Abstract
Thalidomide enhances rituximab-mediated, antibody-dependent, cell-mediated cytotoxicity. We therefore conducted a phase 2 study using thalidomide and rituximab in symptomatic Waldenstrom macroglobulinemia (WM) patients naive to either agent. Intended therapy consisted of daily thalidomide (200 mg for 2 weeks, then 400 mg for 50 weeks) and rituximab (375 mg/m2 per week) dosed on weeks 2 to 5 and 13 to 16. Twenty-five patients were enrolled, 20 of whom were untreated. Responses were complete response (n = 1), partial response (n = 15), and major response (n = 2), for overall and major response rate of 72% and 64%, respectively, on an intent-to-treat basis. Median serum IgM decreased from 3670 to 1590 mg/dL (P < .001), whereas median hematocrit rose from 33.0% to 37.6% (P = .004) at best response. Median time to progression for responders was 38 months. Peripheral neuropathy to thalidomide was the most common adverse event. Among 11 patients experiencing grade 2 or greater neuropathy, 10 resolved to grade 1 or less at a median of 6.7 months. Thalidomide in combination with rituximab is active and produces long-term responses in WM. Lower doses of thalidomide (ie, ≤ 200 mg/day) should be considered given the high frequency of treatment-related neuropathy in this patient population. This trial is registered at www.clinicaltrials.gov as #NCT00142116.
Introduction
Monoclonal antibodies have been successfully used to treat patients with B-cell malignancies, including Waldenstrom macroglobulinemia (WM). Most of these efforts have focused on the use of rituximab, a chimeric human IgG1 monoclonal antibody, which targets CD20, which is widely expressed in WM.1,2 Studies using standard-dose rituximab therapy have demonstrated activity in WM, with overall response rates of 27% to 35% and median durations of response from 8 to 27+ months.2-7 More recently, the use of extended schedule rituximab has been evaluated wherein patients received 8 infusions of rituximab (375 mg/m2 per week) at weeks 1 to 4 and 12 to 16. Overall response rates of 44% to 48% were observed in these studies, with median durations of response from 16+ to 29+ months.8,9 Among WM patients receiving rituximab as monotherapy, lower response rates have been observed in those patients with high serum IgM (> 6000 mg/dL) and beta-2 microglobulin (B2M; > 3.0 mg/L) levels, as well as homozygous expression of phenylalanine at amino acid position 158 on CD16 (FcγRIIIA-158).8-10
Studies combining rituximab with chemotherapy have also been explored in WM.11 The combination of nucleoside analogs plus rituximab has yielded major response rates of 70% to 90%,12-15 whereas the combinations of CHOP-R (cyclophosphamide, adriamycin, vincristine, prednisone, rituximab) or DC-R (dexamethasone, cyclophosphamide, rituximab) have resulted in response rates of 80% to 90%.16-18 Median time to progression (TTP) in excess of 3 years has been reported with these combinations. Although these combinations have produced more impressive responses, greater toxicity has also been reported in WM patients, with the use of nucleoside analogs causing prolonged neutropenia, stem cell damage, disease transformation, and secondary myelodysplasia/acute leukemia.11,12,19
In an effort to augment monoclonal antibody responses in WM patients while averting short- and long-term chemotherapy-induced toxicities, we sought the development of immunomodulatory agents for combination therapy with rituximab. Thalidomide is an immunomodulatory agent that induces the elaboration of immunostimulatory cytokines, including interleukin-2 and interferon-γ.20 Importantly, thalidomide induces the expansion of natural killer (NK) cells, which are important effectors of in vivo rituximab activity, as well as increased antibody-dependent, cell-mediated cytotoxicity (ADCC) induced by rituximab.20-24 As monotherapy, thalidomide has modest activity in WM patients, producing response rates of 25%, whereas the combination of thalidomide plus steroids and/or clarithromycin produces response rates of 40%.25-27 In view of these considerations, we carried out a phase 2 study of thalidomide and rituximab and here present outcome and long-term follow-up.
