Abstract
Graft rejection has been defined as the mirror image of graft-versus-host disease, which is biologically characterized primarily as a Th1-type process. As such, we reasoned that graft rejection would represent a Th1 response amenable to Th2 modulation. Indeed, adoptive transfer of host Th1-type cells mediated rejection of fully MHC-disparate murine bone marrow allografts more effectively than host Th2-type cells. Furthermore, STAT1-deficient host T cells did not differentiate into Th1-type cells in vivo and failed to mediate rejection. We next hypothesized that donor Th2 cell allograft augmentation would prevent rejection by modulation of the host Th1/Th2 balance. In the setting of donor Th2 cell therapy, host–anti-donor allospecific T cells acquired Th2 polarity, persisted posttransplantation, and did not mediate rejection. Abrogation of rejection required donor Th2 cell IL-4 secretion and host T-cell STAT6 signaling. In conclusion, T cell–mediated marrow graft rejection primarily resembles a Th1-type process that can be abrogated by donor Th2 cell therapy that promotes engraftment through a novel mechanism whereby cytokine polarization is transferred to host T cells.
Introduction
Graft rejection limits the success of allogeneic bone marrow transplantation (BMT) because current strategies of preventing rejection primarily involve donor T cells (which initiate graft-vs-host disease [GVHD]) and/or intensive host conditioning (which promotes transplantation-related morbidity and mortality). It is therefore important to better understand the T-cell biology underlying graft rejection to develop alternative methods of ensuring genetically disparate transplantation.
Although clinical graft rejection and GVHD are “mirror images,”1 their biologic overlap is less well documented. Host and donor T cells have long been known to mediate graft rejection2 and GVHD,3 respectively. In murine acute GVHD, the Th1/Th2 immune regulation paradigm is relevant because adoptively transferred donor Th2 cells inhibit Th1-mediated GVHD.4,5 More recently, we found that ex vivo rapamycin generates Th2 cells (Th2R cells) that potently inhibit murine GVHD through an IL-4–dependent mechanism.6
Although both CD4+ and CD8+ host T cells mediate graft rejection,7 the relative contribution of CD4+ Th1/Th2 or CD8+ Tc1/Tc2 subsets has not been clarified. Furthermore, the molecular basis of graft rejection after allogeneic BMT remains poorly defined. Host T-cell use of granzyme B cytolysis during rejection was relatively dispensable in one model.8 Similarly, rejection occurred independent of perforin or fas ligand (fasL)9 or combination blockade of perforin, fasL, and TNF-family molecules.10 We recently associated the presence or absence of host T cell–allospecific IFN-γ secretion with graft rejection or alloengraftment, respectively.11 These IFN-γ findings are consistent with data from an in vitro model of rejection12 but fall short of providing direct evidence for Th1/Th2 immune regulation of graft rejection. In this project, our first objective was to determine whether host Th1- and Th2-type T cells differentially mediate rejection. To accomplish this, we used a host T-cell add-back model13 to evaluate ex vivo costimulated and polarized host T cells in a manner analogous to our previous GVHD studies.14
The role of donor T cells in preventing rejection has been more extensively studied than the role of host T cells in mediating rejection. Initial research indicated an important graft-facilitating role of alloreactive or nonalloreactive “veto-type” donor CD8+ T cells.15-17 Alloengraftment was generally attributed to clonal deletion of alloreactive host T cells18,19 ; in vitro studies identified a role for donor T-cell CD820 and fasL20,21 in the deletion process, whereas in vivo studies demonstrated a role for perforin or fasL.22 Previously, we found that donor cytotoxic CD8+ T cells secreting Th2 cytokines (Tc2 cells) were particularly effective in preventing rejection.23,24 Initially, the contribution of donor CD4+ T cells to engraftment was characterized as less robust than the CD8+ T-cell contribution.15 However, recent studies illustrated the capacity of donor CD4+ regulatory T (Treg) cells to promote engraftment25,26 ; however, a molecular mechanism for Treg cell promotion of engraftment was not elucidated. Moreover, we recently found that allograft augmentation with donor CD4+ Th2R cells potently abrogated fully MHC-disparate hematopoietic stem cell graft rejection with minimal GVHD.11
Given this background, our second objective was to evaluate the molecular mechanism whereby donor Th2R cells prevent rejection. Th2 cells typically modulate immunity in great part through interleukin-4 (IL-4) secretion, which influences other immune effectors to adopt Th2 polarization.27 We therefore hypothesized that Th2R cell abrogation of rejection might depend upon donor IL-4 production and thereby promote host Th2 polarity as a mechanism of engraftment distinct from previously described clonal deletion mechanisms.
Methods
Mice
Female C57BL/6 (B6, H-2Kb), BALB/c (H-2Kd), and C57BL/6 (B6, H-2Kb, CD45.1 or CD45.2 congenic) mice were obtained from Frederick Cancer Research Facility (Frederick, MD). 2C-transgenic mice on the B6 background that express TCR allospecific for Ld were bred by Phil Lucas (NCI). B6 mice genetically deficient in STAT1, IL-4, and STAT6 and BALB/c mice genetically deficient in STAT4, STAT6, and IL-4 were obtained from Taconic (Hudson, NY) and The Jackson Laboratory (Bar Harbor, ME). Mice were maintained in a specific pathogen-free facility and treated according to an animal protocol approved by the NCI Animal Care and Use Committee.
Cells
Bone marrow cells were harvested and T cell–depleted (Miltenyi CD90 [Thy1.2] microbeads). Host T cells for postirradiation add-back were obtained from the spleen and were purified (pan-T-cell selection kit; Miltenyi Biotec, Auburn, CA). Splenic CD8+ T cells from 2C TCR- transgenic mice were purified (CD8 enrichment kit; StemCell Technologies, Vancouver, BC). For the generation of Th1/Tc1, Th2/Tc2, Th2, or Th2R cells, spleen cells from C57BL/6 or BALB/c mice were harvested, red cells were lysed (ACK buffer; Quality Biologicals, Gaithersburg, MD), and B cells were depleted (goat anti–mouse magnetic bioparticles; Polysciences, Warrington, PA). For Th2 cultures, CD4 cells were then purified (CD4 selection kit; StemCell Technologies). Anti-CD3, anti-CD28–coated beads (“CD3/CD28 beads”) were produced as previously detailed14 ; T cells were costimulated (3:1 ratio of beads to T cells) in complete medium (CM) consisting of RPMI 1640 (Mediatech, Manassas, VA), 10% fetal calf serum (FCS; Gemini Bio-Products, West Sacramento, CA), pen-strep-glut, nonessential amino acids (Invitrogen), 2-ME (5 × 10−5 M; Invitrogen, Carlsbad, CA), and N-acetyl-cysteine (3.3 mM; Bristol-Myers Squibb, New York, NY). All recombinant cytokines were obtained from PeproTech (Rocky Hill, NJ). For Th2/Tc2 and purified CD4+Th2 cell generation, CM was supplemented with rhIL-2 (20 IU/mL), rhIL-7 (20 ng/mL), and rmIL-4 (1000 IU/mL); for Th1/Tc1 cell generation, CM was supplemented with rhIL-2 (20 IU/mL), rhIL-7 (20 ng/mL), rm IL-12 (10 ng/mL), and rat anti–mouse IL4 (clone 11B11, 10 μg/mL; obtained from FCRF). For Th2R cell generation, cultures were additionally supplemented with rapamycin (10 μM; sirolimus; Sigma-Aldrich, St Louis, MO). Cytokine- and rapamycin-replete media was added daily to maintain T cells at 0.2 to 1.0 × 106 cells/mL.
Host T cells used in the add-back experiments were at least 99% enriched for T cells and were typically composed of CD4+ and CD8+ T cells at a 1.8:1.0 ratio. For experiments that used 2C-transgenic T cells, the add-back comprised a 1:1 mixture of purified CD8+ 2C cells and CD45.1+ splenic T cells (with the splenic T cells containing CD4+ and CD8+ T cells in a 1.8:1.0 ratio). For experiments with ex vivo–expanded Th2/Tc2 and Th1/Tc1 host T cells, CD8+ cells preferentially expanded relative to CD4+ cells, thereby resulting in CD4:CD8 ratios of 1.3:1.0 (Th2/Tc2 cells) and 0.8:1.0 (Th1/Tc1 cells).
