was no significant difference in OS between thalidomide-naive and thalidomide-exposed patients (median of 36.1 vs 33.3, P = .20).
Twelve patients, whose only prior treatment was thalidomide, received lenalidomide plus dexamethasone with their only prior treatment being thalidomide. Of these, 10 (83%) responded to lenalidomide plus dexamethasone, with a CR/VGPR rate of 42%, and a median TTP of 13.6 months. For the 22 patients whose responses to prior thalidomide therapy were not evaluable and were therefore not included in the T1, T2, or T3 subgroups, 46% responded (≥ PR) to lenalidomide plus dexamethasone with a median TTP of 8.5 months, and median PFS of 8.5 months.
Adverse events
In thalidomide-naive patients, grade 3 or 4 deep vein thrombosis or pulmonary embolism (DVT/PE) was more common in patients treated with lenalidomide plus dexamethasone than in those who had received dexamethasone alone (10% vs 4%, P < .05; Table 4). In thalidomide-exposed patients, the incidences of grade 3 or 4 DVT/PE were 15% and 3% for those treated with lenalidomide plus dexamethasone and those on dexamethasone alone, respectively (P < .05; Table 4). Among patients treated with lenalidomide plus dexamethasone, DVT/PE rates (all grade 3 or 4) were similar, irrespective of the presence or absence of prior thalidomide treatment (15% vs 10%, respectively; P = .17; Table 4).
Anticoagulation usually involves prophylactic low-molecular-weight heparin (either low dose or full dose), or warfarin orally with the targeted INR range between 2 and 3. Antithrombotic prophylaxis is usually with oral aspirin. We do not have data to support the choices of anticoagulation. Our data suggest to use prophylactic therapeutic anticoagulation in thalidomide-exposed patients receiving lenalidomide plus dexamethasone rather than not to use any forms of anticoagulation.
The frequencies of grade 3 or 4 neutropenia and thrombocytopenia were higher for patients receiving lenalidomide plus dexamethasone than for those receiving dexamethasone alone, irrespective of prior thalidomide treatment (Table 4).
In thalidomide-naive patients, the occurrence of grade 3 or 4 neutropenia was 32% with lenalidomide plus dexamethasone, higher than that of dexamethasone alone (4%, P < .05). In thalidomide-exposed patients, the frequency of grade 3 or 4 neutropenia was 41% with lenalidomide plus dexamethasone, higher than that of dexamethasone alone (2%, P < .05). In patients treated with lenalidomide plus dexamethasone, the frequency of grade 3 or 4 neutropenia was not significantly different between thalidomide-naive and thalidomide-exposed patients (32% vs 41%, P > .05).
In thalidomide-naive patients, the rate of febrile neutropenia was 3% with lenalidomide plus dexamethasone, much higher than that of dexamethasone alone (0%). In thalidomide-exposed patients, similar differences exist (2% vs 0%). Therefore, lenalidomide plus dexamethasone was associated with higher frequencies of febrile neutropenia either in thalidomide-naive patients or thalidomide-exposed patients.
In thalidomide-naive patients, the rate of grade 3 or 4 nonneutropenic infections was 16% with lenalidomide plus dexamethasone, higher than that with dexamethasone alone (7%, P < .05). However, similar differences between lenalidomide plus dexamethasone and dexamethasone alone in the thalidomide-exposed group of patients did not reach statistical difference (14% vs 9%, P > .05).
In patients treated with lenalidomide plus dexamethasone, the frequencies of grade 3 or 4 anemia were similar for those with and without prior thalidomide exposure (P > .05 for all; Table 4).
In thalidomide-naive patients, the occurrence of grade 3 or 4 thrombocytopenia was 11% with lenalidomide plus dexamethasone, similar to that of dexamethasone alone (5%, P > .05). In thalidomide-exposed patients, the frequency of grade 3 or 4 thrombocytopenia was 17% with lenalidomide plus dexamethasone, much higher than that of dexamethasone alone (8%, P < .05). This was likely to be due to a combination of the myelosuppression by lenalidomide plus dexamethasone and/or the impact of a greater number of prior lines of therapy and longer duration from of disease on the marrow. (Table 1).
