Abstract
The phagocyte NADPH oxidase (NOX2) is critical for the bactericidal activity of phagocytic cells and plays a major role in innate immunity. We showed recently that NOX2 activity in mouse dendritic cells (DCs) prevents acidification of phagosomes, promoting antigen cross-presentation. Inorder to investigate the role of NOX2 in the regulation of the phagosomal pH in human DCs, we analyzed the production of reactive oxygen species (ROS) and the phagosomal/endosomal pH in monocyte-derived DCs and macrophages (MØs) from healthy donors or patients with chronic granulomatous disease (CGD). As expected, we found that human MØs acidify their phagosomes more efficiently than human DCs. Accordingly, the expression of the vacuolar proton ATPase (V-H+-ATPase) was higher in MØs than in DCs. Phagosomal ROS production, however, was also higher in MØs than in DCs, due to higher levels of gp91phox expression and recruitment to phagosomes. In contrast, in the absence of active NOX2, the phagosomal and endosomal pH decreased. Both in the presence of a NOX2 inhibitor and in DCs derived from patients with CGD, the cross-presentation of 2 model tumor antigens was impaired. We conclude that NOX2 activity participates in the regulation of the phagosomal and endosomal pH in human DCs, and is required for efficient antigen cross-presentation.
Introduction
The phagocyte NADPH oxidase (NOX2) belongs to the NOX family of reactive oxygen species (ROS)–generating proteins and plays a critical role in the bactericidal activity of phagocytic cells. This enzymatic complex includes 2 membrane-bound subunits that form cytochrome b558 and are the catalytic core of the enzyme (gp91phox and p22phox), and cytosolic subunits (p47phox, p67phox, p40phox and a small G protein rac), which regulate the enzymatic activity.1,2 The functional importance of NOX2 in host defense against infections is demonstrated by the absence or dysfunction of one or more of its components in individuals suffering from chronic granulomatous disease (CGD), a rare genetic disorder characterized by recurrent and often life-threatening infections by bacteria and fungi.3,4
Even though the role of NOX2 in innate immune responses has been widely described, its possible importance in adaptive immune responses is only beginning to be considered. Indeed, recent studies have focused on dendritic cells (DCs), the most efficient antigen-presenting cells and key players at the interface between innate and adaptive immunity.5-7 An important feature of adaptive immunity is cross-presentation, the ability of antigen-presenting cells to present internalized, exogenous antigens on MHC class I molecules.8,9 Cross-presentation is not only important for the initiation of immune responses against tumors and viruses that do not infect DCs, but it is also critical for the maintenance of peripheral tolerance to self-antigens.10
We have recently demonstrated that gp91phox−/− mouse bone marrow–derived DCs show an increased acidification of phagosomes, which results in excessive protein degradation and ineffective antigen cross-presentation.5 In contrast, and in opposition to what occurs in murine bone marrow–derived macrophages (BMMØs), the low levels of ROS produced by NOX2 consume protons in the phagosomal lumen and maintain the pH around 7.5. Moreover, it has been shown that inhibiting endosomal acidification with chloroquine or NH4Cl improves the efficiency of cross-presentation in human DCs, both in vitro and in vivo.11
Based on these results, we intended to gain insight into the function of NOX2 activity in human monocyte–derived DCs from healthy donors, as well as from gp91phox-deficient CGD patients. Here, we evaluate the participation of NOX2 in the regulation of endosomal and phagosomal pH in human monocyte–derived DCs, and investigate how these events impact on antigen cross-presentation. We also discuss the eventual role of NOX2 in the development of adaptive immune responses in humans.
Methods
Antibodies
The following antibodies were used: fluorescein isothiocyanate (FITC)–labeled mouse anti–human CD1a, CD80, CD86, HLA-A2, HLA-A,B,C, HLA-DR, IgG1 κ isotype control (BD Pharmingen, San Diego, CA); PE-labeled mouse anti–human CD14 and IgG1 κ isotype control (BD Pharmingen); Alexa Fluor 594–labeled goat anti–mouse IgG (Molecular Probes, Eugene, OR); horseradish peroxidase (HRP)–conjugated donkey anti–rabbit IgG, HRP-conjugated goat anti–mouse IgG (Jackson Immunoresearch, West Grove, PA); mouse anti–human α-tubulin (Calbiochem, Gibbstown, NJ); rabbit polyclonal anti–human rab5 (Abcam, Paris, France); rabbit polyclonal anti–human lamp-1, antihuman Erk-2, and anti–human NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA); rat polyclonal anti–human gp96 (Assay Designs, Ann Arbor, MI); mouse anti–human Melan A (Novocastra Laboratories, Newcastle, United Kingdom). The antibodies anti–human gp91phox and anti–human p47 were kindly provided by Dr J. El-Benna, (INSERM U773, Hôpital Bichat, Paris, France). The antibody anti–human V-H+-ATPase (16 kDa transmembrane subunit) was kindly provided by Dr Mhairi Skinner (Department of Molecular and Cellular Biology, University of Guelph, Guelph, ON).
DC and MØ generation
Peripheral blood mononuclear cells (PBMCs) from healthy donors were obtained from buffy coats provided by the Etablissement français du sang, site Crosatier, Paris, France. In the case of patients with CGD, heparinized blood was obtained from 12 gp91phox-deficient patients with CGD (age range: 5-26 years), with their informed consent and in accordance with the Declaration of Helsinki. Diagnosis was based on the analysis of the granulocyte respiratory burst, by nitroblue tetrazolium reduction and dihydrorhodamine-123 oxidation. Approval for these studies was obtained from the Clinical Research Committee and the Ethics Committee of Hospital Garrahan. Anti-CD14–conjugated magnetic microbeads (Miltenyi Biotec, Auburn, CA) were used to purify monocytes from PBMCs from controls or patients with CGD. Immature DCs were generated, as described,12 by culturing monocytes in RPMI 1640 Glutamax medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS; Biowest, Nuaillé, France), 100 ng/mL human recombinant GM-CSF (AbCys SA, Paris, France), and 40 ng/mL human recombinant IL-4 (AbCys SA) during 5 days. MØs were generated by culturing monocytes in RPMI 1640 Glutamax medium supplemented with 5% human serum AB (AbCys) and 40 ng/mL human recombinant M-CSF (AbCys) during 7 days.
T cells
The HLA-A2–restricted CD8+ T-cell clones LT12 and LT47 were generated and maintained in culture as previously described.13
Induction of apoptotic melanoma cells
HLA-A2–negative Mel888 melanoma cell line was cultured in RPMI 1640 Glutamax medium supplemented with 10% FCS and treated with oxaliplatin (5 μg/mL; Sanofi-Aventis, Paris, France) during 24 hours as described.14 The treatment resulted in a population containing 85% annexin V–positive propidium iodide–negative cells as assessed by annexin V–FITC apoptosis detection kit (BD Pharmingen) followed by FACS analysis.