Graft rejection model
For B6-into-BALB/c and BALB/c-into-B6 transplantation, recipients were lethally irradiated on day −2 (1050 or 1100 cGy, respectively; 137Cs gamma radiation, gamma cell 40; Atomic Energy of Canada, Chalk River, ON). Wild-type (WT), cytokine-polarized, 2C TCR-transgenic, or molecule-deficient host T cells were administered (intravenously) on day −1; the type and dose of such host T-cell infusions are detailed in each figure legend. TCD bone marrow (BM) cells (10 × 106 cells) were administered (intravenously) on day 0. Some cohorts received a separate intravenous injection of donor Th2 or Th2R cells on day 0; all Th2 cell infusions were at a dose of 107 cells per recipient, except for the “high-dose” cohort that received 5 × 107 cells per recipient.
Flow cytometry
Spleen and bone marrow (harvested from femurs and tibia, bilaterally) were harvested at different times after BMT, and single-cell suspensions were labeled with anti–H-2Kb, -H-2Kd, -CD45.1, -CD8, and -CD4 conjugated with FITC, PE, APC, PE-Cy5, or Cascade Blue (PharMingen, San Jose, CA). For tracking of the 2C TCR-transgenic T cells, 1 of 2 methods were used depending on availability of the 1B2 clonotypic antibody, namely method 1 (anti–H-2Kb, -H-2Kd, -CD45.1, -CD8, -CD4, -Vβ8 conjugated with FITC, PE, APC, PE-Cy5, Cascade Blue, and APC-Cy5, respectively) and method 2 (anti–H-2Kb, -H-2Kd, -1B2, -CD8, -CD45.1, and -Vβ8 conjugated with FITC, PE, APC, PE-Cy5, Cascade Blue, and APC-Cy5, respectively). For chimerism analysis, post-BMT blood, spleen, and bone marrow cells were labeled with anti–H-2Kb and anti–H-2Kd. Six- to 7-color flow cytometry was performed on an LSR II instrument using FACSDiva software (BD Biosciences, San Jose, CA). Live events (10 000-200 000) were acquired with Hoechst 33 258 exclusion of dead cells.
Quantification of HVGR using cytokine capture flow cytometry
Posttransplant splenic single cells were adjusted to 0.5 × 106 cells/mL and either not stimulated or stimulated with B6 DC, or BALB/c dendritic cells (DCs) generated by culturing marrow cells for 6 days in rmGM-CSF and rmIL-4 (each at 1000 IU/mL; PeproTech); bacterial LPS (1 μg/mL; Calbiochem) was added to the final 24 hours of DC culture. Cultured bone marrow cells were evaluated by surface flow cytometry: both syngeneic and allogeneic cells were found to express a DC profile consistent with that previously reported,28 including up-regulation of CD11c, CD80, CD86, and MHC class II molecules (data not shown). Expanded DCs were washed and used at a spleen cell to DC ratio of 10:1. After 24 hours, supernatants were collected for cytokine analysis, and cells were evaluated by cytokine capture flow cytometry using IFN-γ catch reagent (Miltenyi) followed by 45 minutes of incubation (RPMI 1640 with 10% FCS) at 37°C (slow rotation). Cells were washed with cold buffer, labeled with IFN-γ detection Ab (APC) and previously described surface Abs, washed, and analyzed. Flow cytometric frequency data were multiplied by splenic cell yields to obtain the absolute number of cytokine-secreting cells per spleen; allospecific values were calculated by subtracting values obtained after syngeneic stimulation.
Cell purification by flow sorting
Adoptively transferred 2C TCR-transgenic T cells were isolated from transplant recipients at days 8 and 11 after BMT by flow sorting using anti–H-2Kb, -H-2Kd, -CD45.1, -CD8, and -Vβ8 antibodies conjugated with FITC, PE, APC, PE-Cy5, and APC-Cy5, respectively, to obtain a purity of more than 95%.
Determination of cytokine phenotype
Recipient spleen cells were harvested and red cells were lysed (ACK buffer); spleen cells were adjusted to 0.5 × 106 cells/mL in 24-well plates and stimulated with B6 or BALB/c DC (spleen cell to DC ratio, 10:1) or CD3/CD28 beads for 24 hours. Ex vivo–expanded Th1/Tc1 and Th2/Tc2 cells were harvested on day 6 of culture, washed, adjusted to 0.5 × 106 cells/mL in 24-well plates, and restimulated with CD3/CD28 beads for 24 hours. Resultant culture supernatants were evaluated for cytokine content by multiplex bead array (Bio-Rad, Hercules, CA).
Statistics
Survival analysis was performed according to the Kaplan-Meier method, and survival curves were compared using log-rank testing. Flow and cytokine data were analyzed using Student 2-tailed t tests. Values of P less than .05 were considered statistically significant.
Results
Host Th1/Tc1 cells mediate increased rejection relative to host Th2/Tc2 cells
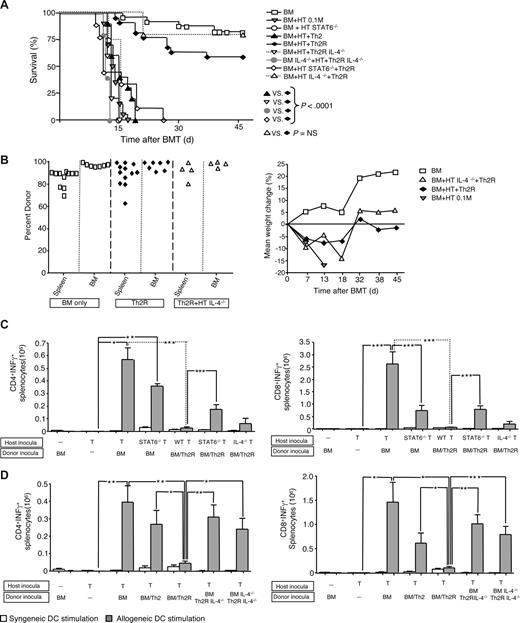
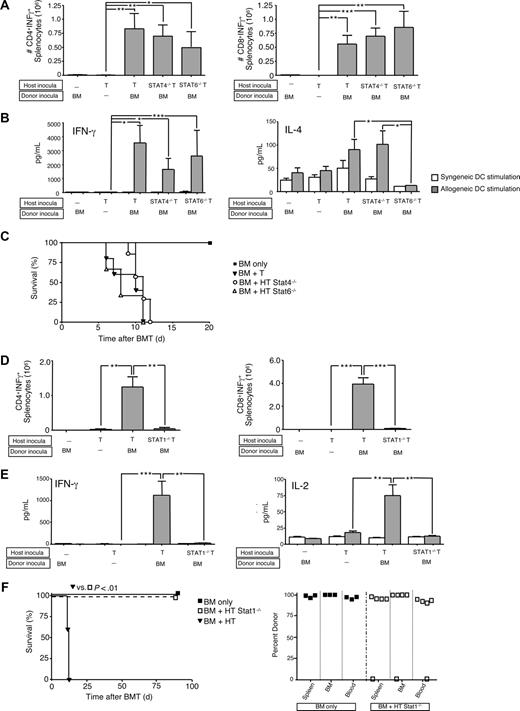
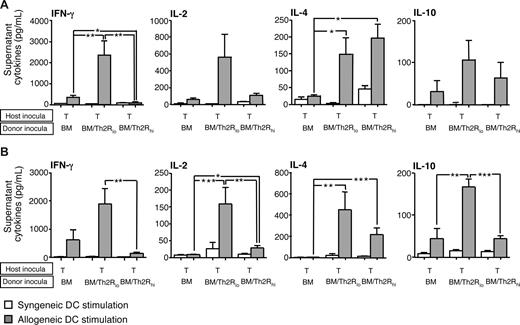
First, we evaluated whether cytokine polarized T-cell subsets differentially mediated rejection. Relative to Th1/Tc1 cells, Th2/Tc2 cells had minimally reduced IFN-γ, reduced IL-2, and increased IL-4 and IL-10 before adoptive transfer (Figure 1A). Mirroring our results in the donor–anti-host direction,14 nonpolarized and Th1/Tc1 host T cells acquired host–anti-donor allospecific IFN-γ secretion upon in vivo encounter with MHC-mismatched donor BM cells (Figure 1B cytokine capture assay); in contrast, host Th2/Tc2 cells acquired minimal allospecificity in vivo. These findings were corroborated by supernatant assay: recipients of nonpolarized or Th1/Tc1 host T cells secreted allospecific IFN-γ and IL-2 (Figure 1C); by comparison, Th2/Tc2 cell recipients secreted increased IL-4 and reduced IFN-γ and IL-2. Host Th1/Tc1 and Th2/Tc2 cells differentially mediated graft rejection: each recipient of nonpolarized or Th1/Tc1 cells underwent lethal graft rejection, whereas host Th2/Tc2 cell recipients typically had long-term survival and full donor alloengraftment (Figure 1D).
