Abstract
Calcium and its major downstream effector, calcium/calmodulin-dependent protein kinase II (CaMKII), are found to be important for the functions of immune cells. Lipopolysaccharide (LPS) has been shown to induce intracellular calcium release in macrophages; however, whether and how CaMKII is required for Toll-like receptor (TLR) signaling remain unknown. Here we demonstrate that TLR 4, 9, and 3 ligands markedly induce intracellular calcium fluxes and activate CaMKII-α in macrophages. Selective inhibition or RNA interference of CaMKII significantly suppresses TLR4, 9, 3-triggered production of interleukin-6 (IL-6), tumor necrosis factor-α, and interferon-α/β (IFN-α/β) in macrophages. Coincidently, overexpression of constitutively active CaMKII-α significantly enhances production of the above cytokines. In addition to the activation of mitogen-activated protein kinase and nuclear factor κB pathways, CaMKII-α can directly bind and phosphorylate transforming growth factor β–activated kinase 1 (TAK1) and IFN regulatory factor 3 (IRF3; serine on 386) via the N-terminal part of its regulatory domain. Therefore, CaMKII can be activated by TLR ligands, and in turn promotes both myeloid differentiating factor 88 and Toll/IL-1 receptor domain-containing adaptor protein-inducing IFN-β–dependent inflammatory responses by directly activating TAK1 and IRF3. The cross-talk with the calcium/CaMKII pathway is needed for full activation of TLR signaling in macrophages.
Introduction
On recognition of pathogenic components, Toll-like receptors (TLRs) are activated, leading to a variety of signaling events that initiate innate immunity and activate immune cells to produce proinflammatory cytokines and type I interferon (IFN).1,2 Most of the members of the TLR family, with the exception of TLR3, trigger immune response via the conserved myeloid differentiation factor 88 (MyD88)–dependent pathway, which involves MyD88, interleukin-1 (IL-1) receptor–associated kinase 1 (IRAK1), tumor necrosis factor (TNF) receptor–associated factor 6 (TRAF6), transforming growth factor-β (TGF-β)–activated kinase 1 (TAK1), downstream mitogen-activated protein kinases (MAPKs), and nuclear factor κB (NF-κB).2,3 Toll/IL-1 receptor–domain-containing adaptor protein inducing IFN-β (TRIF) has been found to induce the expression of type I IFN in response to TLR4 and TLR3 ligands, which associates with TANK-binding kinase 1 (TBK1) and activates downstream IFN regulatory factor 3 (IRF3). In addition, the TRIF pathway also contributes to TLR3- and TLR4-activated proinflammatory cytokine production.3,4
TLR activation is essential for provoking the innate immune response and enhancing adaptive immunity against invading pathogens. Less efficient activation of the TLR response may not evoke potent anti-infection or antitumor immunity; however, excessive activation of TLR may also induce immunopathologic processes, such as endotoxin shock and autoimmune diseases. How to manipulate or control the TLR response for prevention and treatment of inflammatory and immunologic diseases largely depends on the understanding of the molecular basis for TLR responses. Up to now, molecular mechanisms for the initiation and regulation of TLR responses remain to be fully understood. In addition to the MyD88- or TRIF-dependent pathway, ligation of TLRs has been found to activate various other intracellular signaling molecules, such as phosphatidylinositol 3-kinase (PI3K)/AKT5 and MAPK kinase kinase (MEKK3).6 These molecules are potentially involved in TLR signaling through different mechanisms and required for TLR-mediated full activation of immune cells, especially for antigen-presenting cells, such as macrophages and dendritic cells. This raises one important question that other signaling molecules involved in TLR signaling should be further clarified. So, cross-talks between TLR signaling and other signal pathways have attracted much attention in recent years.
Calcium (Ca2+) functions as a major second messenger that regulates a broad range of important cellular processes.7 Many of the cellular responses to the Ca2+ signal are induced or modulated by a family of multifunctional Ca2+/calmodulin-dependent protein kinases (CaMKs), among which CaMKII is a ubiquitous serine/threonine protein kinase encoded by 4 separate genes (α, β, γ, and δ).8 In the resting cells, CaMKII exists as an oligomeric complex of 8 to 12 subunits with autoinhibition of catalytic activity by the autoinhibitory domain. Binding of Ca2+/CaM relieves autoinhibition, resulting in activation of CaMKII and intersubunit phosphorylation (Thr286, for CaMKII-α). This phosphorylation prevents the autoinhibitory domain from reassociating with the kinase domain and results in Ca2+/CaM-independent kinase activity.9 In addition, the phosphorylated subunit shows a 1000-fold increase in the affinity for CaM because of a marked change in the off rate. This unique mechanism allows CaMKII to molecularly potentiate transient increases of Ca2+ and apparently enables detection of the frequency of such transients.10 CaMKII is eventually inactivated by removal of this phosphate by a CaMK-dedicated phosphatase.11 CaMKII can phosphorylate up to 50 different substrates, such as cAMP response element–binding protein (CREB), signal transducer and activator of transcription 1 (STAT1), which are involved in diverse cellular functions.8,12,13 In addition, CaMKII has been found to play important roles in the immune responses, such as T-cell activation,14 maturation, and antigen presentation of dendritic cells.15
Lipopolysaccharide (LPS) has been shown to elicit Ca2+ flux in murine macrophages,16,17 which is important for TNF-α production17,18 ; CaMKII was also reported to enhance platelet-activating factor-primed and LPS-induced TNF-α production in THP-1 cells,19 suggesting a possible involvement of Ca2+ flux and CaMKII in TLR4 signaling. However, the exact roles of Ca2+ and its major downstream kinase CaMKII in TLR-triggered production of proinflammatory cytokine and type I IFN, and the cross-talk between the Ca2+/CaMKII pathway and TLR signaling are still poorly characterized. In the present study, we provide evidence that the TLR4, TLR3, and TLR9 ligands can significantly trigger the elevation of intracellular Ca2+ and activation of CaMKII in macrophages, and in turn CaMKII promotes both MyD88- and TRIF-dependent proinflammatory cytokine and type I IFN production by directly binding and activating TAK1 and IRF3. Consistently, CaMKII inhibitor can decrease in vivo production of proinflammatory cytokines and IFN-β and increase the resistance to septic shock in lethal LPS-challenged mice. Therefore, positive cross-talk with the Ca2+/CaMKII pathway is required to the full activation of TLR responses in macrophages.