Phagosomal pH measurement
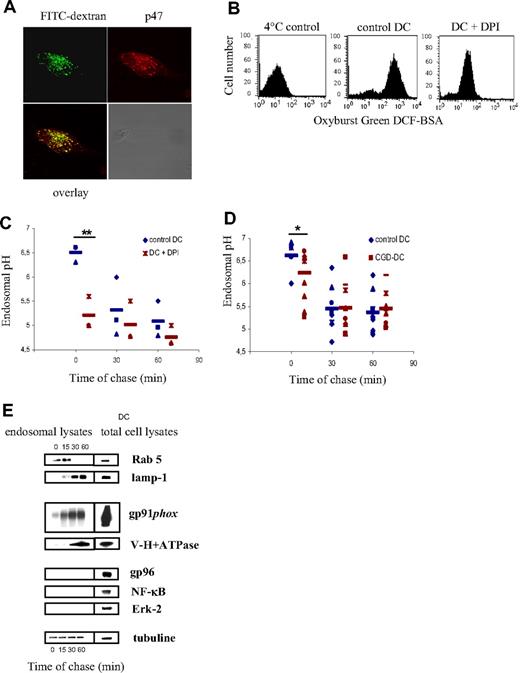
Phagosomal pH was measured as previously described.5 Briefly, cells were pulsed with 3 μm polybeads amino (Polysciences, Warrington, PA) covalently coupled with FITC (Sigma; pH sensitive) and FluoProbes 647 (pH insensitive; Molecular Probes) for 15 minutes at 37°C and extensively washed with cold phosphate-buffered saline (PBS). Cells were then incubated at 37°C (“chased”) for the indicated periods of time and immediately analyzed by FACS (FACSCalibur), using a FL1 (FITC)/FL4 (FluoProbes 647) gate selective for cells that have phagocytosed 1 latex bead. The ratio of the mean fluorescence intensity (MFI) emission between both dyes was determined. Values were compared with a standard curve obtained by resuspending the cells that had phagocytosed beads in CO2 independent medium (Invitrogen) at a fixed pH (ranging from pH 5 to pH 8) containing 0.1% Triton X-100. Cells were immediately analyzed by FACS to determine the emission ratio of both fluorescent dyes at each pH value. To evaluate the effect of blocking NOX2 activity, 10 μM diphenylene iodonium (DPI; Calbiochem) was added to DCs 30 minutes before the pulse and during the chase.
Endosomal pH measurement
Endosomal pH was measured using FITC-labeled 40 kDa dextran and Fluoprobes647-labeled 40 kDa dextran (Molecular Probes). Cells were pulsed with both labeled dextrans (1 mg/mL) for 10 minutes at 37°C and extensively washed with cold PBS. Cells were then “chased” for the indicated periods of time and immediately analyzed by FACS, using a FL1/FL4 gate selective for cells that have endocytosed. The ratio of the mean fluorescence intensity (MFI) emission between both dyes was determined. A control of dextran attached to cells was performed keeping cells at 4°C during endocytosis. Values were compared with a standard curve obtained by resuspending the cells that had endocytosed dextran in CO2 independent medium (GIBCO) at a fixed pH (ranging from pH 5 to pH 8) containing 0.01% Triton X-100. Cells were immediately analyzed by FACS to determine the emission ratio of both fluorescent dyes at each pH value. To evaluate the effect of blocking NOX2 activity, 10 μM DPI was added to DCs 30 minutes before the pulse and during the chase.
NOX2 activity measurement
NOX2 total and phagosomal activity were measured by chemiluminiscence, as previously described.15 DCs or MØs (0.5 × 106) were resuspended in 200 μL CO2-independent medium (Invitrogen) containing 10 μM luminol (Sigma) and 5 U of horseradish peroxidase (Sigma). Cells were stimulated with 0.5 μg/mL PMA and chemiluminiscence was measured over 180 minutes. To analyze phagosomal NOX2 activity, luminol was covalently coupled to 3 μm polybeads amino. Beads were first treated with 8% glutaraldehyde in PBS, overnight at room temperature and with agitation. Afterward, beads were washed with PBS and incubated with 100 mM luminol in DMSO during 4 hours at room temperature, subsequently washed with 0.5 M glycine and PBS and stored in PBS. DCs or MØs were pulsed with luminol-coupled beads for 15 minutes and then extensively washed with PBS. In order to obtain the same percentage of phagocytosis between DCs and MØs, the dilution of beads used for the pulse was higher for MØs than for DCs. Cells were resuspended in 200 μL of CO2-independent medium containing 5 U horseradish peroxidase and placed in the luminometer at 37°C for chemiluminiscence measurement over 180 minutes. When indicated, 0.5 μg/mL PMA was added before starting the measurement.
In order to analyze endosomal NOX2 activity, DCs were pulsed (10 minutes, 37°C) with 0.5 mg/mL bovine serum albumin (BSA) coupled to 2′,7′-dichlorodihydrofluorescein diacetate, succinimidyl ester (Oxyburst Green H2DCFDA, SE; Molecular Probes) according to the manufacturer's instructions, and washed with PBS. The H2DCF moiety is nonfluorescent until oxidized to dichlorofluorescein (DCF). Oxidation of the conjugate with BSA was detected by FACS. Controls were performed pulsing DCs at 4°C.
Cross-presentation assays
In order to study cross-presentation of tumor antigens by human DCs, we set up an in vitro assay using polypeptidic melanoma-associated antigen precursors MelanA/MART-1 or gp100 (“short” and “long” peptides), or apoptotic melanoma cells. The “long” peptides are 26 amino acids long and have been previously used as precursors for in vitro analysis of proteasomal activity.13 Each synthetic “long” peptide includes one HLA A2-binding “short” epitope recognized by the CTL clone LT12 (in the case of MelanA) or LT47 (in the case of gp100).
Briefly, 20 000 HLA-A2+ DCs were pulsed for 2 hours with either long or short MelanA/MART-1 or gp100 peptides in the presence of RPMI medium supplemented with FCS at a low concentration, washed 3 times with PBS, and cocultured with the corresponding HLA-A2–restricted T-cell clone at a 1:1 ratio. Coculture was performed in 96-well plates, during 5 hours. In the case of apoptotic melanoma cells, 30 000 HLA-A2+ DCs were pulsed with 30 000, 10 000, 3000, and 1000 apoptotic HLA-A2–negative Mel888 cells during 5 hours and cocultured with the HLA-A2–restricted T-cell clone LT12 at a 1:1 ratio. Coculture was performed in 96-well plates, during 30 hours.
Presentation and cross-presentation of MelanA/MART-1 or gp100 antigens were assessed by the antigen-specific response of the T-cell clone LT12 or LT47. T-cell clone activation was evaluated by enzyme-linked immunosorbent assay (ELISA) quantification of IFN-γ present in the coculture supernatant following appropriate dilution.
Alternatively, 3-μm polybeads amino (Polysciences) were coated with 750 μM MelanA long peptide and different numbers of HLA-A2+ DCs (1000, 3000, and 10 000) were pulsed for 2 hours. Coculture was performed as described in this subsection.
In order to inhibit NOX2 activity, HLA-A2+ DCs were pretreated with DPI (10 μM) for 45 minutes, and washed after the pulse.
Phagosome purification
DCs and MØs were incubated for 15 minutes with 3-μm magnetic latex beads (Invitrogen; pulse), then chased for different periods of time and disrupted in homogeneization buffer.16 Magnetic phagosomes were removed from the postnuclear supernatant using a magnet, washed 3 times in cold PBS, and lysed. After 15 minutes at 4°C, the magnetic beads were removed by centrifugation.