Methods
Patients with a clinicopathologic diagnosis of WM,28 who were naive to rituximab and thalidomide, who had CD20-positive disease as determined by previous bone marrow immunohistochemistry or flow cytometry, and who required therapy based on consensus guidelines29 were eligible for this study. A monoclonal IgM protein, a minimum IgM level more than or equal to 2 times the upper limit of normal, a baseline platelet count of more than or equal to 25 000/μL, an absolute neutrophil count of more than or equal to 500/μL, a serum creatinine of less than 2.5 mg/dL (unless nephropathy was attributable to their WM), a serum total bilirubin and serum glutamicoxalacetic transaminase of less than 2.5 times the upper limit of normal, and an Eastern Cooperative Oncology Group performance status of 0 to 2 were required for entry. No chemotherapy, steroid therapy, or radiation therapy within 30 days of study entry was permitted. Patients who were pregnant or lactating, had serious comorbid disease, had any uncontrolled bacterial, fungal, or viral infection, or had an active second malignancy were not eligible. All men and women of reproductive potential were required to agree to use an acceptable method of birth control before and during treatment as well as for 6 months after completion of study treatment and be willing to comply with the FDA-mandated STEPS program.
All patients provided informed written consent in accordance with the Declaration of Helsinki, and the Dana-Farber/Harvard Cancer Center institutional review board approved the protocol. Intended therapy consisted of thalidomide administered at a starting dose of 200 mg by mouth each day for 2 weeks, and then escalated to 400 mg by mouth each day for a total treatment period of 52 weeks. Thalidomide was held for each occurrence of grade more than or equal to 2 nonhematologic and/or grade more than or equal to 3 hematologic toxicity, and dose restart at 50% of previous dose was permitted to 50 mg daily when toxicities resolved to less than grade 2 for nonhematologic or less than grade 3 for hematologic toxicities, respectively. Rituximab was administered at 375 mg/m2 once weekly during weeks 2 to 5 and weeks 13 to 16, for a total of 8 infusions. Patients who did not tolerate the first cycle (4 infusions) of rituximab therapy were removed from the study and not replaced. Twenty-five patients were enrolled in this study, which used a Simon 2-stage design. Using 80% power and alpha set at 0.05, 11 patients were to be included in the first stage of the 2-stage design to test the null hypothesis that the probability of response is less than or equal to 50% vs the alternative that probability of response more than or equal to 75%. For the first stage, after rituximab plus thalidomide is administered to 11 patients, the study would have been terminated if 6 or fewer patients respond. If 7 or more patients responded in the first stage of the trial, an additional 14 patients were to be enrolled and treated during stage II. If, after study completion, the total number responding is greater than 16, the null hypothesis that rituximab plus thalidomide treatment produces a response rate less than 50% will be rejected.
Response determination
A baseline evaluation was obtained for enrollment within 30 days before initiation of therapy. Patients underwent restaging studies at 3, 6, and 12 months and thereafter every 3 months until progression of disease. As part of their response evaluation, all patients underwent history and physical examination; laboratory studies consisting of a complete blood count and differential, serum IgM levels, B2M levels; and a bone marrow biopsy and aspiration. Response determinations were made using modified consensus panel criteria from the Third International Workshop on WM,11,30 and response rates were determined on an evaluable basis. A complete response was defined as having resolution of all symptoms, normalization of serum IgM levels with complete disappearance of IgM paraprotein by immunofixation, and resolution of any adenopathy or splenomegaly. Patients achieving a major response and a minor response were defined as achieving a more than or equal to 50% and more than or equal to 25% reduction in serum IgM levels, respectively. Patients with stable disease were defined as having less than 25% change in serum IgM levels, in the absence of new or increasing adenopathy or splenomegaly and/or other progressive signs or symptoms of WM. Progressive disease was defined as occurring when a greater than 25% increase in serum IgM level occurred from the lowest attained response value or progression of clinically significant disease-related symptom(s). Time to disease progression (TTP) was calculated from the start of therapy using the Kaplan-Meier method.
Analysis of peripheral blood effector cells and FcγRIIIA polymorphisms
Statistical analysis
Comparison of pretreatment and posttreatment parameters was performed using a 2-tailed Student t test on Microsoft Excel software. The impact of FcγRIIIA-158 polymorphisms, serum IgM, and B2M levels on clinical responses was assessed by 2-tailed Fisher exact test (VassarStats). A P value less than or equal to .05 was deemed to be significant for the aforementioned studies.