Host Th1/Tc1 cells mediate increased rejection relative to host Th2/Tc2 cells. (A) BALB/c host T cells were costimulated and expanded in IL-4 or IL-12 to generate Th2/Tc2 or Th1/Tc1 cells, respectively. On culture day 6, cells were restimulated; supernatants were tested for cytokine content (ng/mL). (B-D) B6-into-BALB/c transplantation consisted of lethal host irradiation (1050 cGy; day −2), and some combination, as indicated, of host T cells that were nonpolarized [“HT”], polarized to type I cytokines [“HTh1/Tc1”], or polarized to type II cytokines [“HTh2/Tc2”] (T-cell dose of 0.1 × 106; day −1); and donor BM cells (day 0). On day 8 after BMT, spleen cells were isolated and stimulated with syngeneic (BALB/c) or allogeneic (B6) DCs for 24 hours. The total number of host CD4+ and CD8+ cells producing allospecific IFN-γ was determined by flow cytometry using anti–H-2d, anti–H-2b, -CD4, or -CD8, and antibody capture of IFN-γ (panel B). Resultant supernatants were tested for cytokine content (panel C; pg/mL). Results are mean plus or minus SEM of n = 10 per cohort. *P < .05; **P < .01; ***P < .001. (D) Recipient mice were followed for 90 days after BMT: data shown are overall survival (left panel) and peripheral blood donor chimerism (right panel). Survival data were pooled from 2 independent experiments (total of n = 10 recipients per cohort).
Host Th1/Tc1 cells mediate increased rejection relative to host Th2/Tc2 cells. (A) BALB/c host T cells were costimulated and expanded in IL-4 or IL-12 to generate Th2/Tc2 or Th1/Tc1 cells, respectively. On culture day 6, cells were restimulated; supernatants were tested for cytokine content (ng/mL). (B-D) B6-into-BALB/c transplantation consisted of lethal host irradiation (1050 cGy; day −2), and some combination, as indicated, of host T cells that were nonpolarized [“HT”], polarized to type I cytokines [“HTh1/Tc1”], or polarized to type II cytokines [“HTh2/Tc2”] (T-cell dose of 0.1 × 106; day −1); and donor BM cells (day 0). On day 8 after BMT, spleen cells were isolated and stimulated with syngeneic (BALB/c) or allogeneic (B6) DCs for 24 hours. The total number of host CD4+ and CD8+ cells producing allospecific IFN-γ was determined by flow cytometry using anti–H-2d, anti–H-2b, -CD4, or -CD8, and antibody capture of IFN-γ (panel B). Resultant supernatants were tested for cytokine content (panel C; pg/mL). Results are mean plus or minus SEM of n = 10 per cohort. *P < .05; **P < .01; ***P < .001. (D) Recipient mice were followed for 90 days after BMT: data shown are overall survival (left panel) and peripheral blood donor chimerism (right panel). Survival data were pooled from 2 independent experiments (total of n = 10 recipients per cohort).
STAT1-deficient host T cells fail to mediate graft rejection
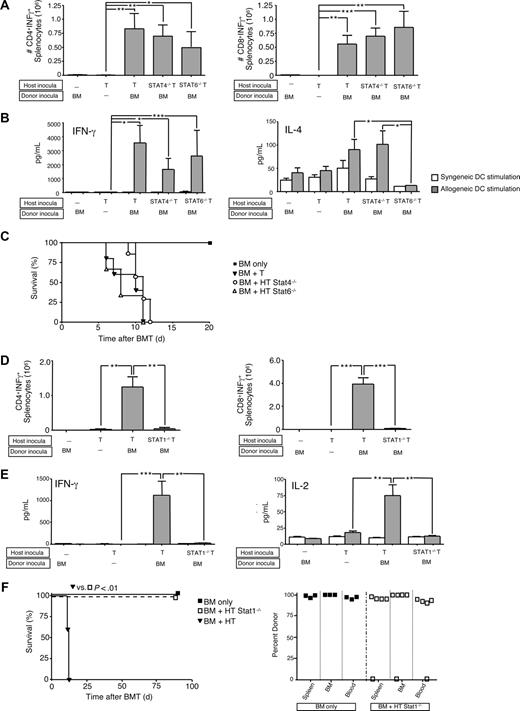
Differential use of signal transduction and activators of transcription (STAT) molecules can help elucidate complex T-cell biology. Specifically, IL-12 promotes Th1 differentiation through STAT429 signaling, whereas IL-4 promotes Th2 differentiation via STAT6.30 In a previous study, donor T cells impaired in Th1 polarity (STAT4-deficient [−/−]) mediated reduced lethal GVHD relative to donor T cells with Th2 impairment (STAT6−/−); of note, each STAT-deficient T-cell population contributing to GVHD with a differential target tissue distribution.31 Because we found Th1/Tc1 and Th2/Tc2 host T cells to differentially mediate graft rejection, we hypothesized that STAT4−/− T cells would not cause rejection. To the contrary, wild-type (WT), STAT4−/−, and STAT6−/− host CD4+ and CD8+ T cells all acquired allospecific IFN-γ secretion in vivo (Figure 2A capture assay; Figure 2B supernatant assay). Predictably, STAT6−/− host T cells had reduced IL-4 secretion after in vivo allosensitization (Figure 2B); the cohorts were not different in terms of IL-2 or IL-10 secretion (not shown). Host T cells from WT, STAT4−/−, and STAT6−/− mice all yielded graft rejection resulting in post-BMT lethality (Figure 2C).
Graft rejection requires host T-cell expression of STAT1 but not STAT4 or STAT6. (A-C) B6-into-BALB/c transplantation consisted of lethal host irradiation (1050 cGy; day −2), and some combination, as indicated, of wild-type (WT; “HT”), STAT4-deficient (−/−), or STAT6−/− host T cells (T-cell dose, 106; day −1); and B6 donor BM cells (day 0). Results shown are mean plus or minus SEM of n = 5 to 10 per cohort. On day 8 after BMT, spleen cells were isolated and stimulated with syngeneic (BALB/c) or allogeneic (B6) DCs for 24 hours. The total number of host CD4+ and CD8+ cells producing allospecific IFN-γ was determined by flow cytometry (panel A). Resultant supernatants were tested for cytokine content (panel B; pg/mL). *P < .05; **P < .01; ***P < .001. Overall survival of recipient mice is shown (panel C). (D-F) BALB/c-into-B6 transplantation consisted of lethal irradiation (1100 cGy; day −2), and some combination, as indicated, of WT (“HT”) or STAT1−/− host T cells (T-cell dose, 106; day −1) and BALB/c donor BM cells (day 0). Results shown are mean plus or minus SEM of n = 5 to 10 per cohort. On day 8 after BMT, spleen cells were isolated and stimulated with syngeneic or allogeneic DCs; total number of host CD4+ and CD8+ cells producing allospecific IFN-γ was determined by flow cytometry (panel D) and resultant supernatants were tested for cytokine content (panel E; pg/mL). *P < .05; **P < .01; ***P < .001. (F) Overall survival is shown (left panel; n = 10 per cohort); day 90 after BMT donor chimerism in spleen, BM, and blood is shown (right panel).
Graft rejection requires host T-cell expression of STAT1 but not STAT4 or STAT6. (A-C) B6-into-BALB/c transplantation consisted of lethal host irradiation (1050 cGy; day −2), and some combination, as indicated, of wild-type (WT; “HT”), STAT4-deficient (−/−), or STAT6−/− host T cells (T-cell dose, 106; day −1); and B6 donor BM cells (day 0). Results shown are mean plus or minus SEM of n = 5 to 10 per cohort. On day 8 after BMT, spleen cells were isolated and stimulated with syngeneic (BALB/c) or allogeneic (B6) DCs for 24 hours. The total number of host CD4+ and CD8+ cells producing allospecific IFN-γ was determined by flow cytometry (panel A). Resultant supernatants were tested for cytokine content (panel B; pg/mL). *P < .05; **P < .01; ***P < .001. Overall survival of recipient mice is shown (panel C). (D-F) BALB/c-into-B6 transplantation consisted of lethal irradiation (1100 cGy; day −2), and some combination, as indicated, of WT (“HT”) or STAT1−/− host T cells (T-cell dose, 106; day −1) and BALB/c donor BM cells (day 0). Results shown are mean plus or minus SEM of n = 5 to 10 per cohort. On day 8 after BMT, spleen cells were isolated and stimulated with syngeneic or allogeneic DCs; total number of host CD4+ and CD8+ cells producing allospecific IFN-γ was determined by flow cytometry (panel D) and resultant supernatants were tested for cytokine content (panel E; pg/mL). *P < .05; **P < .01; ***P < .001. (F) Overall survival is shown (left panel; n = 10 per cohort); day 90 after BMT donor chimerism in spleen, BM, and blood is shown (right panel).