Methods
Mice and reagents
C57BL/6 mice (6-8 weeks) were obtained from Joint Ventures Sipper BK Experimental Animal (Shanghai, China). LPS (0111:B4) was from Sigma-Aldrich (St Louis, MO). Poly(I:C) and KN62 was from Calbiochem (San Diego, CA). All animal experiments were undertaken in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of Secondary Military Medical University (Shanghai, China). Endotoxin level in poly(I:C) was less than 0.0125 endotoxin units/mg, as measured by Limulus Amoebocyte Lysate assay. CpG oligodeoxynucleotide (ODN) was synthesized and repurified as described previously.20 Endotoxin level in CpG ODN was less than 0.015 endotoxin units/mg CpG ODN. O,O′-Bis(2-aminophenyl)ethyleneglycol-N,N,N′,N′-tetraacetic acid, tetraacetoxymethyl ester (BAPTA-AM) was from Dojindo (Kumamoto, Japan). Antibodies specific to hemagglutinin (HA)–tag, total-, and phospho–extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), p38, and IRF3 were from Cell Signaling Technology (Danvers, MA). Antibodies specific to TAK1, CaMKII-α, and phospho–CaMKII-α were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Flag and anti–β-actin antibodies were from Sigma-Aldrich (St Louis, MO).
Cell culture and transfection
Mouse macrophage cell line RAW264.7 and human HEK293 cell line were obtained from ATCC (Manassas, VA) and cultured as described.20 The cells were transfected with JetPEI (Illkirch, France). Thioglycolate-elicited mouse peritoneal macrophages were prepared and cultured as described previously,20 and nucleofected with the Mouse Macrophage Nucleofect Kit using Amaxa Nucleofector II Biosystems (Amaxa Biosystems, Gaithersburg, MD).
Plasmid constructs
cDNA encoding mouse CaMKII-α was amplified from mRNA of RAW264.7 cells by reverse-transcribed polymerase chain reaction (RT-PCR), and cloned into the pcDNA3.1 expression vector (Invitrogen, Carlsbad, CA). The Flag-tagged expression vectors of domain-truncated mutants of CaMKII-α, including CaMKII260 (amino acids 1-260), CaMKII290 (amino acids 1-290), CaMKII300 (amino acids 1-300), CaMKII261 (amino acids 261-478), CaMKII301 (amino acids 301-478), and CaMKII-Δ (amino acids 261-300 deleted), were constructed by PCR cloning and PCR mutation. HA-tagged IRF3, IRF3N140 (amino acids 1-140), and IRF3C141 (amino acids 141-427) constructs were generated by PCR. HA-tagged TAK1 plasmid was a kind gift from Dr Kunihiro Matsumoto (Nagoya University, Nagoya, Japan). IRF3 reporter plasmids were kind gifts from Dr Takashi Fujita (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). MyD88 and TRIF constructs, IFN-β, TNF-α, and NF-κB luciferase reporter plasmids were described previously.20 Mouse cDNA encoding residues 380-427 of IRF3 was cloned into pGEX-4T3 vector (GE Healthcare, Little Chalfont, United Kingdom) to generate glutathione S-transferase (GST)–IRF3 wild-type (wt) construct. Various mutants of GST-IRF3 were generated from GST-IRF3 wt plasmid. All constructs were confirmed by DNA sequencing.
Ca2+ imaging
Ca2+ imaging was performed as described previously with minor modification.21 RAW264.7 cells or mouse peritoneal macrophages were plated on coverslips and loaded for 1 hour at 37°C with 3 μM Fluo-3/AM (Invitrogen) in the presence of 0.02% pluronic acid in Dulbecco modified Eagle medium (DMEM). Cells were washed with phosphate-buffered saline (PBS) and incubated at 37°C for 30 minutes in DMEM. Fluo-3/AM fluorescence imaging was recorded at baseline and scanned at 10-second intervals using a Leica TCS SP2 confocal laser microscope (Leica, Wetzlar, Germany) under a 10×/0.40 CS objective lens. Images were analyzed using Leica Confocal software and processed with Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA). Data were collected as the average fluorescence detected from 15 cells per microscope field at each time point.
RNA interfering
The sequences of small-interfering RNA (siRNA) targeting CaMKII-α were 5′-CACCACCATTGAGGACGAA-3′ (CaMKII-α siRNA1) and 5′-ACAGGAAATTATCAAAGTG-3′ (CaMKII-α siRNA2). The control small RNA sequence was 5′-AATCAGTCACGTTAATGGTCG-3′. siRNA duplexes were transfected into RAW264.7 cells or mouse peritoneal macrophages using Genesilencer Transfection Reagent (Genlantis, San Diego, CA) according to the standard protocol.
Detection of IL-6, TNF-α, and IFN-β
IL-6, TNF-α, and IFN-β levels in the culture supernatants and serum were measured with enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer's protocols.
Real-time quantitative PCR
Total RNA was extracted with TRIzol reagent (Invitrogen) following the manufacturer's instructions. Real-time quantitative RT-PCR (Q-PCR) analysis was performed using LightCycler (Roche Diagnostics, Indianapolis, IN) and the SYBR RT-PCR Kit (Takara, Kyoto, Japan). The primers used for mouse β-actin and IFN-4α analysis were described as previously.20 Data were normalized by the level of β-actin expression in each sample.
Assay of luciferase reporter gene expression
RAW264.7 cells or HEK293 cells were cotransfected with the mixture of indicated luciferase reporter plasmid, pRL-TK-Renilla luciferase plasmid, and indicated amounts of CaMKII290, MyD88, TRIF, or IRF3 construct. Total amounts of plasmid DNA were equalized with empty control vector. After 24 or 36 hours, the cells were left untreated or treated with LPS or poly(I:C). Luciferase activities were measured using the Dual-Luciferase Reporter Assay system (Promega, Madison, WI) according to the manufacturer's instructions. Data are normalized for transfection efficiency by dividing Firefly luciferase activity with that of Renilla luciferase.
Immunoblot and immunoprecipitation
The nuclear and cytoplasmic extracts were prepared with NE-PER nuclear and cytoplasmic extraction reagents (Pierce Chemical, Rockford, IL). Immunoblot and immunoprecipitation were performed as described previously.20 In some immunoprecipitation experiments, a unique horseradish peroxidase–conjugated secondary antibody, mouse TrueBlot ULTRA (eBioscience, San Diego, CA) was used, which can minimize the reactivity with the heavy and light chains of the immunoprecipitating antibody.
GST pull-down assay
GST-tagged CaMKII (amino acids 1-300; Santa Cruz Biotechnology) was incubated with RAW264.7 cell lysates at 4°C for 30 minutes, further incubated with glutathione-Sepharose 4B (Pierce Chemical) for 2 hours in immunoprecipitated buffer containing Nonidet P40. After washing 3 times, the precipitates were subjected to Western blot analysis.
Expression of GST fusion proteins
wt and mutant GST-IRF3 380-427 were expressed in Escherichia coli and purified by GST affinity chromatography (Pierce Chemical).
Assay for CaMKII activity
Endogenous CaMKII-α was immunoprecipitated from cell lysates with anti–CaMKII-α antibody and its activity was determined with the CaMKII kinase assay system (New England Biolabs, Ipswich, MA) containing 5 μCi [γ-32P] adenosine triphosphate (GE Healthcare). The 32P incorporation was determined with a liquid scintillation counter (Beckman Coulter, Fullerton, CA).