In thalidomide-naive patients, the rate of grade 3 or 4 neuropathy was 0.4% with lenalidomide plus dexamethasone, similar to that with dexamethasone alone (0.5%, P > .05). However, in thalidomide-exposed patients, the rate of grade 3 or 4 neuropathy tended to be higher (3%) in patients treated with lenalidomide plus dexamethasone, but was not significantly different from that noted in patients treated with dexamethasone alone (1%, P = .06, Table 4). This trend could be due to the addition of lenalidomide to therapy in patients with prior thalidomide exposure or to the greater number of prior lines of therapy and longer duration of disease prior to protocol entry (Table 1).
Dosing
In patients who received prior thalidomide treatment and in those who were thalidomide naive, the median daily doses were 25 mg lenalidomide or placebo, and 40 mg dexamethasone in both the lenalidomide plus dexamethasone and dexamethasone alone groups. In thalidomide-naive patients and those who had received prior thalidomide treatment, dose reductions were more common in those treated with lenalidomide plus dexamethasone compared with dexamethasone alone. Prior thalidomide treatment did not influence the number of patients needing a dose reduction (Table 5).
Discussion
Results from this posthoc subgroup analysis of data pooled from the phase 3 randomized clinical trials, MM-009 and MM-010, showed that lenalidomide plus dexamethasone was more effective than dexamethasone alone in the treatment of patients with relapsed or refractory MM, irrespective of prior thalidomide exposure. Furthermore, lenalidomide plus dexamethasone was active in patients who had relapsed on or had never previously responded to thalidomide.
Preliminary results have shown that lenalidomide plus dexamethasone at first relapse resulted in a higher OR rate (65% vs 58%) and a longer median TTP (16.4 vs 9.5 months) than when the treatment was used later, after multiple relapses.18 Patients in the prior thalidomide groups (T1-T3) were generally treated later after diagnosis (ie, in a later phase of the disease) and had received more prior therapies compared with patients not previously treated with thalidomide. It is interesting to note that in the small number of patients who received lenalidomide plus dexamethasone as second-line therapy, immediately after thalidomide treatment, the combination resulted in an OR (83% for the 12 patients) much higher than lenalidomide plus dexamethasone used later in the treatment, after other additional therapies. This further supports the significant role of lenalidomide plus dexamethasone as second-line treatment, regardless of prior exposure to thalidomide.
Despite receiving more prior therapies and a longer duration of disease since diagnosis, 54% of patients with prior thalidomide exposure responded to lenalidomide plus dexamethasone treatment. Furthermore, our subgroup analysis demonstrated that even in the truly thalidomide-refractory subgroup of patients, 50% responded to lenalidomide plus dexamethasone, with 5% achieving CR. To our knowledge, these are the highest response rates reported in patients who were resistant to thalidomide.
As reported in the primary studies, a superior OR rate, TTP, and PFS after lenalidomide plus dexamethasone treatment compared with dexamethasone alone was observed, regardless of the number of prior therapies or prior exposure to thalidomide.14,15 In the present analysis, the longest TTP and PFS were observed among patients without prior thalidomide exposure who received lenalidomide plus dexamethasone. However, all the thalidomide-exposed subgroups, including those relapsed on or who were refractory to thalidomide, also benefited significantly from lenalidomide plus dexamethasone treatment. It is worth noting that the more favorable efficacy results for thalidomide-naive patients compared with thalidomide-exposed patients were not only observed in the lenalidomide plus dexamethasone group, but also in the dexamethasone alone group. Moreover, the lower efficacy of lenalidomide plus dexamethasone in thalidomide-exposed patients is likely because this is a more heavily pretreated group in general. These results indicate that although there might be some degree of cross-resistance between thalidomide and lenalidomide, there are still benefits for all patients regardless of prior thalidomide exposure.