STAT1 conveys type I and type II interferon receptor signaling and proximally mediates Th1 polarization independent of IL-12/STAT4.32,33 We therefore hypothesized that STAT1−/− host T cells would be Th1 deficient and thereby mediate reduced rejection. Indeed, adoptively transferred STAT1−/− host T cells had markedly reduced allospecific IFN-γ secretion (Figure 2D capture assay; Figure 2E supernatant assay) and IL-2 secretion after BMT (Figure 2E); IL-4 and IL-10 did not differ between recipients of STAT1−/− and WT host T cells (not shown). Remarkably, STAT1−/− T cells mediated greatly reduced rejection (Figure 2E survival and chimerism). Of note, no significant difference was found between the control cohort and the cohort that received host STAT1−/− T cells (Figure 2F) in terms of cell composition in the spleen (% CD3+ cells: 18% ± 2% vs 22% ± 1.5%; p = NS), bone marrow (% Gr-1+ cells: 35.7% ± 0.8% vs 37.4% ± 1.2%; p = NS), or blood (% Gr-1+ cells: 13.2% ± 0.9% vs 15.9% ± 0.7%, p = NS).
Donor Th2R cell therapy restricts, but does not eliminate, allospecific host T cells
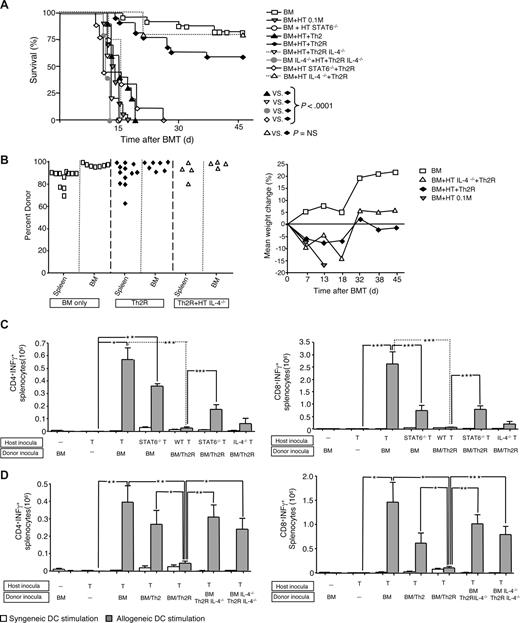
Recently, we found that ex vivo rapamycin generated donor Th2R cells with an apoptosis-resistant phenotype that prevented fully MHC-disparate hematopoietic stem cell graft rejection11 and prevented GVHD through an IL-4–dependent mechanism.6 Because Th2/Tc2 and STAT1−/− host T cells mediated reduced host-versus-graft reactivity and reduced graft rejection, we hypothesized that donor Th2R cells would operate by transfer of cytokine polarization to host T cells. Such a mechanism would represent a form of “infectious”34 transplantation tolerance distinct from previously described in vitro models of graft facilitation that emphasized donor T-cell clonal deletion of alloreactive host T cells through lytic mechanisms18-21 ; these prior models used TCR transgenic CD8+ T cells that are Vβ8-restricted and mediate an H-2b-anti–H-2d rejection response. Our first objective was to rule out the possibility that donor Th2 cell therapy operated strictly through a clonal deletion mechanism. We therefore established an in vivo model whereby postirradiation host T-cell add-back inocula contained allospecific transgenic T cells that could be tracked by either a clone specific antibody (1B2) or Vβ8 restriction (Figure 3A).
Donor Th2 cell abrogation of rejection restricts but does not eliminate allospecific host T cells. (A) Spleen cells from 2C TCR-transgenic mice were evaluated by flow cytometry for coexpression of the clonotypic TCR (using 1B2 antibody) and Vβ8; a representative example is shown. (B-D) BALB/c-into-B6 transplantation consisted of lethal irradiation (1100 cGy; day −2), and some combination, as indicated, of host T cells (day −1; 1:1 mix of 2C and CD45.1-congenic WT T cells [each population, 0.05 × 106 cells]) and BALB/c donor BM cells with or without donor Th2 cells generated ex vivo in rapamycin (Th2R cells; day 0). The ratio of donor Th2 cells to host T cells was either 100:1 (“BM/Th2Rlo”) or 500:1 (“BM/Th2Rhi”). At day 8 after BMT, the number of allospecific host T cells in the spleen (panel B) and BM (panel C) was enumerated by flow cytometry. Allospecific host T cells were identified using H-Kb+, H-2Kd−, CD45.1−, CD8+, Vβ8+ (experiment number 1; panels B and C left) or H-Kb+, H-2Kd−, CD45.1−, CD8+, Vβ8+, 1B2+ (experiment number 2; panels B and C right). *P < .05; **P < .01; ***P < .001. (D) Longitudinal tracking of allospecific host T cells by Vβ8 analysis at days 11, 14, 21, and 45 after BMT in the spleen (left panel) and BM (right panel) in transplant recipients of high-dose donor Th2R cells (n = 5-8 recipients at each time point). (E) In a third experiment, B6 hosts received lethal irradiation (day −2), and some combination, as indicated, of host T cells (“HT”; 1:1 mix of 2C TCR-transgenic and CD45.1-congenic WT T cells; each population, 0.01 × 106 cells; day −1), and donor BM with or without donor Th2R cells (day 0; donor Th2R cell: host T-cell ratio, 500:1). Mice were followed for survival analysis for 45 days (left panel; n = 10 per cohort) and weight loss (middle panel); donor chimerism in the spleen, BM, and blood was determined at day 45 after BMT (right panel).
Donor Th2 cell abrogation of rejection restricts but does not eliminate allospecific host T cells. (A) Spleen cells from 2C TCR-transgenic mice were evaluated by flow cytometry for coexpression of the clonotypic TCR (using 1B2 antibody) and Vβ8; a representative example is shown. (B-D) BALB/c-into-B6 transplantation consisted of lethal irradiation (1100 cGy; day −2), and some combination, as indicated, of host T cells (day −1; 1:1 mix of 2C and CD45.1-congenic WT T cells [each population, 0.05 × 106 cells]) and BALB/c donor BM cells with or without donor Th2 cells generated ex vivo in rapamycin (Th2R cells; day 0). The ratio of donor Th2 cells to host T cells was either 100:1 (“BM/Th2Rlo”) or 500:1 (“BM/Th2Rhi”). At day 8 after BMT, the number of allospecific host T cells in the spleen (panel B) and BM (panel C) was enumerated by flow cytometry. Allospecific host T cells were identified using H-Kb+, H-2Kd−, CD45.1−, CD8+, Vβ8+ (experiment number 1; panels B and C left) or H-Kb+, H-2Kd−, CD45.1−, CD8+, Vβ8+, 1B2+ (experiment number 2; panels B and C right). *P < .05; **P < .01; ***P < .001. (D) Longitudinal tracking of allospecific host T cells by Vβ8 analysis at days 11, 14, 21, and 45 after BMT in the spleen (left panel) and BM (right panel) in transplant recipients of high-dose donor Th2R cells (n = 5-8 recipients at each time point). (E) In a third experiment, B6 hosts received lethal irradiation (day −2), and some combination, as indicated, of host T cells (“HT”; 1:1 mix of 2C TCR-transgenic and CD45.1-congenic WT T cells; each population, 0.01 × 106 cells; day −1), and donor BM with or without donor Th2R cells (day 0; donor Th2R cell: host T-cell ratio, 500:1). Mice were followed for survival analysis for 45 days (left panel; n = 10 per cohort) and weight loss (middle panel); donor chimerism in the spleen, BM, and blood was determined at day 45 after BMT (right panel).