Endosome purification
DCs and MØs were incubated for 30 minutes with magnetic nanoparticles (8-nm diameter; kindly provided by J. Roger, Université Paris VI, CNRS, Paris, France) at 4°C (pulse), then chased for different periods of time at 37°C, washed and disrupted in homogenization buffer, as described.17 Magnetic endosomes were removed from the postnuclear supernatant using a magnet, washed 3 times in cold PBS, and lysed. After 15 minutes at 4°C, the magnetic particles were removed by centrifugation.
Immunoblotting
Phagosomes, endosomes, or whole-cell lysates (5 μg/lane) were analyzed by 4% to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; NuPAGE, Invitrogen). Proteins were transferred to PVDF membranes (Immobilon, Millipore) and incubated with primary antibodies and HRP-conjugated secondary antibodies. Development was performed with ECL Western blotting reagents (GE Healthcare, Little Chalfont, United Kingdom).
Immunofluorescence
In order to detect NOX2 in endosomes, DCs were pulsed with 70 kDa FITC-dextran during 10 minutes at 37°C. After extensive washing with cold PBS the cells were placed on poly-L-lysine–coated (Sigma) coverslips at 37°C in a 5% CO2 atmosphere for 10 minutes. Cells were then washed and fixed with 2% paraformaldehyde for 10 minutes at 4°C. After washing, cells were permeabilized with 0.05% saponin in PBS, 0.2% BSA for 30 minutes and incubated with an antihuman p47 antibody. Cells were subsequently washed and incubated with Alexa Fluor 594 goat anti–rabbit IgG (Molecular Probes). Immunofluorescence images were acquired with a Zeiss confocal microscope (LSM Axivert 720) using 63×/1.4 NA oil-immersion objective and analyzed with LSM Image software.
Statistical analysis
Statistical analysis was performed using the nonparametric Wilcoxon test for paired or impaired data, as indicated in the text. This test applies to nonnormally distributed data and compares pairs of values from the same population (same blood donor; paired data) or values from different populations (impaired data).
Results
Phagosomal pH in human DCs and MØs
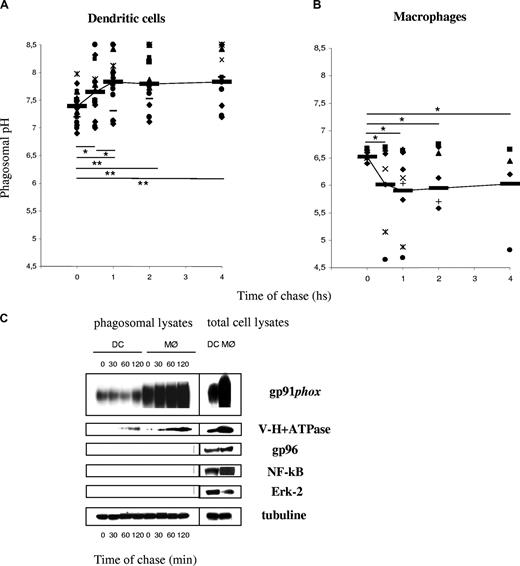
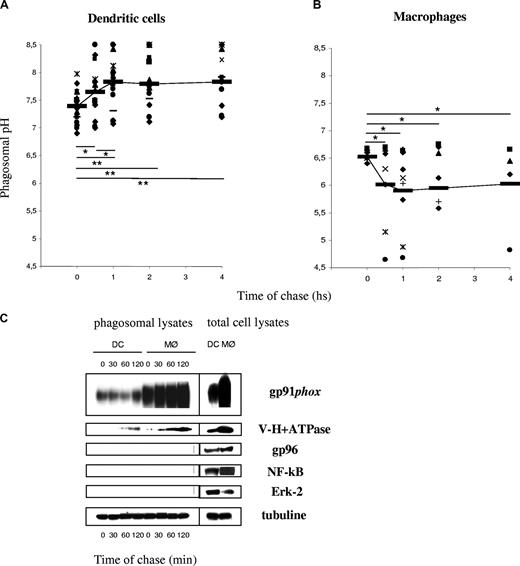
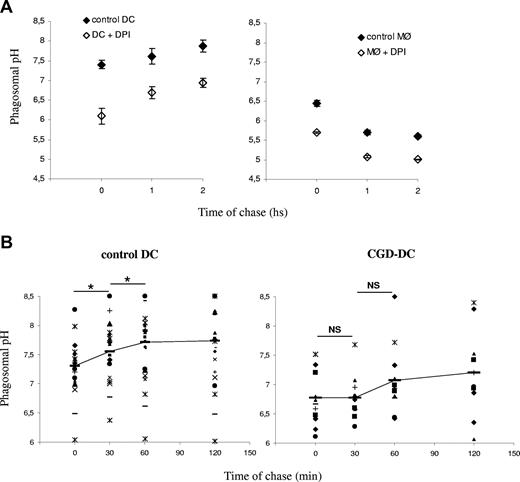
We showed previously that the phagosomal pH in mouse DCs, in contrast to mouse MØs, fails to acidify in the first 3 hours after phagocytosis.5 As shown in Figure 1A, the average phagosomal pH in DCs increases from an initial value of 7.4 plus or minus 0.25 (after a 10-minute phagocytosis pulse) to 7.8 plus or minus 0.5 (after an additional 1 h chase; P = .006). All the healthy donors displayed higher phagosomal pH after 1 hour of phagocytosis, as compared with the pH value directly after the pulse. The phagosomal pH in human MØs acidifies rapidly, reaching pH values below 6 after 30 minutes of phagocytosis (Figure 1B), as expected.18,19 We conclude that the phagosomal lumen in DCs alkalinizes in the first few hours after phagocytosis, whereas the phagosomal pH in MØs acidifies efficiently.
Phagosomal pH increases in human DCs, in contrast to human MØs, after latex bead phagocytosis. (A) DC phagosomal pH kinetics after a 15-minute phagocytosis pulse and the indicated time points of chase. (B) MØ phagosomal pH kinetics after a 15-minute phagocytosis pulse and the indicated time points of chase. Each dot represents a different blood donor. Slashes show average pH. Statistics were performed using the nonparametric Wilcoxon test for paired data. *P < .05; **P < .01. (C) Phagosomal membrane expression of gp91phox and V-H+-ATPase assessed by Western blot in human DCs and MØs, after different time points of chase following magnetic bead phagocytosis and phagosome isolation. Purity of the fractions was assessed by evaluating the expression of gp96, Erk-2, and NF-κB.
Phagosomal pH increases in human DCs, in contrast to human MØs, after latex bead phagocytosis. (A) DC phagosomal pH kinetics after a 15-minute phagocytosis pulse and the indicated time points of chase. (B) MØ phagosomal pH kinetics after a 15-minute phagocytosis pulse and the indicated time points of chase. Each dot represents a different blood donor. Slashes show average pH. Statistics were performed using the nonparametric Wilcoxon test for paired data. *P < .05; **P < .01. (C) Phagosomal membrane expression of gp91phox and V-H+-ATPase assessed by Western blot in human DCs and MØs, after different time points of chase following magnetic bead phagocytosis and phagosome isolation. Purity of the fractions was assessed by evaluating the expression of gp96, Erk-2, and NF-κB.