In vitro kinase assay
Purified GST-IRF3 protein or recombinant GST-TAK1 protein (Abnova, Taiwan) was incubated with recombinant active CaMKII-α in the aforementioned CaMKII kinase assay system at 30°C for 30 minutes. Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography. For determining the activation of TAK1 by CaMKII-α, 5 μg of myelin basic protein (MBP; Upstate Biotechnology, Charlottesville, VA) was added into the aforementioned reaction mixture followed by incubation for another 20 minutes. Samples were analyzed by immunoblotting with anti–phospho-MBP antibody.
Statistical analysis
Statistical significance was determined by the Student t test or analysis of variance for multiple comparisons of normal distributions, with a value of P less than .05 considered to be statistically significant. Survival curves were compared by using the log-rank test.
Results
TLR ligands induce intracellular Ca2+ release and CaMKII activation in macrophages
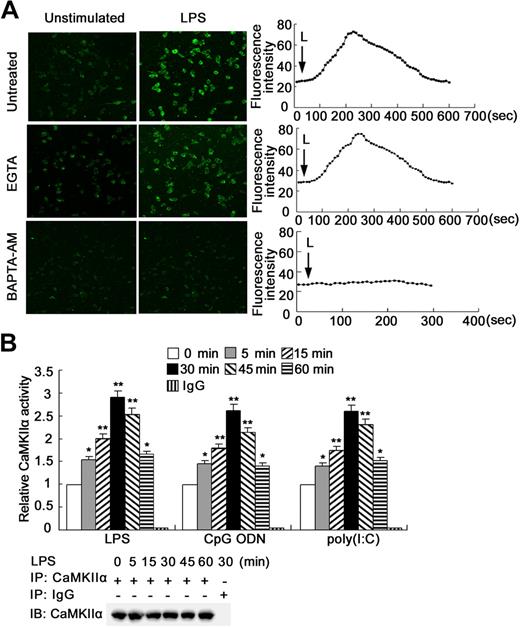
To determine whether Ca2+ fluxes can be elicited by different TLR ligands in macrophages, RAW264.7 cells were loaded with Fluo-3/AM and stimulated with ligands of TLR4, TLR9, or TLR3, respectively. An immediate and 2- to 3-fold increase in fluorescence intensity was noted by 200 seconds after LPS stimulation, which returned to baseline by approximately 8 minutes (Figure 1A). In the presence of the intracellular Ca2+ chelator BAPTA-AM, the LPS-induced Ca2+ mobilization was blocked, whereas ethyleneglycoltetraacetic acid had no such effect, indicating that the source of Ca2+ released was from intracellular Ca2+ stores. Stimulation with CpG ODN or poly(I:C) also induced a similar Ca2+ mobilization from internal Ca2+ stores (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, 3 kinds of TLR ligands evoked Ca2+ release with similar pattern in mouse peritoneal macrophages (data not shown). These data demonstrate that activation of TLR4, 9, 3 caninduce Ca2+ fluxes in macrophages.
TLR ligands induce intracellular Ca2+ release and CaMKII activation in macrophages. (A) RAW264.7 cells were loaded with Fluo 3/AM and stimulated with 0.1 μg/mL LPS in the absence or presence of 1.5 mM ethyleneglycoltetraacetic acid or 6 μM BAPTA-AM, then imaged by Leica TCS SP2 confocal microscopy under a 10×/0.40 CS objective lens at 10-second intervals. The basal (left panel) and peak (middle panel) fluorescence is displayed. Graphs (right panel) showed changes in mean fluorescence intensity from 15 cells per microscopic field over time. Data are representative of 3 independent experiments (original magnification ×100). L indicates LPS. (B) Cell extracts of TLR ligand–stimulated RAW264.7 cells were immunoprecipitated with anti–CaMKII-α antibody. The immunoprecipitates were subjected to CaMKII activity assay with autocamtide-2 as substrate or immunoblotted with anti–CaMKII-α antibody. Data are shown as mean plus or minus SD of 3 independent experiments. *P < .05, **P < .01 vs unstimulated cells.
TLR ligands induce intracellular Ca2+ release and CaMKII activation in macrophages. (A) RAW264.7 cells were loaded with Fluo 3/AM and stimulated with 0.1 μg/mL LPS in the absence or presence of 1.5 mM ethyleneglycoltetraacetic acid or 6 μM BAPTA-AM, then imaged by Leica TCS SP2 confocal microscopy under a 10×/0.40 CS objective lens at 10-second intervals. The basal (left panel) and peak (middle panel) fluorescence is displayed. Graphs (right panel) showed changes in mean fluorescence intensity from 15 cells per microscopic field over time. Data are representative of 3 independent experiments (original magnification ×100). L indicates LPS. (B) Cell extracts of TLR ligand–stimulated RAW264.7 cells were immunoprecipitated with anti–CaMKII-α antibody. The immunoprecipitates were subjected to CaMKII activity assay with autocamtide-2 as substrate or immunoblotted with anti–CaMKII-α antibody. Data are shown as mean plus or minus SD of 3 independent experiments. *P < .05, **P < .01 vs unstimulated cells.
Because Ca2+ fluxes can be initiated by TLR ligands in macrophages, CaMKII, as a major biochemical decoder of intracellular Ca2+ oscillations, may be activated. Although the expression of α isoform of CaMKII (CaMKII-α) remained almost unchanged (Figure S2A), a rapid and marked increase of CaMKII-α activity in macrophages could be observed after stimulation with LPS, CpG ODN, or poly(I:C) (Figure 1B), which was confirmed by the significant increase of CaMKII-α phosphorylation (T286; Figure S2B). The notable increase of CaMKII activity was also observed in mouse peritoneal macrophages stimulated with LPS, CpG ODN, or poly(I:C), respectively (data not shown). So, TLR ligands can induce Ca2+ fluxes and activate CaMKII-α in macrophages, suggesting that activation of the calcium/CaMKII pathway is a common and universal process during TLR responses. This raises the possibility that the Ca2+/CaMKII pathway may be potentially involved in TLR signaling.