In experiment number 1 (cell tracking by Vβ8 analysis; performed at day 8 after transplantation), allospecific host T cells expanded in response to the allograft (Figure 3B left panel, spleen; Figure 3C left panel, BM). Given the supraphysiologic number of allospecific host T cells in this model, we analyzed the engraftment effect of “low-dose” and “high-dose” donor Th2R cells that yielded donor Th2R to host T-cell ratios of 100:1 and 500:1, respectively. Unexpectedly, low-dose Th2R cells actually increased allospecific host T-cell expansion; however, high-dose Th2R cells markedly attenuated allospecific host T-cell expansion relative to rejection controls. In experiment number 2, high-dose Th2R cell inhibition of host allospecific T-cell expansion was observed in spleen (Figure 3B right panel) and BM (Figure 3C right panel) by both 1B2 and Vβ8 analysis.
These results indicated that donor Th2R cell therapy restricted but did not eliminate the allospecific host T-cell repertoire. To confirm and extend this observation, allospecific host T cells in high-dose Th2R cell recipients were serially tracked at days 11, 14, 21, and 45 after transplant: allospecific host T-cell number was relatively stable in spleen (Figure 3D left panel) and progressively diminished in BM (Figure 3D right panel). The mean percentage of 2C cells in the spleen at days 11, 14, 21, and 45 after BMT was 0.8%, 0.9%, 0.2%, and 0.17%, respectively; similarly, the mean percentage of 2C cells in the bone marrow at days 11, 14, 21, and 45 after BMT were 2.7%, 0.7%, 0.15%, and 0.17%, respectively. Rejection controls underwent graft rejection and post-BMT lethality within 2 weeks after transplantation (Figure 3E left panel). By comparison, high-dose Th2R cell recipients typically survived through day 45 after BMT; such recipients had donor chimerism in spleen, BM, and blood (Figure 4E right panel) and stable weight loss consistent with moderate ongoing GVHD (Figure 4E middle panel).
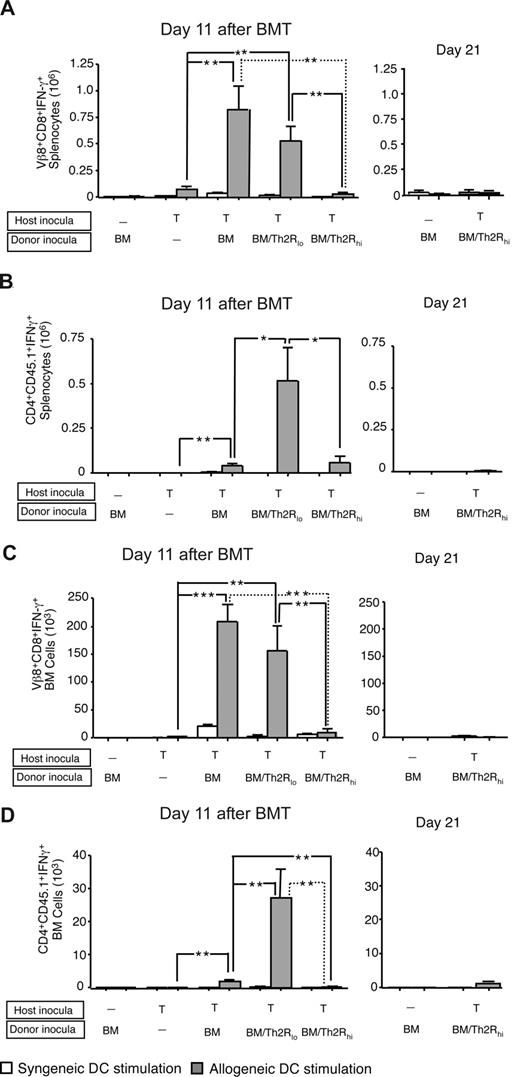
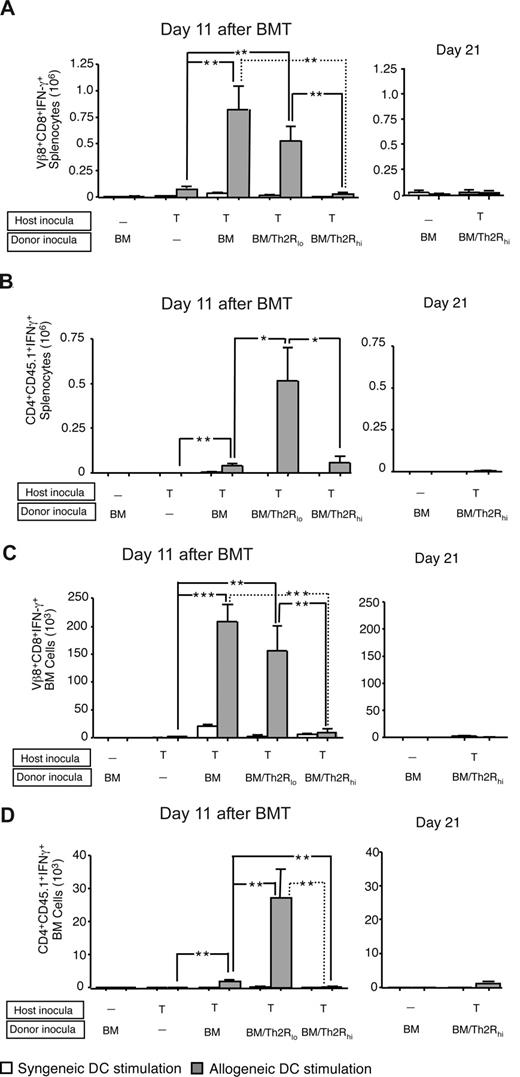
Donor Th2R cell abrogation of rejection reduces allospecific host T-cell effector function. BALB/c-into-B6 transplantation consisted of lethal irradiation (1100 cGy; day −2), and some combination, as indicated, of host T cells (day −1; 1:1 mix of 2C TCR-transgenic and CD45.1-congenic WT T cells; each population, 0.05 × 106 cells); and BALB/c donor BM cells with or without donor Th2R cells (day 0). The ratio of donor Th2 cells to host T cells was either 100:1 (“BM/Th2Rlo”) or 500:1 (“BM/Th2Rhi”). On days 11 and 21 after BMT, spleen, and BM cells were isolated and stimulated with syngeneic (B6) or allogeneic (BALB/c) DCs for 24 hours. The total number of host Vβ8+CD8+ cells or CD4+CD45.1+ cells producing allospecific IFN-γ in the spleen (panels A and B, respectively) and BM (panels C and D, respectively) was evaluated by flow cytometry at both day 11 after BMT (left panels) and day 21 after BMT (right panels). Results shown are mean plus or minus SEM of n = 10 per cohort. *P < .05; **P < .01; ***P < .001.
Donor Th2R cell abrogation of rejection reduces allospecific host T-cell effector function. BALB/c-into-B6 transplantation consisted of lethal irradiation (1100 cGy; day −2), and some combination, as indicated, of host T cells (day −1; 1:1 mix of 2C TCR-transgenic and CD45.1-congenic WT T cells; each population, 0.05 × 106 cells); and BALB/c donor BM cells with or without donor Th2R cells (day 0). The ratio of donor Th2 cells to host T cells was either 100:1 (“BM/Th2Rlo”) or 500:1 (“BM/Th2Rhi”). On days 11 and 21 after BMT, spleen, and BM cells were isolated and stimulated with syngeneic (B6) or allogeneic (BALB/c) DCs for 24 hours. The total number of host Vβ8+CD8+ cells or CD4+CD45.1+ cells producing allospecific IFN-γ in the spleen (panels A and B, respectively) and BM (panels C and D, respectively) was evaluated by flow cytometry at both day 11 after BMT (left panels) and day 21 after BMT (right panels). Results shown are mean plus or minus SEM of n = 10 per cohort. *P < .05; **P < .01; ***P < .001.
Donor Th2R cells inhibit alloreactive host T-cell effector function
Next, we characterized the in vivo influence of donor Th2R cells on allospecific host T-cell effector function. At day 11 after BMT, spleen and BM of rejection controls had increased allospecific IFN-γ secretion emanating from Vβ8-restricted host T cells (Figure 4A,C left panels) and, to a lesser extent, from nontransgenic CD45.1+ host T cells (Figure 4B,D left panels). Transgenic host T-cell IFN-γ secretion was dramatically reduced in high-dose, but not low-dose, Th2R cell recipients in both spleen and BM. The relatively low frequency of IFN-γ secreting nontransgenic CD45.1+ host T cells was dramatically increased in low-dose Th2R cell recipients, but not high-dose Th2R cell recipients. At day 21 after BMT, only bone marrow transplant controls and high-dose Th2R cell recipients had engrafted: similar to day 11 post-BMT results, recipients of high-dose Th2R cells had minimal in vivo persistence of transgenic (Figure 4A,C right panels) or nontransgenic host T cells (Figure 4B,D right panels) capable of allospecific IFN-γ secretion.