There was a higher incidence of DVT for the thalidomide-exposed patients who were treated with lenalidomide and dexamethasone. We believe that all these patients should receive prophylactic anticoagulation. Anticoagulation usually involves prophylactic low-molecular-weight heparin (either low dose or full dose), or warfarin orally with the targeted INR range between 2 and 3. We do not have data from these 2 trials to support the choices of anticoagulation or antithrombotic therapy. Our data suggest to use prophylactic therapeutic anticoagulation in thalidomide-exposed patients receiving lenalidomide plus dexamethasone or antithrombotic therapy rather than not to use any forms of anticoagulation or antithrombotic therapy. Currently, for patients with prior history of DVT/PE, we use full anticoagulation as described in “Adverse events” with either full-dose low-molecular-weight heparin or warfarin with a targeted INR between 2 and 3. For patients receiving lenalidomide plus dexamethasone without prior history of DVT/PE, with or without prior history of thalidomide exposure, we use antithrombotic medications such as aspirin.
In either thalidomide-naive or thalidomide-exposed patients with myeloma, there were higher rates of neutropenia with lenalidomide plus dexamethasone compared with that of dexamethasone alone. These significantly higher rates of neutropenia were translated into higher frequencies of neutropenia fevers and severe nonneutropenia infections with lenalidomide plus dexamethasone therapy than with dexamethasone alone.
The incidence of peripheral neuropathy was low in both groups of patients with and without prior thalidomide exposure, but a trend toward a higher incidence of peripheral neuropathy was observed in thalidomide-exposed patients treated with lenalidomide plus dexamethasone. These results indicate that toxicities may be increased in patients receiving lenalidomide plus dexamethasone after prior thalidomide exposure. However, it must be noted that the thalidomide-exposed patients were more heavily pretreated than the thalidomide-naive patients.
In conclusion, our secondary analysis of data pooled from two phase 3 trials showed that treatment with lenalidomide plus dexamethasone was superior to dexamethasone alone in relapsed or refractory MM patients with or without prior thalidomide exposure. Prior thalidomide might have contributed to inferior TTP and PFS, without affecting OS in patients treated with lenalidomide + dexamethasone or dexamethasone alone.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Raymond Alexanian for his input in the analysis and the preparation of the paper.
The MM-009 and MM-010 studies were funded by Celgene Corporation, Summit, NJ; editorial support for the preparation of this paper was also funded by Celgene.
Authorship
Contribution: M.W., M.A.D., C.C., M.T.C., M.A., A.S., S.V.R., and D.M.W. enrolled patients; Z.Y. and M.O. analyzed results and made the figures; M.W., M.A.D., C.C., M.T.C., M.A., A.S., S.V.R., Z.Y., M.O., J.B.Z., R.D.K., and D.M.W. wrote the paper; the authors were fully responsible for content and editorial decisions for this paper.
Conflict-of-interest disclosure: M.W. received honoraria from Celgene and received research funding for this project from Celgene; M.A.D. received honoraria from Celgene; C.C. received honoraria and has done consultant work for Celgene; M.T.C. received honoraria for lectures from Janssen-Cilag and Pharmion; Z.Y., M.O., J.B.Z., and R.D.K. are employees of Celgene; D.M.W. received grant support and lecture fees from Celgene. All other authors declare no competing financial interests.
Correspondence: Michael Wang, M. D. Anderson Cancer Center, Department of Lymphoma/Myeloma, 1515 Holcombe Blvd, Unit 429, Houston, TX 77030; e-mail: miwang@mdanderson.org.
References
Author notes
Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, Atlanta, GA, June 4, 200619; the 48th Annual Meeting of the American Society of Hematology, Orlando, FL, December 10, 200620; and the XIth International Myeloma Workshop, Kas Island, Greece, June 26, 2007.21