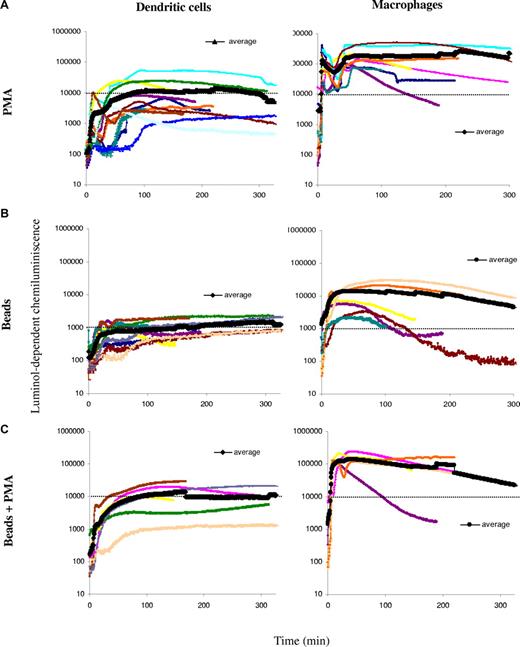
NOX2 activity in human DCs and MØs
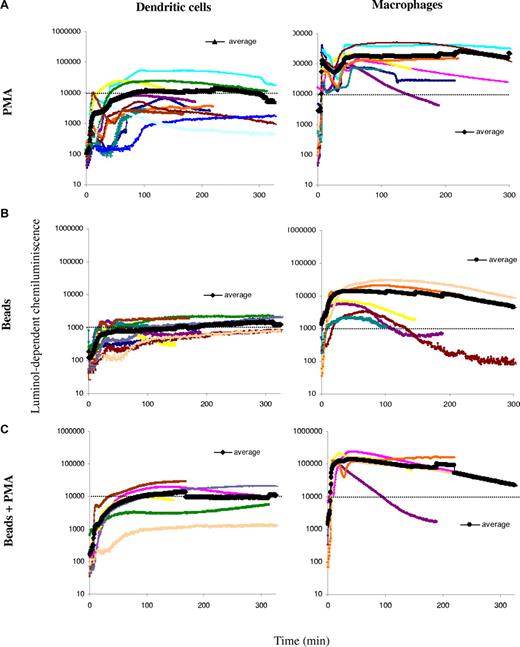
Murine DCs fail to acidify their phagosomes due to an active mechanism of phagosomal alkalinization. Active alkalinization is due to NOX2 assembly in the phagosomal membrane in DCs.5 In murine resting BMMØs, low levels of NOX2 activity result in active acidification. In order to investigate NOX2 activity in human DCs and MØs, ROS production was measured by chemiluminiscence (Figure 2). We first analyzed total ROS production after stimulation with PMA in a series of 10 healthy donors. As shown in Figure 2A, ROS production in DCs ranged between 103 and 7 × 104 arbitrary units at the plateau, with an average around 104. ROS production in MØs was, in average, 10-fold higher than in DCs. ROS production in phagosomes, with or without PMA stimulation, followed a similar pattern: MØs produce, on average, 10-fold more ROS than DCs (Figure 2B,C). This high production of ROS is consistent with higher levels of gp91phox expression and recruitment to phagosomes in MØs, as compared with DCs (a representative Western blot analysis of total lysates and purified phagosomes is shown in Figure 1C). We conclude that, in contrast to BMMØs, human MØs produce higher levels of ROS than DCs. The strong acidification observed in MØs suggests that ROS production in MØs does not affect acidification, in contrast to what has been described in neutrophils and in mouse DCs.5,20 This apparent discrepancy between NOX2 expression/activity and phagosomal pH in human DCs versus MØs, can be partially explained by the differential expression and phagosomal recruitment of the vacuolar-type H+ ATP-ase (V-H+-ATPase) in both cell types. Indeed, MØs expressed higher levels of the V-H+-ATPase, which was recruited to phagosomes earlier and in higher amounts, as compared with DCs (Figure 1C).
ROS production in human DCs and MØs. ROS production was measured over time using luminol-dependent chemiluminiscence, after stimulation with PMA and/or phagocytosis of luminol-coated beads in DCs (left panels) and MØs (right panels) generated in parallel from a series of healthy volunteers. Thick lines show the average of all experiments. (A) Total ROS production after cell stimulation with 0.5 μg/mL PMA in DCs or MØs from the same donors. (B) DC and MØ phagosomal ROS production after a 15-minute pulse with luminol-coated beads. (C) DC and MØ phagosomal ROS production after a 15-minute pulse with luminol-coated beads followed by the addition of 0.5 μg/mL PMA.
ROS production in human DCs and MØs. ROS production was measured over time using luminol-dependent chemiluminiscence, after stimulation with PMA and/or phagocytosis of luminol-coated beads in DCs (left panels) and MØs (right panels) generated in parallel from a series of healthy volunteers. Thick lines show the average of all experiments. (A) Total ROS production after cell stimulation with 0.5 μg/mL PMA in DCs or MØs from the same donors. (B) DC and MØ phagosomal ROS production after a 15-minute pulse with luminol-coated beads. (C) DC and MØ phagosomal ROS production after a 15-minute pulse with luminol-coated beads followed by the addition of 0.5 μg/mL PMA.
DPI induces a decrease of the phagosomal pH in DCs and MØs
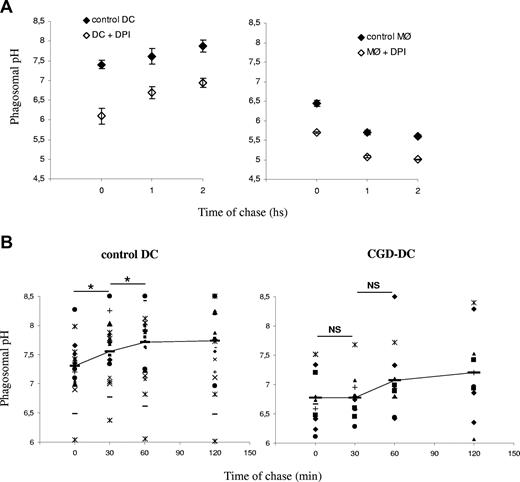
The potential influence of phagosomal ROS production on the phagosomal pH was first investigated using a specific inhibitor of flavoproteins, DPI, which, among other flavoproteins, completely blocks NOX2 at low concentrations. Pretreatment of DCs or MØs with DPI induced a marked decrease in phagosomal pH in 3 different healthy donors (Figure 3A). In the DPI-treated DCs, the phagosomal pH increased over time, but did not become alkaline, like in untreated DCs. These results suggest that ROS production in phagosomes, presumably by NOX2, contributes to pH regulation in both DCs and MØs. These results also show that even in the absence of ROS production (which was efficiently blocked by DPI), the phagosomal pH in DCs is much higher than in MØs.