Th2R cells transfer cytokine phenotype to allospecific host T cells
These results indicated that donor Th2R cell therapy reduced host T-cell acquisition of the Th1 cytokine phenotype that was associated with rejection. We next hypothesized that reduction in host Th1-type immunity would associate with a commensurate increase in Th2-type immunity within allospecific host T cells. To address this, we characterized the cytokine phenotype of posttransplant Vβ8+ allospecific host T cells purified by flow sorting. At day 8 and day 11 after BMT (Figure 5A,B, respectively), allospecific host T cells in rejection controls primarily secreted IFN-γ. Purified allospecific host T cells from low-dose Th2R cell recipients had increased secretion of not only IL-4 and IL-10, but also of IFN-γ and IL-2. In marked contrast, allospecific host T cells from high-dose Th2R cell recipients uniquely secreted reduced Th1 cytokines and increased Th2 cytokines. Therefore, consistent with our hypothesis, donor Th2R cells indeed transferred cytokine polarization to host T cells. Furthermore, these results indicate that host T-cell acquisition of Th2 cytokine production was not alone sufficient for effective abrogation of rejection, but must be accompanied by concomitant elimination of host Th1 cytokine secretion capacity.
Allospecific host T cells adopt a Th2 cytokine phenotype during rejection abrogation by donor Th2 cells. BALB/c-into-B6 transplantation consisted of lethal host irradiation (1100 cGy; day −2), and a combination, as indicated, of host T cells (day 1; 1:1 mix of 2C TCR-transgenic and CD45.1-congenic WT T cells; each population, 0.05 × 106 cells) and BALB/c donor BM cells with or without donor Th2R cells (day 0). The ratio of donor Th2 cells to host T cells was either 100:1 (“BM/Th2Rlo”) or 500:1 (“BM/Th2Rhi”). Spleen cells were harvested on day 8 after BMT (A) and day 11 after BMT (B), and allospecific host T cells were purified by flow sorting for H-2Kb+CD8+Vβ8+CD45.1− cells. Purified host T cells were stimulated with anti–CD3/CD28-coated beads for 24 hours; resultant supernatants were tested for cytokine content (pg/mL). Results shown are mean plus or minus SEM of n = 5 per cohort for each time point. *P < .05; **P < .01; ***P < .001.
Allospecific host T cells adopt a Th2 cytokine phenotype during rejection abrogation by donor Th2 cells. BALB/c-into-B6 transplantation consisted of lethal host irradiation (1100 cGy; day −2), and a combination, as indicated, of host T cells (day 1; 1:1 mix of 2C TCR-transgenic and CD45.1-congenic WT T cells; each population, 0.05 × 106 cells) and BALB/c donor BM cells with or without donor Th2R cells (day 0). The ratio of donor Th2 cells to host T cells was either 100:1 (“BM/Th2Rlo”) or 500:1 (“BM/Th2Rhi”). Spleen cells were harvested on day 8 after BMT (A) and day 11 after BMT (B), and allospecific host T cells were purified by flow sorting for H-2Kb+CD8+Vβ8+CD45.1− cells. Purified host T cells were stimulated with anti–CD3/CD28-coated beads for 24 hours; resultant supernatants were tested for cytokine content (pg/mL). Results shown are mean plus or minus SEM of n = 5 per cohort for each time point. *P < .05; **P < .01; ***P < .001.
Donor Th2R cell abrogation of graft rejection requires an intact IL-4/STAT6 axis
We next hypothesized that therapeutic transfer of donor polarization to host T cells would be mediated by IL-4. Indeed, transfer of IL-4–deficient donor Th2R cells, either alone or in combination with IL-4–deficient BM, resulted in graft rejection and post-BMT lethality (Figure 6A). Therapeutic donor IL-4 secretion was mediated in an infectious manner requiring host T-cell expression of STAT6. Because host T-cell IL-4 secretion was not required for alloengraftment, the protective effect of donor IL-4 was directly mediated. One cohort received donor Th2 cells grown without ex vivo rapamycin: consistent with our previous results,11 only rapamycin-generated donor Th2R cells prevented rejection. Surviving Th2R cell recipients had donor chimerism in spleen, BM, and blood at day 45 after BMT (Figure 6B left panel) and a stable pattern of weight loss consistent with moderate ongoing GVHD (Figure 6B right panel). Finally, the importance of the IL-4/STAT6 axis to donor Th2 cell therapy was reflected in the immunologic end-point performed at day 8 after BMT: elimination of host CD4+ and CD8+ T-cell allospecific IFN-γ secretion only occurred with intact donor Th2R cell IL-4 secretion and host T-cell STAT6 signaling (Figures 6C,D).
Th2 cell abrogation of rejection requires donor Th2 cell IL-4 and host T-cell STAT6. BALB/c-into-B6 transplantation consisted of lethal host irradiation (1100 cGy; day −2), and some combination, as indicated, of WT, STAT6−/−, or IL-4−/− host T cells (day −1, “HT”) and BALB/c donor BM (WT or IL-4−/−) with or without donor Th2R cells generated from WT or IL-4−/− donors (day 0). The ratio of donor Th2R cells to host T cells was 100:1 (10 × 106 cells: 0.1 × 106 cells); donor Th2 cells were generated in the presence or absence of rapamycin (Th2R or Th2 cells, respectively). Transplant recipients were monitored until day 45 for survival (A) and weight loss (panel B right); surviving mice were evaluated at day 45 for donor chimerism in the spleen and BM (panel B left). On day 8 after BMT, spleen cells were isolated and stimulated with syngeneic (B6) or allogeneic (BALB/c) DCs for 24 hours. The total number of host CD4+ and CD8+ cells in the spleen producing allospecific IFN-γ was determined by flow cytometry. Results from molecule-deficient host and donor cells are shown in panels C and D, respectively; results shown are mean plus or minus SEM of n = 10 per cohort. *P < .05; **P < .01; ***P < .001.
Th2 cell abrogation of rejection requires donor Th2 cell IL-4 and host T-cell STAT6. BALB/c-into-B6 transplantation consisted of lethal host irradiation (1100 cGy; day −2), and some combination, as indicated, of WT, STAT6−/−, or IL-4−/− host T cells (day −1, “HT”) and BALB/c donor BM (WT or IL-4−/−) with or without donor Th2R cells generated from WT or IL-4−/− donors (day 0). The ratio of donor Th2R cells to host T cells was 100:1 (10 × 106 cells: 0.1 × 106 cells); donor Th2 cells were generated in the presence or absence of rapamycin (Th2R or Th2 cells, respectively). Transplant recipients were monitored until day 45 for survival (A) and weight loss (panel B right); surviving mice were evaluated at day 45 for donor chimerism in the spleen and BM (panel B left). On day 8 after BMT, spleen cells were isolated and stimulated with syngeneic (B6) or allogeneic (BALB/c) DCs for 24 hours. The total number of host CD4+ and CD8+ cells in the spleen producing allospecific IFN-γ was determined by flow cytometry. Results from molecule-deficient host and donor cells are shown in panels C and D, respectively; results shown are mean plus or minus SEM of n = 10 per cohort. *P < .05; **P < .01; ***P < .001.
Discussion
This report for the first time illustrates that host T cell–mediated rejection of MHC-disparate bone marrow allografts resembles a Th1-type process amenable to modulation by donor Th2-type cells. These data shed new light onto the biology of graft rejection and provide important mechanistic information pertaining to donor Th2 cell therapy for facilitation of alloengraftment. Therefore, after the initial characterization that graft rejection can be defined as the mirror image of graft-versus-host disease,1 our findings reveal that the T-cell biology underpinning these reactions also reflects one another in terms of the Th1/Th2 paradigm.
We demonstrated by 2 separate approaches that Th1-type host T cells preferentially mediated graft rejection. First, we found that unmanipulated host T cells and cytokine polarized Th1/Tc1 cells acquired allospecific IFN-γ secretion in vivo and mediated graft rejection with similar kinetics; in marked contrast, Th2/Tc2 polarized host T cells yielded reduced IFN-γ allospecificity and reduced rejection. These are the first data to directly indicate the importance of host T-cell cytokine phenotype as a determinant of marrow allograft rejection.