Results
Patient and disease characteristics
The clinical features of the 25 patients enrolled in this study are summarized in Table 1. Of the 25 patients enrolled on study, 20 were previously untreated. Of the 5 previously treated patients, 4 (16%) and 1 (4%) of patients demonstrated relapsed disease or disease refractory to their prior therapy, respectively. Six (24%) of the 25 patients were documented by clinical history and/or examination to have grade 1 sensory neuropathy attributable to their WM at baseline. Anti–myelin-associated glycoprotein and antiganglioside M1 (GM1) antibody studies were obtained on these patients, of whom only one patient was positive for the anti–myelin-associated glycoprotein antibody. The median cumulative dose of thalidomide administered for the intended 52-week treatment period among all enrolled patients was 19 950 mg (range, 2100-96 000 mg). Twenty-one and 2 patients completed 8 and 4 infusions of rituximab therapy, respectively, and therefore were evaluable for response. Both unevaluable patients died before completing the first 4 weeks of rituximab therapy resulting from events deemed unrelated to protocol therapy: (1) complications associated with chronic obstructive pulmonary disease and (2) autoimmune myopathy and cardiomyopathy. The circumstances of both deaths on study were reviewed by the Drug and Safety Monitoring Board, which deemed the events to be unrelated to protocol therapy.
Clinical response to therapy
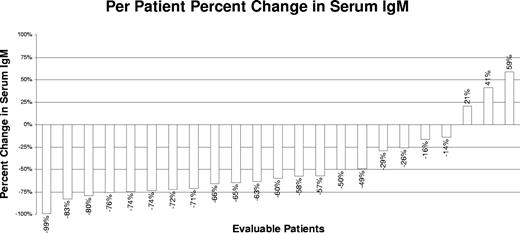
The individual changes in serum IgM levels at best response for all evaluable patients are shown in Figure 1. Median serum IgM levels for all evaluable patients declined from 3670 mg/dL (range, 924-8610 mg/dL) to 1590 mg/dL (range, 36-5230 mg/dL) at best response (P = 3.9 × 10−5). Pretherapy, 18 of 23 (78.3%) patients demonstrated a serum IgM level more than or equal to 3000 mg/dL; at best response, only 6 of 23 (26.1%) cases had an IgM level more than or equal to 3000 mg/dL. On an intent-to-treat basis, 18 (72%) patients demonstrated at least a minor response as their best response. Of these patients, 16 (64%) patients achieved a major response and 2 (8%) patients achieved a minor response. One complete remission was observed among major responders. Sixteen of 20 (80%) of the untreated patients demonstrated a response (including 14 major responders); whereas among previously treated patients, 2 of 5 (40%) responded (both major responders; P = .11 by Fisher exact t test). Improvements in baseline WM-related sensory neuropathy were observed in 3 of 6 patients, including resolution in 2 patients, whereas in one patient the sensory neuropathy worsened. For responding patients, the median time to best response was 18.9 months (range, 3.8-41.4 months), and the median time for a 25% reduction in serum IgM among responders was 4.7 months (range, 0.724.0 months). Among major responders, the median time to achieving a 50% reduction in serum IgM was 7.9 months (range, 2.8-21.8 months), which was in line with our previous experience with extended rituximab monotherapy.8
Individual changes (%) in serum IgM levels after treatment with thalidomide and rituximab.
Individual changes (%) in serum IgM levels after treatment with thalidomide and rituximab.