Blockade of CaMKII activation or silencing of CaMKII expression attenuates TLR-activated proinflammatory cytokine and type I IFN production in macrophages
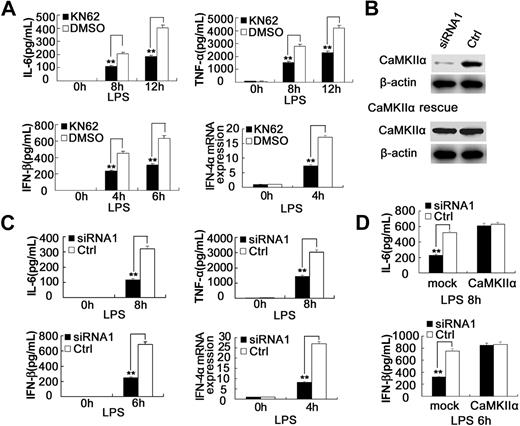
KN62, a selective inhibitor of CaMKII, can effectively block the activation of CaMKII by binding to an unidentified site on CaMKII and interfering with calmodulin binding.22 KN62 is also a potent inhibitor of CaMKIV23 ; however, CaMKIV is not expressed in RAW264.7 cells or mouse peritoneal macrophages as determined by RT-PCR and Western blot (data not shown). To explore the role of CaMKII in TLR signaling, we examined the effects of CaMKII inhibitor KN62 on the production of cytokines in macrophages stimulated with TLR ligands. KN62 could inhibit LPS-induced IL-6 production in a dose-dependent manner, whereas 15 μM KN62 could achieve the maximum inhibitory effect (∼ 50%) without cytotoxicity as measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays; 20 μM KN62 showed similar inhibitory effect. Based on these data (Figure S3A,B), we selected 15 μM KN62 for all the following experiments. In addition to inhibition of IL-6, KN62 could significantly suppress LPS-induced production of TNF-α, IFN-α, and IFN-β in RAW264.7 cells (Figure S3C) and mouse peritoneal macrophages (Figure 2A). Furthermore, KN62 significantly inhibited CpG ODN–induced IL-6, TNF-α production, as well as poly(I:C)–induced IL-6, TNF-α, and IFN-β production in RAW264.7 cells (Figure S3D,E).
Blockade of CaMKII activation with KN62 or silencing of CaMKII expression attenuates TLR4-activated proinflammatory cytokine and type I IFN production in macrophages. (A) Mouse peritoneal macrophages (4 × 105) were pretreated with 15 μM KN62 for 30 minutes followed by stimulation with 0.1 μg/mL LPS for the indicated time. The production of IL-6, TNF-α, or IFN-β was measured by ELISA, and IFN-4α mRNA expression was measured by Q-PCR. (B) (Top) RAW264.7 cells were transfected with control small RNA (Ctrl) or CaMKII-α siRNA 1. After 48 hours, CaMKII-α and β-actin expression in the cells was detected by immunoblot. (Bottom) CaMKII-α–silenced RAW264.7 cells were transfected with CaMKII-α full-length expression vector. After 36 hours, CaMKII-α and β-actin expression in the cells was detected by immunoblot. Similar results were obtained in 3 independent experiments. (C) Mouse peritoneal macrophages (4 × 105) were transfected with control small RNA (Ctrl) or CaMKII-α siRNA1. After 48 hours, the cells were stimulated with 0.1 μg/mL LPS for the indicated time. IL-6, TNF-α, or IFN-β in the supernatants was measured by ELISA, and IFN-4α mRNA expression was measured by Q-PCR. (D) RAW264.7 cells (1.5 × 105) were transfected with control small RNA (Ctrl) or CaMKII-α siRNA1. After 36 hours, the cells were transfected with full-length CaMKII-α plasmid. Thirty-six hours later, the cells were stimulated with 0.1 μg/mL LPS for the indicated time. IL-6 and IFN-β in the supernatants were measured by ELISA. Data are shown as mean plus or minus SD of 3 independent experiments. **P < .01.
Blockade of CaMKII activation with KN62 or silencing of CaMKII expression attenuates TLR4-activated proinflammatory cytokine and type I IFN production in macrophages. (A) Mouse peritoneal macrophages (4 × 105) were pretreated with 15 μM KN62 for 30 minutes followed by stimulation with 0.1 μg/mL LPS for the indicated time. The production of IL-6, TNF-α, or IFN-β was measured by ELISA, and IFN-4α mRNA expression was measured by Q-PCR. (B) (Top) RAW264.7 cells were transfected with control small RNA (Ctrl) or CaMKII-α siRNA 1. After 48 hours, CaMKII-α and β-actin expression in the cells was detected by immunoblot. (Bottom) CaMKII-α–silenced RAW264.7 cells were transfected with CaMKII-α full-length expression vector. After 36 hours, CaMKII-α and β-actin expression in the cells was detected by immunoblot. Similar results were obtained in 3 independent experiments. (C) Mouse peritoneal macrophages (4 × 105) were transfected with control small RNA (Ctrl) or CaMKII-α siRNA1. After 48 hours, the cells were stimulated with 0.1 μg/mL LPS for the indicated time. IL-6, TNF-α, or IFN-β in the supernatants was measured by ELISA, and IFN-4α mRNA expression was measured by Q-PCR. (D) RAW264.7 cells (1.5 × 105) were transfected with control small RNA (Ctrl) or CaMKII-α siRNA1. After 36 hours, the cells were transfected with full-length CaMKII-α plasmid. Thirty-six hours later, the cells were stimulated with 0.1 μg/mL LPS for the indicated time. IL-6 and IFN-β in the supernatants were measured by ELISA. Data are shown as mean plus or minus SD of 3 independent experiments. **P < .01.
We also investigated whether CaMKII knockdown could regulate TLR-triggered proinflammatory cytokine and type I IFN production in macrophages. As shown in Figure 2B and Figure S4B, endogenous CaMKII-α expression in RAW264.7 cells was significantly down-regulated by siRNA 1 (82%) and, to a lesser extent (∼ 79%), by siRNA 2. Similar results were obtained in mouse peritoneal macrophages (data not shown). Compared with control siRNA, CaMKII-α siRNA 1 significantly decreased LPS-induced production of IL-6, TNF-α, IFN-α, and IFN-β in RAW264.7 cells (Figure S4A) and mouse peritoneal macrophages (Figure 2C). CaMKII-α siRNA 2 also markedly decreased LPS-induced production of IL-6 and IFN-β in RAW264.7 cells, although these inhibitory effects were less significant than that of siRNA 1 (Figure S4C). CaMKII-α knockdown also suppressed CpG ODN–induced IL-6, TNF-α production, as well as poly(I:C)–induced IL-6, TNF-α, and IFN-β production in RAW264.7 cells (Figure S4D,E). To exclude the possible off-target effects of RNA interfering, we transfected CaMKII-α–silenced RAW264.7 cells with CaMKII-α full-length expression vector and found that CaMKII-α overexpression could rescue CaMKII-α silence-induced inhibition of proinflammatory cytokine production by macrophages (Figure 2B,D). Because TLR9 triggers proinflammatory cytokine production through the MyD88-dependent pathway, TLR3 triggers type I IFN and proinflammatory cytokine production through the TRIF-dependent pathway, whereas TLR4 triggers both pathways, these data indicate that CaMKII enhances both MyD88-dependent and TRIF-dependent cytokine production in macrophages.