Second, we found that host T cells deficient in STAT1, which provides an afferent signal for Th1 polarization,33 were devoid of IFN-γ allospecificity in vivo and had greatly diminished rejection capacity. Previous attempts to identify a molecular mechanism of marrow graft rejection have been elusive10 ; to our knowledge, STAT1 is the only molecule now identified to be essential for mediation of marrow rejection. This result is consistent with a recent report that found STAT1 to be essential for memory CD8+ T-cell expansion and persistence in vivo.35 Of note, host T cells deficient in STAT4, which is a more distal signaling pathway in Th1 development,36 had fully intact IFN-γ allospecificity and competently mediated rejection. Interruption of host T-cell Th1 differentiation in our model therefore appears to be effective only at the proximal STAT1 checkpoint. Our results suggest that STAT1-specific inhibitors might facilitate MHC-disparate marrow allografts in a manner similar to an approach used in a rat cardiac allograft model.37
The present results extend our recent finding that donor CD4+ Th2R cell therapy represents a new approach to prevent graft rejection11 and further support our conclusion that host T cell–mediated rejection is primarily a Th1-type process. That is, effective donor Th2 cell therapy was associated not only with host T-cell adoption of a Th2 phenotype but also with the absence of Th1 differentiation. Several new findings have potential relevance for ongoing clinical trials using the strategy of allograft augmentation with donor Th2R cells. First, donor Th2R cell dose was an important determinant of alloengraftment: in fact, recipients of a suboptimal Th2R cell dose actually had increased host T-cell IFN-γ allospecificity concomitant with Th2 polarization. By comparison, an effective dose of donor Th2R cells yielded both Th2 polarization and inhibition of Th1 cytokines after transplantation. These findings are consistent with previous observations in rodent models of heart and kidney transplantation, where CD4-depleting antibody therapy promoted transplant tolerance only when both Th2 dominance and down-regulation of Th1 development occurred simultaneously.38,39 Further experiments will seek to better understand the mechanism whereby an effective dose of donor Th2R cells both promotes host Th2 polarity and limits host Th1 polarity; for example, it will be important to characterize the effect of donor Th2 cell dose on host T-cell expression of the Th2 transcription factor GATA-3, which is known to be essential for the inhibition of Th1 response.40 It is also possible that effective Th2 cell therapy may require not only host T-cell Th2 polarization via STAT6, but also involve some additional mechanism, such as consumption of cytokines required for Th1 differentiation.41
Second, similar to our recent results,11 we again observed that the manufacturing method was critical for success of the Th2 cell therapy: specifically, ex vivo expansion in rapamycin, which generates an antiapoptotic Th2R cell with enhanced in vivo survival,11 was required for Th2 cell rejection abrogation. Because Treg cells have been shown to prevent graft rejection25,26 and ex vivo rapamycin has been shown to preferentially expand Treg cells,42 it is reasonable to question whether Th2R cells might be functioning similar to a Treg cell population. However, our experiments performed to date do not suggest that a great deal of mechanistic overlap exists. First, we have previously characterized the Th2R cell product as being relatively devoid of Foxp3+ cells (< 1%).11 Second, in the current experiments, we found that the Th2R cell population functioned more analogous to a helper T cell, as evidenced by Th2R cell capacity to promote the expansion of host T cells at a sub-therapeutic dose. And third, we found that donor Th2R cells prevented rejection by a mechanism that resulted in the activation of host T cells toward a Th2-type effector status through an IL-4/STAT6 signaling pathway. By comparison, the IL-4 pathway is not typically implicated in Treg cell mechanisms; in fact, IL-4 and STAT6 signaling prevents the induction of Treg cells.43
Our finding that donor Th2R cells prevent rejection via an IL-4/STAT6 axis provides a new mechanism of donor T-cell facilitation of engraftment that appears distinct from previously implicated clonal deletion mechanisms. Using TCR transgenic allospecific host T cells and functional T-cell tracking by multicolor surface and cytokine-capture flow cytometry, we found that allospecific host T cells under the influence of donor Th2R cells had greatly diminished clonal expansion; however, such cells persisted in vivo with greatly reduced Th1 effector function and adoption of a Th2 cytokine profile. In previous studies, the fate of minor histocompatibility (HC) antigen specific host T cells has been studied during in vivo graft rejection.44 To our knowledge, the current results represent the initial study to track major HC-antigen specific T cells in vivo during rejection and the first to demonstrate that alloreactive host T cells can be shunted toward a Th2 phenotype in vivo. The mechanism of tolerance that we have identified is somewhat similar to that which occurs after total lymphoid irradiation, which was at least in part dependent upon host T-cell IL-4 secretion.45
In conclusion, host T cell–mediated rejection of fully MHC-disparate allografts can be primarily characterized as a Th1-type process. Host Th1 differentiation and subsequent rejection can be prevented by a proximal block at the STAT1 checkpoint or by potent donor Th2R cell therapy that infectiously forces host T-cell differentiation toward a relatively absolute Th2 polarity via a STAT6 pathway. Donor Th2R cell therapy therefore represents a vehicle for delivery of IL-4 in the relevant microenvironment of donor and host T-cell activation for modulation of both GVHD and graft rejection, thereby holding promise as a novel translational approach to allogeneic transplantation across genetic barriers.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Phil Lucas and Nyana Singh, both of NCI Experimental Transplantation and Immunity Branch, for providing the 2C- transgenic mice and 1B2 antibody.
This work was supported by the Center for Cancer Research, NCI, Intramural Research Program.
National Institutes of Health
Authorship
Contribution: J.M., J.F., K.R., N.B., and V.K. performed research and analyzed data; J.M., K.R., and D.H.F. wrote the manuscript; J.F. and S.A. assisted in the manuscript writing; and J.M. and D.H.F. designed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jacopo Mariotti, Experimental Transplantation and Immunology Branch, NCI, NIH, Clinical Research Center 3-East Labs, 3-3330, Bethesda, MD 20892; e-mail mariottj@mail.nih.gov.