Reduced alkalinization of the phagosomal pH in the absence of NOX2 activity. (A) DC (left) and MØ (right) phagosomal pH kinetics in the presence or absence of 10 μM DPI. The average and standard deviation (SD) of 3 independent experiments (3 different donors) are shown. (B) Control DC (left) and CGD-DC (right) phagosomal pH kinetics after a 15-minute phagocytosis pulse and the indicated time points of chase. Dots represent different blood donors. Slashes show average pH. Statistics were performed using the nonparametric Wilcoxon test for paired data. *P < .05; **P < .01
Reduced alkalinization of the phagosomal pH in the absence of NOX2 activity. (A) DC (left) and MØ (right) phagosomal pH kinetics in the presence or absence of 10 μM DPI. The average and standard deviation (SD) of 3 independent experiments (3 different donors) are shown. (B) Control DC (left) and CGD-DC (right) phagosomal pH kinetics after a 15-minute phagocytosis pulse and the indicated time points of chase. Dots represent different blood donors. Slashes show average pH. Statistics were performed using the nonparametric Wilcoxon test for paired data. *P < .05; **P < .01
DCs from patients with CGD fail to alkalinize their phagosomes
In order to directly address the eventual role of NOX2 in phagosomal pH control, we analyzed DCs derived from a series of 12 patients with CGD. These patients bear mutations in the gp91phox subunit of the NADPH oxidase, the catalytic subunit of NOX2. The diagnosis of these patients is based on the inability of their granulocytes to reduce NBT and to oxidize dihydrorhodamine-123. Because volumes of 50 mL blood were needed for the experiments, patients with CGD who were older than 5 years were selected for the study (range: 5-26 years). Informed consents were signed by the patients or their tutors. Monocytes were isolated from their blood samples, and DCs were differentiated in vitro. The phenotypes of the DCs were indistinguishable from those shown by healthy donors' DCs (the expression of CD1a, CD14, CD80/86, and MHCI/II was evaluated; not shown).
We analyzed the phagosomal pH in CGD-derived DCs, as compared with DCs derived from healthy donors (Figure 3B). In contrast to what we found in control DCs, the phagosomal pH in CGD-DCs did not increase significantly over time. Moreover, the phagosomal pH values were significantly lower (7 ± 0.57 vs 7.9 ± 0.7; P = .009) in CGD-DCs, as compared with control DCs, up to 60 minutes after phagocytosis. We conclude that NOX2 contributes to the alkalinization of phagosomes in human DCs. Nevertheless, reduced ROS production in CGD-DC phagosomes does not result in efficient phagosome acidification, as compared with macrophages, suggesting that other phagosomal pumps or channels (including the V-H+-ATPase), contribute to the differences in acidification in both cell types.
NOX2 activity enhances antigen cross-presentation of particulate antigens
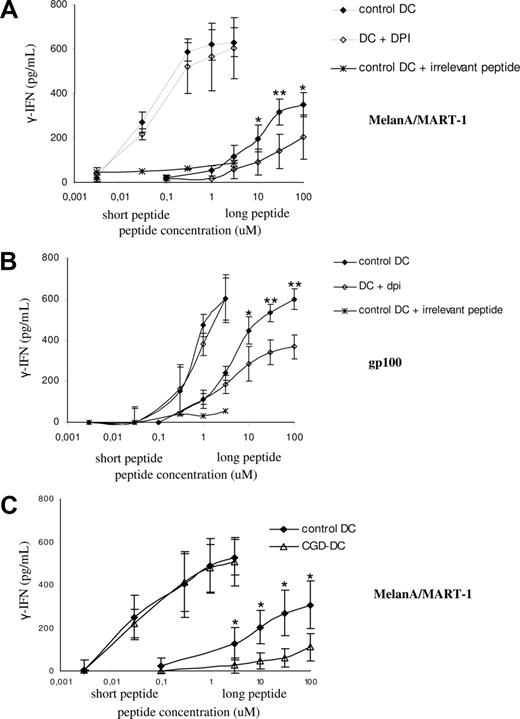
We showed previously that enhanced acidification of the phagocytic pathway impairs cross-presentation in murine DCs.5 The role of acidification in cross-presentation in human DCs was analyzed first using DPI. We set up a cross-presentation assay using a system of long and short peptides and HLA-A2–restricted CTL clones, as described in “Methods.” Cross-presentation of the extended peptides, but not of the short peptides, requires processing by the DCs, as the production of IFN-γ is blocked by proteasome inhibitors or fixation of the DC (F.F., A.R.M., C. Sadaka, S.A., manuscript in preparation).
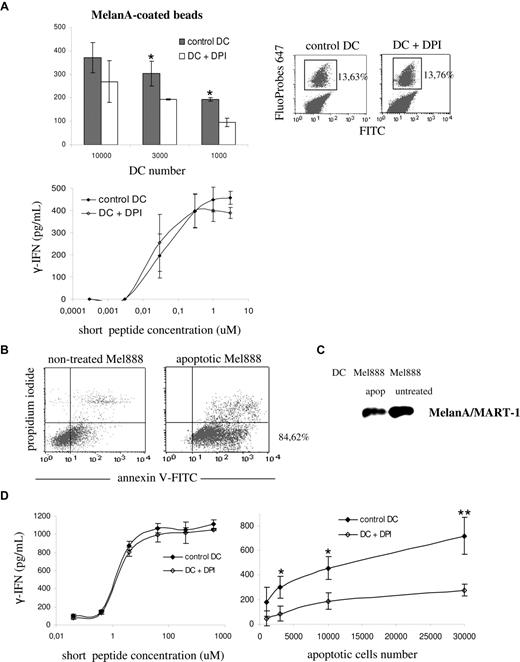
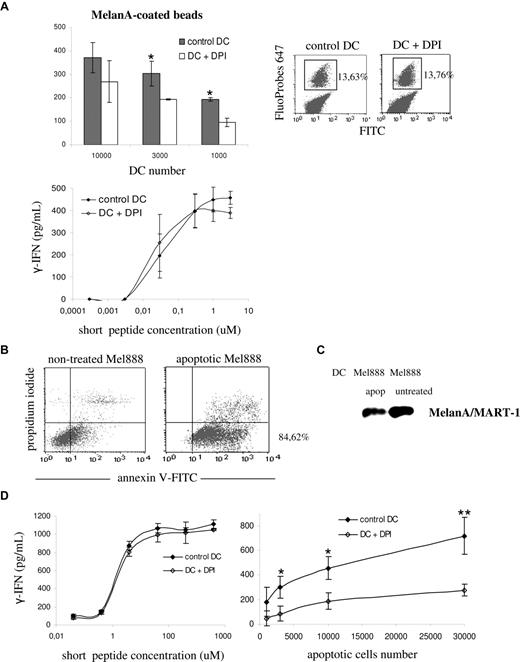
As shown in Figure 4A (top left panel), the presentation of the long MelanA/MART-1 peptide coated to beads was partially blocked by treatment of DCs with DPI. In contrast, DCs presented the short peptide to the T-cell clone in a dose-dependent, DPI-insensitive manner (Figure 4A bottom left panel). Treatment with DPI did not alter the phagocytic activity of DCs, as shown by FACS analysis of FITC/FluoProbes 647–labeled latex beads (Figure 4A right panel). We further extended our observations to the cross-presentation of MelanA/MART-1 present in apoptotic melanoma cells. Overnight treatment with oxaliplatin induced apoptosis in 85% of the cells, as shown by annexin V labeling in Figure 4B. MelanA/MART-1 expression after treatment was confirmed by Western blot (Figure 4C). As shown in Figure 4D, DPI-treatment of DCs had no impact on the presentation of the short peptide to the T-cell clone. In contrast, antigen cross-presentation of MelanA/MART-1 from apoptotic melanoma cells was impaired, as compared with control DCs. These results suggest that, similar to mouse DCs, NOX2 activity is required for cross-presentation in human DCs.