Overexpression of constitutively active CaMKII promotes both MyD88-dependent and TRIF-dependent proinflammatory cytokine and IFN-β production in macrophages
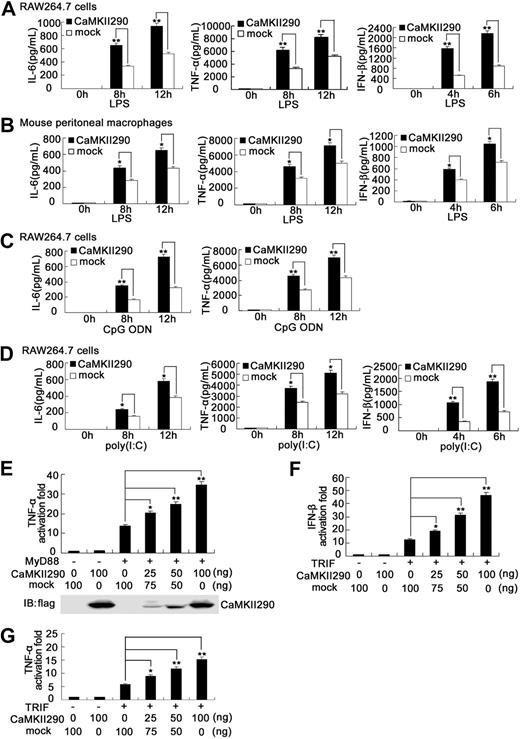
To further confirm the role of CaMKII in TLR responses, we observed the effect of overexpression of constitutively active CaMKII on TLR-triggered inflammatory cytokine and type I IFN production in macrophages. The truncated constitutively active CaMKII-α (CaMKII290) was constructed by the deletion of the autoinhibitory domain in its C-terminal region (amino acids 291-478), which is independent of Ca2+/calmodulin regulation.24,25 CaMKII290 overexpression significantly enhanced LPS-induced production of IL-6, TNF-α, and IFN-β in RAW264.7 cells (Figure 3A) and mouse peritoneal macrophages (Figure 3B). Moreover, CaMKII290 overexpression markedly enhanced CpG ODN–induced production of IL-6, TNF-α (Figure 3C), as well as poly(I:C)–induced production of IL-6, TNF-α, and IFN-β in RAW264.7 cells (Figure 3D). Our further experiments showed that cotransfection of CaMKII290 could markedly enhance MyD88-activated TNF-α as well as TRIF-activated TNF-α and IFN-β reporter gene expression in a dose-dependent manner (Figure 3E-G). These data provide convincing evidence that CaMKII promotes both MyD88-dependent and TRIF-dependent proinflammatory cytokine and IFN-β production in macrophages.
Overexpression of constitutively active CaMKII enhances TLR-triggered proinflammatory cytokine and IFN-β production in macrophages. RAW264.7 cells (1.5 × 105) (A,C,D) were transiently transfected with constitutively active CaMKII plasmid (CaMKII290). Mouse peritoneal macrophages (4 × 105) (B) were nucleofected with CaMKII290 using Amaxa Nucleofector II Biosystems. After 36 hours, the cells were stimulated with 0.1 μg/mL LPS (A,B), 0.3 μM CpG ODN (C), or 10 μg/mL poly(I:C) (D), respectively, for the indicated time. IL-6, TNF-α, or IFN-β in the supernatants was detected by ELISA. Data are shown as mean plus or minus SD of 3 independent experiments. HEK293 cells were cotransfected with 50 ng of MyD88 (E) or TRIF (F,G) expressing plasmid, 50 ng of TNF-α (E,G), or IFN-β (F) luciferase reporter plasmid, 10 ng of pTK-Renilla luciferase, together with indicated amount of CaMKII290 expressing plasmid. Total amounts of plasmid DNA were equalized using empty control vector. After 24 hours of culture, luciferase activity was measured and normalized by Renilla luciferase activity. The expression of CaMKII290 in HEK293 cells was immunoblotted with anti-flag antibody (E). Data are shown as mean plus or minus SD (n = 5) of one typical experiment from 3 independent experiments with similar results. *P < .05; **P < .01.
Overexpression of constitutively active CaMKII enhances TLR-triggered proinflammatory cytokine and IFN-β production in macrophages. RAW264.7 cells (1.5 × 105) (A,C,D) were transiently transfected with constitutively active CaMKII plasmid (CaMKII290). Mouse peritoneal macrophages (4 × 105) (B) were nucleofected with CaMKII290 using Amaxa Nucleofector II Biosystems. After 36 hours, the cells were stimulated with 0.1 μg/mL LPS (A,B), 0.3 μM CpG ODN (C), or 10 μg/mL poly(I:C) (D), respectively, for the indicated time. IL-6, TNF-α, or IFN-β in the supernatants was detected by ELISA. Data are shown as mean plus or minus SD of 3 independent experiments. HEK293 cells were cotransfected with 50 ng of MyD88 (E) or TRIF (F,G) expressing plasmid, 50 ng of TNF-α (E,G), or IFN-β (F) luciferase reporter plasmid, 10 ng of pTK-Renilla luciferase, together with indicated amount of CaMKII290 expressing plasmid. Total amounts of plasmid DNA were equalized using empty control vector. After 24 hours of culture, luciferase activity was measured and normalized by Renilla luciferase activity. The expression of CaMKII290 in HEK293 cells was immunoblotted with anti-flag antibody (E). Data are shown as mean plus or minus SD (n = 5) of one typical experiment from 3 independent experiments with similar results. *P < .05; **P < .01.
CaMKII enhances MAPK, NF-κB, and IRF3 activation in TLR-triggered macrophages
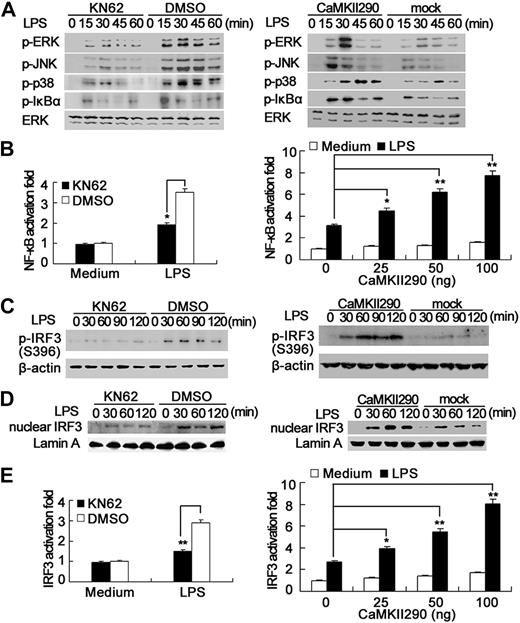
Next, we investigated the mechanisms for the increase of TLR responses by CaMKII. It is well known that activation of MAPK and NF-κB pathways is necessary for proinflammatory cytokine production in TLR signaling. CaMKII inhibitor KN62 impaired LPS-induced phosphorylation of ERK, JNK, p38, and IκBα in RAW264.7 cells; consistently, CaMKII290 overexpression increased LPS-induced phosphorylation of these proteins (Figure 4A). NF-κB luciferase reporter gene assay further confirmed that CaMKII inhibitor KN62 impaired NF-κB activation, whereas CaMKII290 overexpression dose-dependently enhanced NF-κB activation in LPS-stimulated RAW264.7 cells (Figure 4B). CaMKII-α knockdown with specific siRNA also suppressed LPS-induced NF-κB activation in macrophages (data not shown). These results suggest that CaMKII can enhance LPS-induced MAPK and NF-κB activation, thus contributing to the significant increase of proinflammatory cytokine production in LPS-stimulated macrophages by CaMKII.