TTP
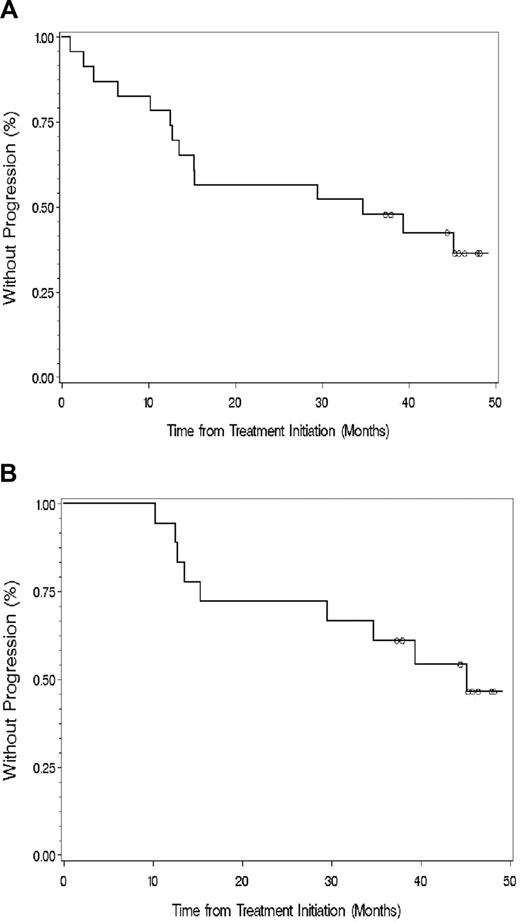
The median TTP for all evaluable patients was 34.8 months (range, 1.0-49.1 months; Figure 2). With a median follow-up of 47.1 months, 10 of the 18 responding patients have progressed. The median TTP for all responding patients was 38.7 months (range, 10.3-49.1 months; Figure 2). Among untreated patients, the median TTP for untreated patients was 36.04 months (range, 2.5-49.1 months); whereas among previously treated patients, the median TTP was 15.25 months (range, 1-45.8 months; P = .36).
Time to progression for (A) all evaluable patients and (B) those who responded to thalidomide and rituximab. ○ denotes patients who had not progressed at last follow-up.
Time to progression for (A) all evaluable patients and (B) those who responded to thalidomide and rituximab. ○ denotes patients who had not progressed at last follow-up.
Changes in hematologic parameters
Before therapy, 6 (26.1%) and 2 (8.7%) of 23 evaluable patients demonstrated a hematocrit of less than or equal to 30% and a platelet count of less than or equal to 100 000/μL, respectively. After therapy, and at best response, 1 (4.3%) and none of the 23 evaluable patients demonstrated a hematocrit of less than or equal to 30% and a platelet count of less than or equal to 100 000/μL, respectively. A significant increase in the median hematocrit was noted for the 23 evaluable patients, from 33.0% (range, 23.6%-42.6%) before therapy to 37.6% (range, 29.3%-44.3%) after therapy (P = .004). Pretherapy and posttherapy median platelet counts remained unaffected by therapy (P = .73).
Toxicities
Dose reduction of thalidomide occurred in all patients and led to premature discontinuation in 14 patients. Discontinuation of thalidomide in these patients occurred at a median of 4.1 months (range, 1.2-10.4 months). Grade more than or equal to 2 toxicities was as follows: peripheral neuropathy (44%), somnolence (12%), confusion (12%), rash (8%), tremors (8%), bradycardia (8%), and palpitations (4%). A total of 7 (28%), 4 (16%), and 4 (16%) experienced grade 3, 2, and 1 peripheral neuropathy, respectively. Among the 11 patients experiencing a more than or equal to grade 2 peripheral neuropathy, neuropathies were first reported at a median of 6.3 months (range, 0.64-11.8 months) after initiation of thalidomide. Resolution to less than or equal to grade 1 occurred in 10 of 11 (91%) patients at a median time of 5.3 months (range, 1-22.5 months) after onset of the neuropathy; complete resolution occurred in 7 of 11 (63.6%) patients at a median time of 8.8 months (range, 2.3-43.7 months). Development of thalidomide related neuropathy occurred in 3 of 6 patients (2 grade 1, 1 grade 3), which was on par with that experienced with non–WM-connected neuropathy patients.
Paradoxical increases in serum IgM levels
Abrupt and paradoxical increases in serum IgM levels have been reported with the use of rituximab in patients with WM, which can aggravate hyperviscosity and contribute to hyperviscosity-related symptoms.7,30,31 For this reason, plasmapheresis was strongly encouraged for patients who had a pretherapy serum viscosity of 3.5 CP or greater. Six patients underwent pretherapy plasmapheresis. Of the 17 patients who did not require prophylactic plasmapheresis before the first 4 infusions of rituximab, an increase in serum IgM was observed in 9 of 17 (52.9%) patients, with a more than or equal to 25% increase in serum IgM in 5 of 17 (29.4%) patients. Of the 21 patients receiving the second 4-week course of rituximab infusions, one patient required prophylactic plasmapheresis. Of 20 patients who did not require prophylactic plasmapheresis before the second course of rituximab, an increase in serum IgM was observed in 7 of 20 (35%) patients, with a more than or equal to 25% increase in serum IgM occurring in only one of 20 (5%) patients.