Efficient cross-presentation of melanoma particulate antigens requires NOX2 activity. (A) Control HLA-A2–positive DCs (gray) and DPI-treated DCs (white) were pulsed with either MelanA/MART-1 long peptides coated to latex beads (top left panel) or MelanA/MART-1 short peptides (bottom panel). DCs were pretreated with 10 μM DPI 30 minutes before the pulse and washed before the coculture with the CTL clone. The percentage of phagocytosis of FITC/FluoProbes 647 latex beads by control and DPI-treated DCs was assessed by FACS (top right panel). Cross-presentation was evaluated by measuring the production of IFN-γ by the HLA-A2–restricted CTL clone LT12 after DC-CTL coculture. (B) HLA-A2–negative Mel888 melanoma cells were treated overnight with oxaliplatin to induce apoptosis. Apoptosis and necrosis were quantified using annexin V–FITC and propidum iodide, respectively, by FACS. (C) Expression of MelanA/MART-1 in whole-cell lysates from apoptotic and nontreated Mel888 cells, assessed by Western blot. (D) Control HLA-A2–positive DCs (black) and DPI-treated DCs (white) were pulsed with either MelanA/MART-1 short peptides (left panel) or different numbers of apoptotic Mel888 cells, as described in “Methods.” Cross-presentation was evaluated as indicated.
Efficient cross-presentation of melanoma particulate antigens requires NOX2 activity. (A) Control HLA-A2–positive DCs (gray) and DPI-treated DCs (white) were pulsed with either MelanA/MART-1 long peptides coated to latex beads (top left panel) or MelanA/MART-1 short peptides (bottom panel). DCs were pretreated with 10 μM DPI 30 minutes before the pulse and washed before the coculture with the CTL clone. The percentage of phagocytosis of FITC/FluoProbes 647 latex beads by control and DPI-treated DCs was assessed by FACS (top right panel). Cross-presentation was evaluated by measuring the production of IFN-γ by the HLA-A2–restricted CTL clone LT12 after DC-CTL coculture. (B) HLA-A2–negative Mel888 melanoma cells were treated overnight with oxaliplatin to induce apoptosis. Apoptosis and necrosis were quantified using annexin V–FITC and propidum iodide, respectively, by FACS. (C) Expression of MelanA/MART-1 in whole-cell lysates from apoptotic and nontreated Mel888 cells, assessed by Western blot. (D) Control HLA-A2–positive DCs (black) and DPI-treated DCs (white) were pulsed with either MelanA/MART-1 short peptides (left panel) or different numbers of apoptotic Mel888 cells, as described in “Methods.” Cross-presentation was evaluated as indicated.
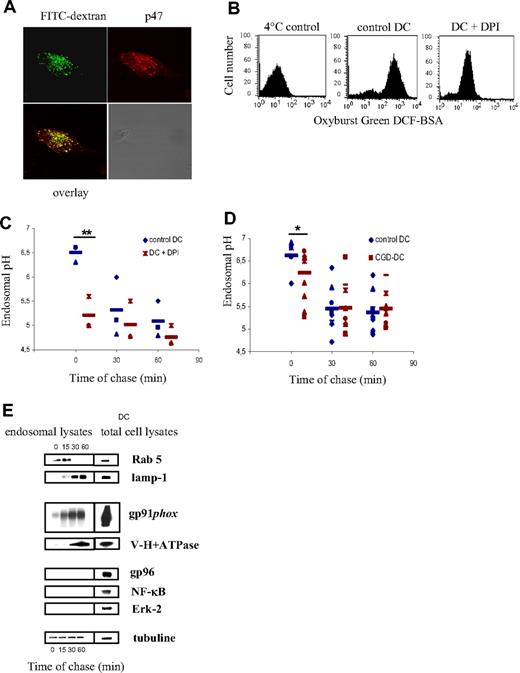
NOX2 also regulates endosomal pH
In addition to phagocytosis, DCs efficiently take up soluble antigens via endocytosis. In order to investigate if NOX2 also assembles on the endosomal membrane in DCs, we pulsed DCs with 70 kDa FITC-labeled dextran and we analyzed the recruitment of the cytosolic NOX2 subunit p47 to FITC dextran–positive endosomes by confocal microscopy. We have detected that p47 colocalizes with FITC dextran after a 10-minute pulse (Figure 5A), showing that NOX2 is also recruited to the endosomal membranes. In order to analyze NOX2 activity in endosomes, DCs were pulsed with BSA coupled to Oxyburst Green H2DCF. The oxidation of the conjugate by endosomal ROS was then measured by FACS. We have detected that control DCs oxidize the conjugate, compared with DCs pulsed at 4°C. In contrast, DCs pretreated with DPI displayed reduced oxidative activity (Figure 5B).
Endosomal pH decreases in normal and CGD-DCs after dextran endocytosis. (A) Colocalization of FITC-dextran (green) and p47 (red) on endosomes of DCs, after a 10-minute endocytic pulse, as detected using confocal microscopy. (B) Endosomal ROS production measured by FACS. Control and DPI-treated DCs were pulsed with Oxyburst Green H2DCF coupled to BSA. Oxidation of the conjugate by endosomal ROS was detected by FACS. (C) DC endosomal pH kinetics after a 10-minute endocytosis pulse and the indicated time points of chase in the presence or absence of 10 μM DPI. The average and SD of 3 independent experiments are shown. Statistics were performed using the nonparametric Wilcoxon test for impaired data. **P < .01. (D) Control DC (blue) and CGD-DC (red) endosomal pH kinetics after a 10-minute endocytosis pulse and the indicated time points of chase. Statistics were performed using the nonparametric Wilcoxon test for paired data. *P < .05. (E) Endosomal membrane expression of Rab 5, lamp-1, gp91phox, and V-H+-ATPase after different time points of chase at 37°C following a 30-minute endocytic pulse at 4°C, as described in “Methods.” Purity of the fractions was assessed by evaluating the expression of gp96, Erk-2, and NF-κB.
Endosomal pH decreases in normal and CGD-DCs after dextran endocytosis. (A) Colocalization of FITC-dextran (green) and p47 (red) on endosomes of DCs, after a 10-minute endocytic pulse, as detected using confocal microscopy. (B) Endosomal ROS production measured by FACS. Control and DPI-treated DCs were pulsed with Oxyburst Green H2DCF coupled to BSA. Oxidation of the conjugate by endosomal ROS was detected by FACS. (C) DC endosomal pH kinetics after a 10-minute endocytosis pulse and the indicated time points of chase in the presence or absence of 10 μM DPI. The average and SD of 3 independent experiments are shown. Statistics were performed using the nonparametric Wilcoxon test for impaired data. **P < .01. (D) Control DC (blue) and CGD-DC (red) endosomal pH kinetics after a 10-minute endocytosis pulse and the indicated time points of chase. Statistics were performed using the nonparametric Wilcoxon test for paired data. *P < .05. (E) Endosomal membrane expression of Rab 5, lamp-1, gp91phox, and V-H+-ATPase after different time points of chase at 37°C following a 30-minute endocytic pulse at 4°C, as described in “Methods.” Purity of the fractions was assessed by evaluating the expression of gp96, Erk-2, and NF-κB.