Activation of CaMKII enhances MAPK, NF-κB, and IRF3 activation in TLR-triggered macrophages. (A) RAW264.7 cells were pretreated with 15 μM KN62 (left panel) for 30 minutes or transfected with CaMKII290 plasmid and then cultured for 36 hours (right panel). The cells were stimulated with 0.1 μg/mL LPS for the indicated time. Phospho-ERK, JNK, p38, IκBα, and total ERK were detected by immunoblot. (B) RAW264.7 cells were transfected with 100 ng of pGL3.5XκB-luciferase, 10 ng of pTK–Renilla luciferase, together without (left panel) or with indicated amount of CaMKII290 plasmid (right panel). After 36 hours, the cells were stimulated with 0.1 μg/mL LPS for 6 hours. Luciferase activity was measured and normalized by Renilla luciferase activity. (C,D) RAW264.7 cells were treated as described in panel A; the whole cell extracts (C) or nuclear extracts (D) were prepared. Phospho- (C) or total-IRF3 (D) was detected by immunoblot. Data are representative of 3 separate experiments. (E) RAW264.7 cells were transfected with 100 ng of IRF3 luciferase reporter plasmids (80 ng of Gal4 luciferase reporter plasmid, 20 ng of Gal4-IRF3–expressing plasmid), 10 ng of pTK-Renilla luciferase, together with indicated amount of CaMKII290 plasmid. After 36 hours, the cells were stimulated with 0.1 μg/mL LPS for 6 hours. Luciferase activity was measured. Data are shown as mean plus or minus SD (n = 5) of one typical result from 3 independent experiments with similar results. *P < .05; **P < .01.
Activation of CaMKII enhances MAPK, NF-κB, and IRF3 activation in TLR-triggered macrophages. (A) RAW264.7 cells were pretreated with 15 μM KN62 (left panel) for 30 minutes or transfected with CaMKII290 plasmid and then cultured for 36 hours (right panel). The cells were stimulated with 0.1 μg/mL LPS for the indicated time. Phospho-ERK, JNK, p38, IκBα, and total ERK were detected by immunoblot. (B) RAW264.7 cells were transfected with 100 ng of pGL3.5XκB-luciferase, 10 ng of pTK–Renilla luciferase, together without (left panel) or with indicated amount of CaMKII290 plasmid (right panel). After 36 hours, the cells were stimulated with 0.1 μg/mL LPS for 6 hours. Luciferase activity was measured and normalized by Renilla luciferase activity. (C,D) RAW264.7 cells were treated as described in panel A; the whole cell extracts (C) or nuclear extracts (D) were prepared. Phospho- (C) or total-IRF3 (D) was detected by immunoblot. Data are representative of 3 separate experiments. (E) RAW264.7 cells were transfected with 100 ng of IRF3 luciferase reporter plasmids (80 ng of Gal4 luciferase reporter plasmid, 20 ng of Gal4-IRF3–expressing plasmid), 10 ng of pTK-Renilla luciferase, together with indicated amount of CaMKII290 plasmid. After 36 hours, the cells were stimulated with 0.1 μg/mL LPS for 6 hours. Luciferase activity was measured. Data are shown as mean plus or minus SD (n = 5) of one typical result from 3 independent experiments with similar results. *P < .05; **P < .01.
IRF3 is the key transcription factor that is activated through the TRIF-dependent pathway and mediates the production of type I IFN in TLR3 and TLR4 signaling.26 IRF3 resides in the cytoplasm in resting cells and, on stimulation, becomes activated via serine/threonine phosphorylation (between residues 385 and 405) leading to its dimerization, nuclear translocation, and association with the coactivators CBP-p300.27 Because activation of IRF3 has been correlated with phosphorylation of Ser396,28 we first observed the effects of CaMKII inhibition or activation on the phosphorylation level of IRF3 (Ser396). Inhibition of CaMKII by KN62 decreased LPS-induced phosphorylation of IRF3 (Ser396); consistently, CaMKII290 overexpression increased its phosphorylation (Figure 4C). In addition, KN62 treatment inhibited LPS-induced nuclear translocation of IRF3 in RAW264.7 cells, whereas CaMKII290 overexpression promoted its nuclear translocation (Figure 4D). By detecting IRF3 luciferase report gene expression in RAW264.7 cells, we found that LPS-induced IRF3 activation was significantly inhibited by KN62 treatment but enhanced by CaMKII290 overexpression in a dose-dependent manner (Figure 4E). Silencing of CaMKII-α expression also impaired IRF3 activation in LPS-stimulated macrophages (data not shown). CaMKII also enhanced poly(I:C)–induced IRF3 activation (Figure S5). So, CaMKII plays an indispensable role in the full activation of IRF3 in TLR3 and TLR4 signaling, thus leading to the significant increase of TRIF-activated IFN-β production by CaMKII.
CaMKII directly binds and phosphorylates TAK1-promoting activation of TAK1
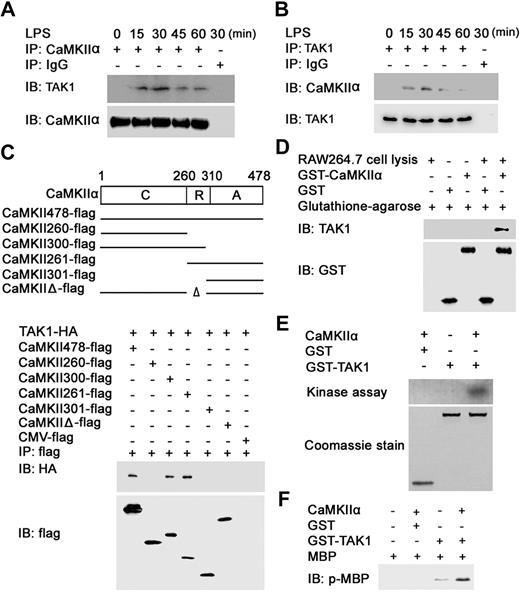
TAK1 is essential to the optimal MAPK, NF-κB activation, and production of proinflammatory cytokines in TLR signaling.29 We wondered whether CaMKII could interact with and activate TAK1. Immunoprecipitation experiments showed that CaMKII-α could interact with TAK1 in LPS-stimulated macrophages (Figure 5A,B), suggesting that endogenous CaMKII-α could interact with TAK1 on TLR4 activation.