Effector cell and humoral immunity studies
Before therapy, and at 3 and 6 months after therapy, peripheral blood effector cell and uninvolved immunoglobulin studies were assessed for 16 patients. After 3 and 6 months of therapy, the absolute median levels of total T cells (CD3+), NK cells (CD16+CD56+), helper T cells (CD4+), and cytotoxic T cells (CD8+) remained unaffected, and no correlation was observed with response. Median serum IgA and IgG levels also remained unaffected at month 3 but modestly declined by month 6 with IgA decreasing from 24 to 20 mg/dL (P = .015) and IgG decreasing from 388 to 348 mg/dL (P = .012).
Impact of FcγRIIIA-158 polymorphisms, serum IgM, and B2M levels on patient responses
The overall response rate and TTP for patients treated with thalidomide and rituximab were determined based on their predicted amino acid polymorphisms in FcγRIIIA-158, which have been shown to be highly predictive of rituximab response in patients with WM.10 The overall response rates were 5 of 7 (71%) for patients with FcγRIIIA-158 F/F and 13 of 16 (81%) for FcγRIIIA-158 V/V or V/F (P = 1.0). TTP for patients with FcγRIIIA-158 F/F and FcγRIIIA-158 V/V or V/F was 32.1 and 37.3 months, respectively (P = .95). We next analyzed the impact of baseline serum IgM levels using the cutoff of 6000 mg/dL, which in previous studies served as a determinant for rituximab response in WM. The overall response rate for patients whose IgM was more than or equal to 6000 mg/dL and less than 6000 mg/dL was 4 of 6 (68%) and 14 of 18 (78%), respectively (P = 1.0). TTP for patients with patients with more than or equal to 6000 mg/dL and less than 6000 mg/dL was 44.4 and 25.0 months, respectively (P = .47). Lastly, we analyzed the impact of B2M levels on response parameters, given the prognostic significance of this variable in predicting overall survival in WM. Eight of 9 (89%) and 10 of 14 (71%) patients with a B2M level of more than or equal to 3 g/dL and less than 3 g/dL, respectively, responded to therapy (P = .61). TTP between these subgroups did not differ significantly at 37.6 and 24.1 months, respectively (P = .32).
Discussion
Although CD20 is expressed on malignant cells from nearly all patients with WM, responses to rituximab are seen in only approximately half of treated patients, even with the use of extended dose schedules. Tumor-related variables, including antigen loss, expression of complement resistance antigens, and large tumor burden, do not appear to account for the heterogeneity in response to rituximab for patients with WM.1,2,7,8 Moreover, we previously reported saturating levels of rituximab on WM cells in patients who had received therapy many months earlier, suggesting that patient-related factors might also account for differential responses to rituximab in WM.32 The possibility that patient-related differences, particularly those effecting ADCC function, might account for variable responses to rituximab in WM was further suggested by studies that correlated NK cell levels, as well as polymorphisms in position 158 of the FcγRIIIA receptor, to rituximab response in indolent non-Hodgkin's lymphoma including WM.10,21-23,33 Given these considerations, we focused our efforts on enhancing rituximab efficacy using agents that augment ADCC activity. In our preclinical studies, thalidomide and its analog lenalidomide enhanced ADCC activity to rituximab, as well as the CD40-directed SGN-40 monoclonal antibody.24 Moreover, in patients receiving thalidomide, increases were noted in circulating NK cells, which is particularly important given their prominence as key effectors in patients treated with rituximab. Here we carried out a clinical trial examining the activity of combination thalidomide and rituximab.
The results of this study demonstrate a high overall (72%) and major response rate (64%) to combination thalidomide and rituximab therapy in WM patients. These results appear to be better than the major response rate reported with either rituximab (40%-44%) or thalidomide (25%) monotherapy. Importantly, the responses in this study were durable and in excess of 3 years. The overall response rate and duration of response for thalidomide and rituximab appear on par with other active cytotoxic agent combination therapies used in WM. Hence, the use of thalidomide and rituximab appears quite appealing as an alternative upfront therapy in WM, given the avoidance of cytotoxic chemotherapy and its stem cell–sparing approach.