In order to measure the endosomal pH in human DCs, we used a mixture of 40 kDa dextran labeled with FITC or with FluoProbes647, as described in “Methods.” In contrast to the phagosomal pH, we found that the endosomal pH decreased over time after endocytosis (Figure 5C). However, similar to the phagosomal pH, the endosomal pH becomes more acidic when cells are pretreated with DPI, suggesting that NOX2 also plays a role in the maintenance of a higher pH in endosomes. This decrease in the endosomal pH induced by DPI is only observed at early time points after endocytosis (Figure 5C), suggesting that NOX2 controls the pH in early, but not in late endosomes.
To ascertain that pH regulation in endosomes is due to NOX2, and not to other flavoenzymes, we analyzed the endosomal pH in DCs from patients with CGD. As shown in Figure 5D, we observed that, consistent with our observations using DPI, the endosomal pH was decreased at early time points after endocytosis in CGD-DCs, as compared with control DCs. At later time points, the endosomal pH was similar in DCs from healthy donors and those from patients with CGD. These results show that NOX2 contributes to the regulation of endosomal pH.
The observation that the endosomal pH is affected only at early time points after endocytosis suggests that NOX2 contributes mainly to the alkalinization of early endosomes, rather than late endosomes and lysosomes. We therefore analyzed the kinetics of recruitment of the gp91phox and the V-H+-ATPase to early and late endosomes. Endosomes were isolated using magnetic nanoparticles after different time points of endocytosis, as described in “Methods.” Rab5 and Lamp-1 were used as markers of early/recycling endosomes and late endosomes/lysosomes, respectively (Figure 5E). Interestingly, after 15 minutes of endocytosis, gp91phox was already recruited to endosomes, whereas the V-H+-ATPase was only detected in endosomes after 30 to 60 minutes of endocytosis. We conclude that the ratio of gp91phox to V-H+-ATPase decreases as endosomes mature, consistent with our observations that NOX2 selectively influences the pH in early endosomes. It is most likely that in late endosomes/lysosomes, elevated levels of acidification by the V-H+-ATPase outweigh alkalinization by NOX2 activity.
Cross-presentation of soluble antigens is impaired in CGD-DCs
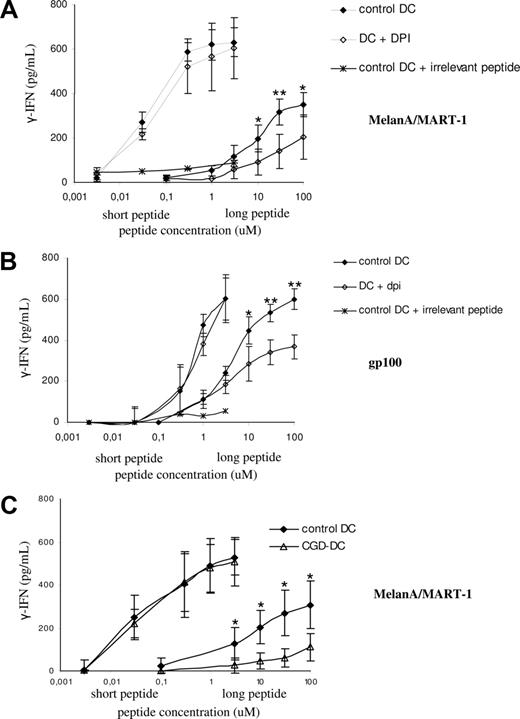
To investigate the role of NOX2 endosomal activity in cross-presentation, we generated DCs from several HLA-A2–positive healthy donors or patients with CGD. As shown in Figure 6A,B, DCs presented the short MelanA and gp100 peptides to the corresponding T-cell clones in a dose-dependent, DPI-insensitive manner. In contrast, the presentation of the long peptides (which requires processing by the DCs; ie, it is proteasome and fixation-sensitive; not shown), was partially blocked by treatment of DCs with DPI, suggesting that NOX2 activity is required for cross-presentation in human DCs. We actually demonstrated a role for NOX2 in cross-presentation using DCs from 4 patients with CGD bearing the HLA-A2 haplotype. As shown in Figure 6C, HLA-A2–positive DCs derived from patients with CGD presented the short peptide with similar efficiency to a series of healthy donors. In contrast, the cross-presentation of the long peptide was impaired in the DCs derived from the patients with CGD. We conclude that mutations in the gp91phox genes that impair the activity of the NADPH oxidase result in a partial defect in the cross-presentation of 2 soluble long peptides.
Antigen cross-presentation of melanoma soluble antigens is dependent on NOX2 activity. (A) Control HLA-A2–positive DCs (black) and DPI-treated DCs (white) were pulsed with the indicated amounts of short or long MelanA/MART1 peptides. Cross-presentation was evaluated by measuring the production of IFN-γ by the HLA-A2–restricted CTL clone LT12 after DC-CTL coculture. The average and SD of 4 independent experiments are shown. (B) Control HLA-A2–positive DCs (black) and DPI-treated DCs (white) were pulsed with the indicated amounts of short or long gp100 peptides, as described in “Methods.” Cross-presentation was evaluated by measuring the production of IFN-γ by the HLA-A2–restricted CTL clone LT47 after DC-CTL coculture. The average and SD of 4 independent experiments are shown. (C) Control HLA-A2–positive DCs (black) and CGD-DCs (white) were pulsed with the indicated amounts of short or long MelanA/MART1 peptides, as described in “Methods.” Cross-presentation was evaluated by measuring the production of IFN-γ by the HLA-A2–restricted CTL clone LT12 after DC-CTL coculture. The average and SD of 4 independent experiments are shown for control and CGD-DCs. Statistics were performed using the nonparametric Wilcoxon test for nonpaired data. *P < .05; **P < .01.
Antigen cross-presentation of melanoma soluble antigens is dependent on NOX2 activity. (A) Control HLA-A2–positive DCs (black) and DPI-treated DCs (white) were pulsed with the indicated amounts of short or long MelanA/MART1 peptides. Cross-presentation was evaluated by measuring the production of IFN-γ by the HLA-A2–restricted CTL clone LT12 after DC-CTL coculture. The average and SD of 4 independent experiments are shown. (B) Control HLA-A2–positive DCs (black) and DPI-treated DCs (white) were pulsed with the indicated amounts of short or long gp100 peptides, as described in “Methods.” Cross-presentation was evaluated by measuring the production of IFN-γ by the HLA-A2–restricted CTL clone LT47 after DC-CTL coculture. The average and SD of 4 independent experiments are shown. (C) Control HLA-A2–positive DCs (black) and CGD-DCs (white) were pulsed with the indicated amounts of short or long MelanA/MART1 peptides, as described in “Methods.” Cross-presentation was evaluated by measuring the production of IFN-γ by the HLA-A2–restricted CTL clone LT12 after DC-CTL coculture. The average and SD of 4 independent experiments are shown for control and CGD-DCs. Statistics were performed using the nonparametric Wilcoxon test for nonpaired data. *P < .05; **P < .01.
Discussion
The work presented here shows that NOX2 expressed in human monocyte–derived DCs contributes to prevent phagosome and endosome acidification. Indeed, DPI-treated and NOX2-defective DCs (ie, derived from monocytes from patients with CGD), displayed increased phagosome and early endosome acidification. As a consequence, the cross-presentation of 2 tumor antigens to CD8+ T cells was impaired. We conclude that NOX2-mediated alkalinization of the phagocytic and endocytic pathways in DCs is required for efficient antigen cross-presentation.