CaMKII directly binds, phosphorylates, and activates TAK1. (A,B) RAW264.7 cells were stimulated with 0.1 μg/mL LPS for the indicated time. Equal amount cell lysates were immunoprecipitated with CaMKII-α (A) or TAK1 (B) antibody and then immunoblotted (IB) with CaMKII-α and TAK1 antibody. (C) HEK293 cells were transfected with TAK1-HA and wt or mutant CaMKII-α–flag construct as indicated. After 24 hours, cell extracts were immunoprecipitated with anti-flag antibody and then immunoblotted with anti-HA and anti-flag antibody. C indicates catalytic domain (amino acids 1-260); R, regulatory domain (amino acids 261-309); A, association domain (amino acids 310-478). (D) GST pull-down assays were performed with recombinant GST-tagged CaMKII-α and cell extracts of RAW264.7 cells. (E) One microgram of recombinant TAK1 protein was incubated with recombinant active CaMKII-α at 30°C for 30 minutes. Samples were separated by SDS-PAGE followed by autoradiography. (F) MBP was added into the reaction mixture as in panel E followed by incubation for another 20 minutes. Samples were analyzed by immunoblotting with anti–phospho-MBP antibody. Data are representative of 3 independent experiments.
CaMKII directly binds, phosphorylates, and activates TAK1. (A,B) RAW264.7 cells were stimulated with 0.1 μg/mL LPS for the indicated time. Equal amount cell lysates were immunoprecipitated with CaMKII-α (A) or TAK1 (B) antibody and then immunoblotted (IB) with CaMKII-α and TAK1 antibody. (C) HEK293 cells were transfected with TAK1-HA and wt or mutant CaMKII-α–flag construct as indicated. After 24 hours, cell extracts were immunoprecipitated with anti-flag antibody and then immunoblotted with anti-HA and anti-flag antibody. C indicates catalytic domain (amino acids 1-260); R, regulatory domain (amino acids 261-309); A, association domain (amino acids 310-478). (D) GST pull-down assays were performed with recombinant GST-tagged CaMKII-α and cell extracts of RAW264.7 cells. (E) One microgram of recombinant TAK1 protein was incubated with recombinant active CaMKII-α at 30°C for 30 minutes. Samples were separated by SDS-PAGE followed by autoradiography. (F) MBP was added into the reaction mixture as in panel E followed by incubation for another 20 minutes. Samples were analyzed by immunoblotting with anti–phospho-MBP antibody. Data are representative of 3 independent experiments.
To investigate which region of CaMKII-α can interact with TAK1, various truncated or deleted mutants of CaMKII-α were constructed according to its known structural and functional domains,8,30 and then cotransfected into HEK293 cells with HA-tagged TAK1. Coimmunoprecipitation revealed that the N-terminal part of the regulatory domain (amino acids 260-300) of CaMKII-α was necessary for the interaction of CaMKII-α and TAK1 (Figure 5C).
To further confirm whether the N terminus of CaMKII-α can directly bind to TAK1, GST pull-down assay was performed by incubating GST-tagged CaMKII-α (amino acids 1-300) with lysates. Blotting with anti-TAK1 antibody displayed that TAK1 could be pulled down with GST-tagged CaMKII-α (Figure 5D).
Then, we tested whether CaMKII could phosphorylate TAK1. As shown in Figure 5E, recombinant active CaMKII-α directly phosphorylated recombinant TAK1 protein in vitro. To determine whether TAK1 can be activated by CaMKII-α, MBP was introduced into the reaction mixture of recombinant active CaMKII-α and TAK1 protein as a substrate of TAK1. Blotting with anti–phospho-MBP antibody showed that phosphorylation of MBP by TAK1 was enhanced in the presence of CaMKII-α (Figure 5F). Taken together, these results suggest that CaMKII-α directly binds and phosphorylates TAK1, which leads to the increased activation of TAK1.
CaMKII directly binds and phosphorylates IRF3-promoting IRF3-activated IFN-β expression
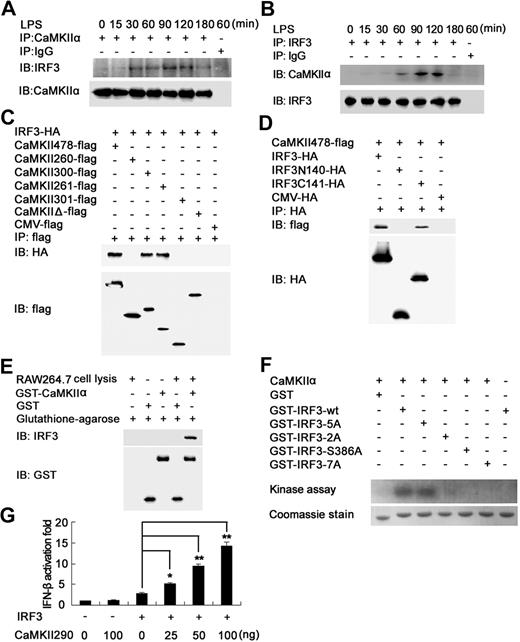
To elucidate the underlying mechanism by which CaMKII enhances TLR3- and TLR4-mediated IRF3 activation, we investigated whether endogenous CaMKII interacted with IRF3. Immunoprecipitation experiments with anti–CaMKII-α antibody showed that IRF3 was coprecipitated with CaMKII-α in LPS-stimulated macrophages (Figure 6A), which confirmed by reverse immunoprecipitation with anti-IRF3 antibody (Figure 6B), suggesting that endogenous CaMKII-α could associate with IRF3 on TLR4 activation. By using immunoprecipitation experiments, we tried to find out whether there exist other molecules of the TLR pathway, with which CaMKII could interact (Figure S6); however, we failed to find out any one except for TAK1 and IRF3.
CaMKII directly binds, phosphorylates IRF3, and enhances IRF3-activated IFN-β expression. (A,B) RAW264.7 cells were stimulated with 0.1 μg/mL LPS for the indicated time. Equal amount cell lysates were immunoprecipitated with CaMKII-α (A) or IRF3 (B) antibody and then detected with CaMKII-α and IRF3 antibody. (C) HEK293 cells were transfected with IRF3-HA and wt or mutant CaMKII-α–flag construct as described in Figure 5C. After 24 hours, cell extracts were immunoprecipitated with anti-flag antibody and then detected with anti-HA and anti-flag antibody. (D) CaMKII-α–flag construct together with HA-tagged IRF3, IRF3N140, or IRF3C141 plasmid were transfected into HEK293 cells. After 24 hours, IRF3 truncates were immunoprecipitated with HA-specific antibody. Precipitated proteins were detected by immunoblot. (E) GST pull-down assays were performed with GST-tagged CaMKII-α and RAW264.7 cell lysates. (F) wt or mutant GST-IRF3 380-427 were used as substrates of recombinant active CaMKII-α. The incorporation of 32P in the IRF3 380-427 was visualized by autoradiography after SDS-PAGE. Residues are as follows: 2A, S385A, S386A; 5A, S396A, S398A, S402A, T404A, S405A; 7A, S385A, S386A, S396A, S398A, S402A, T404A, S405A. Data are representative of 3 independent experiments. (G) HEK293 cells were transfected with 100 ng of IRF3-expressing plasmid, 50 ng of IFN-β luciferase reporter plasmid, 10 ng of pTK-Renilla luciferase, together with indicated amount of CaMKII290 plasmid. After 24 hours, luciferase activity was measured and normalized by Renilla luciferase activity. Data are shown as mean plus or minus SD (n = 5) of 1 typical experiment from 3 independent experiments with similar results. *P < .05; **P < .01.