An important observation in this study was the dose-limiting toxicities, including neuropathies, which necessitated reduction/and or discontinuation of thalidomide in most patients. The dose and schedule of thalidomide should therefore be the subject of further investigation because lower start doses (≤ 200 mg daily), altered delivery schedule (ie, 5 days per week), and more prolonged treatment beyond one year may yield more optimal results. It is interesting, however, that several important predictors of rituximab response (FcγRIIIA-158 polymorphism status, serum IgM and B2M levels) were negated in this study with the combination of thalidomide and rituximab. Further studies in larger patient cohorts will be required to confirm these findings because the patient numbers in this series may have contributed to the lack of significance for these variables.
The combination of thalidomide and rituximab did not appear to negate the paradoxical increases in serum IgM levels observed with the use of rituximab in patients with WM. In this study, we observed that 30% of patients who were not pheresed before initiation of the first course of rituximab treatment had a more than or equal to 25% increase in serum IgM levels, which is on par with that previously reported by us and others.7,31,34 Importantly, however, only 5% of the patients receiving the second course of rituximab had a spike of more than or equal to 25% in serum IgM levels, which may reflect the interim response to therapy for many patients. Therefore, close monitoring of serum IgM levels and symptomatic hyperviscosity should be maintained in patients treated with thalidomide and rituximab, and empiric plasmapheresis should be considered for patients with elevated serum IgM levels. Accordingly, the use of thalidomide plus rituximab should not be used in patients in whom a rapid decrease in serum IgM levels is required. In such patients, other options can be considered, including the use of cytotoxic agent or bortezomib-based therapy.35
Lastly, the use of the thalidomide analog lenalidomide has become increasingly adopted given its higher response rates and fewer nonhematologic toxicities in patients with multiple myeloma, as well as other B-cell disorders. We explored its use in combination with rituximab and observed higher ADCC activity in preliminary studies.24 However, its use in WM patients appears to be problematic because of the development of acute nonhemolytic anemia, signifying potential idiopathic toxicity for WM patients with this agent. Moreover, the response durations among patients who tolerated combined lenalidomide and rituximab were shorter than in this study.36,37 The reason for these discordant findings, particularly because lenalidomide is a more potent immunomodulating agent, remains speculative but may ultimately involve other activities present in thalidomide but not lenalidomide, which benefit WM patients, such as phosphodiesterase-4 inhibition.38 The investigation of other thalidomide analogues, such as CC-4047, in combination with rituximab would also appear warranted.
In conclusion, the results of this study demonstrate that thalidomide in combination with rituximab is highly active and produces long-term responses in patients with WM. The use of this combination as upfront therapy in WM patients appears reasonable, particularly in patients who require nonmyelosuppressive and stem cell–sparing therapy, as well as for patients in whom cytotoxic agents pose undue risks.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Peter and Helen Bing Fund for Waldenstrom Macroglobulinemia at the Dana-Farber Cancer Institute, the Research Fund for Waldenstrom at the Dana-Farber Cancer Institute, and a National Institutes of Health Career Development Award (K23CA087977-03) (S.P.T.).
National Institutes of Health
Authorship
Contribution: S.P.T., A.R.B., and Z.R.H. designed and wrote study; J.D.S., C.J.P., and L.I. oversaw data collection; E.H. and B.T.C. performed basic science correlation studies; K.C.A. assisted in the study design and implementation; and S.P.T., F.M.B., M.P., H.Z., R.B.C., M.M., J. Hill, A.R., L.G., L.C., C.C., S.H.N., D.R.L., H.B., H.S., J. Howard, and P.M. treated study patients and provided study data.
Conflict-of-interest disclosure: S.P.T. and K.C.A. have received research support, consulting fees, and speaking honoraria from Celgene Inc and/or Genentech BioOncology Inc.
Correspondence: Steven P. Treon, Dana-Farber Cancer Institute, M547, 44 Binney Street, Boston, MA 02115; e-mail: steven_treon@dfci.harvard.edu.