![Figure 1. Host Th1/Tc1 cells mediate increased rejection relative to host Th2/Tc2 cells. (A) BALB/c host T cells were costimulated and expanded in IL-4 or IL-12 to generate Th2/Tc2 or Th1/Tc1 cells, respectively. On culture day 6, cells were restimulated; supernatants were tested for cytokine content (ng/mL). (B-D) B6-into-BALB/c transplantation consisted of lethal host irradiation (1050 cGy; day −2), and some combination, as indicated, of host T cells that were nonpolarized [“HT”], polarized to type I cytokines [“HTh1/Tc1”], or polarized to type II cytokines [“HTh2/Tc2”] (T-cell dose of 0.1 × 106; day −1); and donor BM cells (day 0). On day 8 after BMT, spleen cells were isolated and stimulated with syngeneic (BALB/c) or allogeneic (B6) DCs for 24 hours. The total number of host CD4+ and CD8+ cells producing allospecific IFN-γ was determined by flow cytometry using anti–H-2d, anti–H-2b, -CD4, or -CD8, and antibody capture of IFN-γ (panel B). Resultant supernatants were tested for cytokine content (panel C; pg/mL). Results are mean plus or minus SEM of n = 10 per cohort. *P < .05; **P < .01; ***P < .001. (D) Recipient mice were followed for 90 days after BMT: data shown are overall survival (left panel) and peripheral blood donor chimerism (right panel). Survival data were pooled from 2 independent experiments (total of n = 10 recipients per cohort).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-05-154278/7/m_zh80190824740001.jpeg?Expires=1766207072&Signature=VGWOqp~~kyXTjP3ZcG8NRMyuWC87XJG~fXlSwK6eg~XzRJi1eePfzU6UCgPhcUSk81uGe6yFK-~KvHj8jqs9VinQg95I2MhVMWLmR9cHnI6phaoaNVr9tKuwgqrQVczVVbEbG797awiwjdoYen9g0zqt~cUCM8ZeWqOxuIcYjj8TG3AsFs9VrLX~prKyhWUAw4xSWeJNh5wYNRD9imWbXw1g~OpljZwQjELai1lY-RV1eZ~Pl68~NPQc8J4DgmA6jOq4iEZM1drDpJOqujWHVzHxo~6MzN4BBMWjqcexp35wwT~JKiNTD0HzFmzMVLkdPbAZL8a7PXYdWvZ7jMQHdw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Donor Th2 cell abrogation of rejection restricts but does not eliminate allospecific host T cells. (A) Spleen cells from 2C TCR-transgenic mice were evaluated by flow cytometry for coexpression of the clonotypic TCR (using 1B2 antibody) and Vβ8; a representative example is shown. (B-D) BALB/c-into-B6 transplantation consisted of lethal irradiation (1100 cGy; day −2), and some combination, as indicated, of host T cells (day −1; 1:1 mix of 2C and CD45.1-congenic WT T cells [each population, 0.05 × 106 cells]) and BALB/c donor BM cells with or without donor Th2 cells generated ex vivo in rapamycin (Th2R cells; day 0). The ratio of donor Th2 cells to host T cells was either 100:1 (“BM/Th2Rlo”) or 500:1 (“BM/Th2Rhi”). At day 8 after BMT, the number of allospecific host T cells in the spleen (panel B) and BM (panel C) was enumerated by flow cytometry. Allospecific host T cells were identified using H-Kb+, H-2Kd−, CD45.1−, CD8+, Vβ8+ (experiment number 1; panels B and C left) or H-Kb+, H-2Kd−, CD45.1−, CD8+, Vβ8+, 1B2+ (experiment number 2; panels B and C right). *P < .05; **P < .01; ***P < .001. (D) Longitudinal tracking of allospecific host T cells by Vβ8 analysis at days 11, 14, 21, and 45 after BMT in the spleen (left panel) and BM (right panel) in transplant recipients of high-dose donor Th2R cells (n = 5-8 recipients at each time point). (E) In a third experiment, B6 hosts received lethal irradiation (day −2), and some combination, as indicated, of host T cells (“HT”; 1:1 mix of 2C TCR-transgenic and CD45.1-congenic WT T cells; each population, 0.01 × 106 cells; day −1), and donor BM with or without donor Th2R cells (day 0; donor Th2R cell: host T-cell ratio, 500:1). Mice were followed for survival analysis for 45 days (left panel; n = 10 per cohort) and weight loss (middle panel); donor chimerism in the spleen, BM, and blood was determined at day 45 after BMT (right panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-05-154278/7/m_zh80190824740003.jpeg?Expires=1766207072&Signature=sB65929facYrgLtdRi8bUzkb1nhyB2TNRN-k5iYF7THlUBL84XAXyUxCeBUselLIHHe4UQfwGKEYmu1p5HoDXDosuyTK8q7gxKiQ2~xpVg6NB-1QwE0M9qwbQjKFlYV710GUnfr~azw~8LVeZwI8YC2FUyYG8pjfVY-wTP3g2-zb19V7F~5op0YJRvIf72PJp1XXl2VowSX8NP68RUmQwyym~Pc7xQipoAtLBuWFb~WhO3NCw-gZFdLz~D2howJgeDoRA5baxOUvYCXQcH~XKzwHNOF7n0qN7aaO~VG31kgOyHB1nIkn2GVEYbWMbVaz35HnfE8bQ-e2cjJ86EEw9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Host Th1/Tc1 cells mediate increased rejection relative to host Th2/Tc2 cells. (A) BALB/c host T cells were costimulated and expanded in IL-4 or IL-12 to generate Th2/Tc2 or Th1/Tc1 cells, respectively. On culture day 6, cells were restimulated; supernatants were tested for cytokine content (ng/mL). (B-D) B6-into-BALB/c transplantation consisted of lethal host irradiation (1050 cGy; day −2), and some combination, as indicated, of host T cells that were nonpolarized [“HT”], polarized to type I cytokines [“HTh1/Tc1”], or polarized to type II cytokines [“HTh2/Tc2”] (T-cell dose of 0.1 × 106; day −1); and donor BM cells (day 0). On day 8 after BMT, spleen cells were isolated and stimulated with syngeneic (BALB/c) or allogeneic (B6) DCs for 24 hours. The total number of host CD4+ and CD8+ cells producing allospecific IFN-γ was determined by flow cytometry using anti–H-2d, anti–H-2b, -CD4, or -CD8, and antibody capture of IFN-γ (panel B). Resultant supernatants were tested for cytokine content (panel C; pg/mL). Results are mean plus or minus SEM of n = 10 per cohort. *P < .05; **P < .01; ***P < .001. (D) Recipient mice were followed for 90 days after BMT: data shown are overall survival (left panel) and peripheral blood donor chimerism (right panel). Survival data were pooled from 2 independent experiments (total of n = 10 recipients per cohort).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-05-154278/7/m_zh80190824740001.jpeg?Expires=1766545724&Signature=eoKkaTPXnWEXV44hsqyzUZliQGfIUkhB2kIkzyA8C5y4jNP0MG7ZXU4VuVdYjeNRP9s1A3cs93ZjuQb-fHsJKWOgCxhtjtObVGf3GzrNXm8z6eVHFyQo0Q9HrD04~Up1SOI-xky1jeaQ4MMLPLkPhqMPYbOHkdle5rfl5A2Kb~K7YP9uT8ROuHx3eZHdAtjJpWStpWZs1Wtxpp3lfCZOytvOnxRI559oCCldx7p6-2SydJSRF2o-qKXCw8xke6hor-n-CobNMomkhXcDdwzcCon1kejIj8ijqQAkYgPzYbrgxRTssfei7BlEP1q1IYZwnX-MVzRuYefrAV~8fOPKog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Donor Th2 cell abrogation of rejection restricts but does not eliminate allospecific host T cells. (A) Spleen cells from 2C TCR-transgenic mice were evaluated by flow cytometry for coexpression of the clonotypic TCR (using 1B2 antibody) and Vβ8; a representative example is shown. (B-D) BALB/c-into-B6 transplantation consisted of lethal irradiation (1100 cGy; day −2), and some combination, as indicated, of host T cells (day −1; 1:1 mix of 2C and CD45.1-congenic WT T cells [each population, 0.05 × 106 cells]) and BALB/c donor BM cells with or without donor Th2 cells generated ex vivo in rapamycin (Th2R cells; day 0). The ratio of donor Th2 cells to host T cells was either 100:1 (“BM/Th2Rlo”) or 500:1 (“BM/Th2Rhi”). At day 8 after BMT, the number of allospecific host T cells in the spleen (panel B) and BM (panel C) was enumerated by flow cytometry. Allospecific host T cells were identified using H-Kb+, H-2Kd−, CD45.1−, CD8+, Vβ8+ (experiment number 1; panels B and C left) or H-Kb+, H-2Kd−, CD45.1−, CD8+, Vβ8+, 1B2+ (experiment number 2; panels B and C right). *P < .05; **P < .01; ***P < .001. (D) Longitudinal tracking of allospecific host T cells by Vβ8 analysis at days 11, 14, 21, and 45 after BMT in the spleen (left panel) and BM (right panel) in transplant recipients of high-dose donor Th2R cells (n = 5-8 recipients at each time point). (E) In a third experiment, B6 hosts received lethal irradiation (day −2), and some combination, as indicated, of host T cells (“HT”; 1:1 mix of 2C TCR-transgenic and CD45.1-congenic WT T cells; each population, 0.01 × 106 cells; day −1), and donor BM with or without donor Th2R cells (day 0; donor Th2R cell: host T-cell ratio, 500:1). Mice were followed for survival analysis for 45 days (left panel; n = 10 per cohort) and weight loss (middle panel); donor chimerism in the spleen, BM, and blood was determined at day 45 after BMT (right panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-05-154278/7/m_zh80190824740003.jpeg?Expires=1766545724&Signature=egIeuEDP7P9SO5-AihR4nqLG-us8BMkueqjsflVJPfFt3ODLWlv3kg5mSkIcap6WMMKvTroDheGjqBfkswLmzblJiTJ3zrjyfo57cbkehDiNLcYVcJc-BhKZNpOYh8pGKqpKg3PaDI058jhFdb7CpvCDyiMOalwldqXRWIvQAMbUNG8CKpRNYpdt3ZaQLGMjbd8IDz1lt2q8QhkmrelYOTK71-1Gc~UZgPg~WGX8z1rN7YcDcdKFJ3jpDSkkb3khvF-bY2dPTf0FafFvzbYZOPLsTLrELlNQvAw~-c7hzmLcm3D64-CVxN3zNztp-r2Gg7AszCOpiSz7n0atWf6AyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)