These results suggest that patients with CGD may present defects in adaptive immunity. The clinical relevance of this finding, however, has yet to be established. How, then, could an impaired antigen cross-presentation in patients with CGD impact immunopathology? The severity of CGD has been associated with the inability of phagocytes to perform the “respiratory burst” and kill certain microorganisms. CGD is therefore an “innate immunity” disease. Although autoimmunity is not a hallmark of children with CGD, increasing evidence links CGD with a higher risk of developing autoimmunity, especially T-helper type-1 (Th-1) autoimmune diseases, such as sarcoidosis, Crohn disease–like inflammatory bowel disease, and rheumatoid arthritis.19 Henoch-Schoenlein purpura and autoimmune thrombocytopenia have also been described in patients with CGD, and discoid lupus and systemic lupus erythematosus have been reported in mothers carrying the genetic defect and in certain patients.21-23 In mice and rats bearing mutations in the gp91phox or p47phox genes, increased severity of arthritis has also been reported.24,25
The mechanisms linking NADPH oxidase defect and autoimmunity are so far unknown. It has been proposed that the inability of phagocytic cells to completely destroy and clear microbial pathogens would result in an exaggerated and perpetuated inflammatory response that would favor autoimmunity.26 Moreover, NOX2-deficient T cells have a bias toward Th1 differentiation, which could favor the development of autoimmune disorders.27 Our results showing reduced cross-presentation in NOX2-defective DCs suggest an alternative mechanism to link NADPH oxidase defects and autoimmunity. Indeed, if thymic DCs showed a similar dependence on NOX2 expression for cross-presentation, the spectrum of epitopes presented in the thymus in patients with CGD could be affected, resulting in reduced deletion of autoreactive lymphocyte clones at the time of negative selection. This possibility can now be addressed experimentally, especially in animal models of CGD.
Regardless of the actual potential role of a cross-presentation defect in CGD, the analysis of NOX2-defective DCs showed that the NADPH oxidase contributes to the regulation of the phagosomal and endosomal pH. The very wide variability in the endocytic and phagocytic pH among healthy donors made the comparison between them and patients with CGD quite difficult (especially for endosomes). It was quite clear, nevertheless, that the phagosomal pH in the patients was more acidic early after phagocytosis and did not alkalinize, as it did in DCs from healthy donors. In endosomes, the most evident pH differences between healthy donors and patients were observed at early endocytic time points. It is unclear from our experiments if NOX2 assembly in endosomes in constitutive or is induced by the low levels of endotoxin contamination possibly present in the preparations of long peptides or dextran. This issue will be addressed in further studies. The overall conclusion from these experiments is that NOX2 activity contributes to limiting the acidification of phagosomes, and early endosomes. Nevertheless, even in the absence of active NOX2, acidification in DCs was still less potent than in MØs, consistent with the lower levels of V-H+-ATPase and with previous studies in mouse DCs.28
In contrast to our previous observations in murine BMMØs, human MØs display a higher NOX2 activity, even in phagosomes, than DCs. Surprisingly, ROS production in MØ phagosomes does not affect acidification. This phenomenon can be partially explained by the higher expression of the V-ATPase in MØ phagosomes, as compared with DCs. Another possible explanation could be a difference in the phagosomal membrane permeability to protons between MØs and DCs, as it has been recently proposed between MØ and neutrophils.29 Moreover, since the V-ATPase is electrogenic, differences in the extent of acidification could also be caused by variations in the permeability of the phagosomal membrane to counterions, such as chloride. Indeed, it has been shown that phagosomes in macrophages from CFTR-deficient mice are less acidic.30
Quite interestingly, however, and consistent with our previous results in mouse DCs, the differences in endosomal and phagosomal ROS production and pH resulted in a decrease on the efficiency of cross-presentation of long peptides. Short peptides, which do not require further processing by DCs for presentation to CD8+ T cells, were presented with similar efficiencies by DCs from healthy donors or from patients with CGD. Similar results were found after DPI-treatment of DCs, supporting our conclusion that NOX2 activity is required for efficient cross-presentation. The mechanism linking ROS production in endosomes/phagosomes and cross-presentation remains unclear. Differences in proteolytic activity and efficiency of antigen degradation could be involved. Indeed, increased acidification should augment the efficiency of proteolysis in endosomes and phagosomes, thus degrading part of the epitopes that could otherwise be cross-presented. Other mechanisms linking pH and cross-presentation could also be evoked. For example, if the loading of peptides onto MHC class I molecules occurs in the endocytic pathway (which remains a likely possibility), low pH should be detrimental. Other steps of cross-presentation, such as transport to the cytosol, could also require high pH in the lumen of the endocytic pathway.
In conclusion, the defects on adaptive immune responses we had previously observed in DCs from gp91phox-deficient mice are also present in human DCs derived from patients with CGD. The impact of the absence or reduction of NOX2 expression is less striking in humans than in mice, though. In some patients in which the expression of NOX2 is not completely abrogated, residual NOX2 activity could explain this discrepancy. It is also possible that other mechanisms still unraveled become important in phagocytic cells in order to partially compensate for the impairment in NOX2 activity. In any case, our results suggest that CGD could be considered as a disorder that affects both innate and adaptive immune responses. This new view of CGD represents not only a contribution to the understanding of this disorder, but it could also widen the perspectives for the treatment of this disease.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Jamel El-Benna and Pham My-Chan Dang (Hôpital Xavier Bichat, Paris, France) for helpful discussions and for providing the anti-gp91phox and anti-p47 antibodies. We thank Mhairi Skinner (University of Guelph, Guelph, ON) for the provision of the antibody anti–V-H+-ATPase, and J. Roger (Université Paris VI, France) for the provision of the magnetic nanoparticles. We are especially thankful to all the members of U653 Inserm (Paris, France), and Jorge Geffner's laboratory (Buenos Aires, Argentina), for their help with reagents and discussions. We thank Renata Luzzi and Monica Saracco for their technical assistance.
This work was supported by grants from the Ligue National de Lutte contre le Cancer, the Welcome Trust, Biotechnology and Biological Sciences Research Council, Fondation Bettencourt, the Association pour la Recherche contre le Cancer (ARC, prix L. Griffuel), Contrat EC DC-Thera LSBH-CT-2004–512074 “Dendritic cells for novel immunotherapies,” and Contrat EC Cancer Immunotherapy LSHC-CT-2006–518234 “Cancer Immunology and Immunotherapy.” A.M. was supported by ARC and the Institut Curie, France. A.S. was supported by Inserm and the Marie Curie Fellowship Program. M.V. was supported by CONICET and Agencia Nacional de Promoción Científica y Tecnológica, Argentina. S.D.R. was supported by the National Institutes of Health (NIH)/Fogarty International Center (FIC)/ Global Research Initiative Program (GRIP) Grant R01 TW006644.
National Institutes of Health
Authorship
Contribution: A.M., A.S., J.G., S.R., F.F., and S.A. designed the research; A.M., A.S., M.V., and F.F. performed experiments; L.P., O.H., and S.R. provided blood samples from patients with CGD and contributed to discussions; J.G., F.F., and S.A. supervised the research; and A.M. and S.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sebastian Amigorena, Unité 653 Inserm, Institut Curie, 26 rue d'Ulm, 75 005 Paris, France; e-mail: sebastian.amigorena@curie.fr.