CaMKII directly binds, phosphorylates IRF3, and enhances IRF3-activated IFN-β expression. (A,B) RAW264.7 cells were stimulated with 0.1 μg/mL LPS for the indicated time. Equal amount cell lysates were immunoprecipitated with CaMKII-α (A) or IRF3 (B) antibody and then detected with CaMKII-α and IRF3 antibody. (C) HEK293 cells were transfected with IRF3-HA and wt or mutant CaMKII-α–flag construct as described in Figure 5C. After 24 hours, cell extracts were immunoprecipitated with anti-flag antibody and then detected with anti-HA and anti-flag antibody. (D) CaMKII-α–flag construct together with HA-tagged IRF3, IRF3N140, or IRF3C141 plasmid were transfected into HEK293 cells. After 24 hours, IRF3 truncates were immunoprecipitated with HA-specific antibody. Precipitated proteins were detected by immunoblot. (E) GST pull-down assays were performed with GST-tagged CaMKII-α and RAW264.7 cell lysates. (F) wt or mutant GST-IRF3 380-427 were used as substrates of recombinant active CaMKII-α. The incorporation of 32P in the IRF3 380-427 was visualized by autoradiography after SDS-PAGE. Residues are as follows: 2A, S385A, S386A; 5A, S396A, S398A, S402A, T404A, S405A; 7A, S385A, S386A, S396A, S398A, S402A, T404A, S405A. Data are representative of 3 independent experiments. (G) HEK293 cells were transfected with 100 ng of IRF3-expressing plasmid, 50 ng of IFN-β luciferase reporter plasmid, 10 ng of pTK-Renilla luciferase, together with indicated amount of CaMKII290 plasmid. After 24 hours, luciferase activity was measured and normalized by Renilla luciferase activity. Data are shown as mean plus or minus SD (n = 5) of 1 typical experiment from 3 independent experiments with similar results. *P < .05; **P < .01.
To determine the domain of CaMKII-α responsible for CaMKII-α–IRF3 interaction, various flag-tagged truncated or deleted mutants of CaMKII-α, as described in Figure 5C, were cotransfected into HEK293cells with HA-tagged IRF3 for coimmunoprecipitation experiments. The N-terminal part of the regulatory domain (amino acids 260-300) of CaMKII-α was found to be necessary for its interaction with IRF3 (Figure 6C). In addition, GST pull-down assay using GST-tagged CaMKII-α (amino acids 1-300) revealed that the N-terminal of CaMKII-α could directly bind IRF3 (Figure 6E).
IRF3 is composed of N-terminal DNA-binding domain (amino acids 1-140) and C-terminal transactivation domain (amino acids 141-427).26 To map the CaMKII-binding domain in IRF3, coimmunoprecipitation assays were performed and showed that N-terminal truncated mutant of IRF3 (IRF3C141), but not the C-terminal truncated mutant of IRF3 (IRF3N140), could be coprecipitated with CaMKII-α, indicating that C-terminal of IRF3 was responsible for the binding of IRF3 to CaMKII-α (Figure 6D).
Two distinct groups of serine/threonine residues have been implicated in the activation of IRF3. Group 1 includes serines 385 and 386, whereas group 2 includes serines 396, 398, 402, and 405, and threonine 404.27,31 To determine whether CaMKII-α directly phosphorylates IRF3, we performed in vitro CaMKII kinase assay using wt and mutant GST-IRF3 380-427 as a substrate. As shown in Figure 6F, wt IRF3 was phosphorylated by recombinant active CaMKII-α, and replacement of group 2 residues with alanine (5A) led to no decrease in phosphorylation compared with wt IRF3. In contrast, no phosphorylation of IRF3 was observed when S386, group 1 residues (2A) or both group of residues (7A) were replaced by alanines. Thus, S386 of IRF3 is proposed as the primary target of phosphorylation by CaMKII-α.
Then we investigated whether CaMKII activation can enhance IRF3-activated IFN-β expression. Although overexpression of wt IRF3 alone just minimally induced the IFN-β reporter gene expression,32 CaMKII290 overexpression markedly increased IRF3-activated IFN-β reporter gene expression in a dose-dependent manner (Figure 6G), further demonstrating that CaMKII renders the full activation of IRF3 and enhances IRF3-dependent IFN-β expression by directly binding and phosphorylating IRF3.
Blockade of CaMKII activation attenuates endotoxin shock in lethal LPS-challenged mice
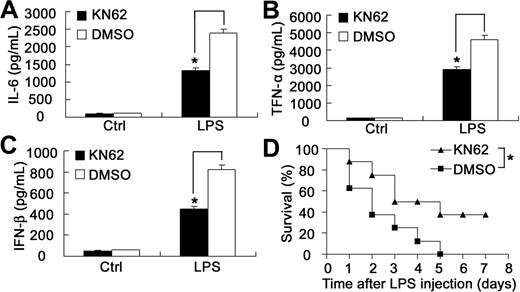
Endotoxin shock is a severe systemic inflammatory response characterized by a progressive release of inflammatory mediators predominantly from monocytes and macrophages mainly including IL-6 and TNF-α.33 IFN-α/β also plays important roles in endotoxin shock.34,35 Considering that blockade of CaMKII activation by KN62 could suppress LPS-induced production of proinflammatory cytokine and IFN-β in macrophages, we wondered whether KN62 could protect mice from endotoxin shock to lethal LPS challenge. As expected, KN62-pretreated mice produced significantly less IL-6, TNF-α, and IFN-β in vivo than control mice (Figure 7A-C). Consistently, all control mice died within 5 days after LPS in vivo administration; however, 37.5% of KN62-pretreated mice still survived (Figure 7D), suggesting that CaMKII blockade could protect mice from endotoxin shock to lethal LPS challenge.
Blockade of CaMKII activation by KN62 protects mice from endotoxin shock after lethal LPS challenge. (A-C) Sex- and age-matched mice were injected intraperitoneally with 25-mg/kg doses of KN62 (the working concentration of KN62 is 7.2 g/L) (n = 8) or equal volume of dimethyl sulfoxide (n = 8) 30 minutes before intraperitoneal administration with 10 mg/kg body weight of LPS. Serum samples were obtained at 1.5 hours after LPS injection. Serum IL-6 (A), TNF-α (B), and IFN-β (C) were quantified by ELISA. Data represent mean plus or minus SD. *P < .05. Three experiments were performed with similar results. (D) Survival curve of mice (n = 8 per group) treated as described in panels A to C. The survival of the LPS-challenged mice was monitored for 7 days. *P < .05. Similar results were obtained in 3 independent experiments.
Blockade of CaMKII activation by KN62 protects mice from endotoxin shock after lethal LPS challenge. (A-C) Sex- and age-matched mice were injected intraperitoneally with 25-mg/kg doses of KN62 (the working concentration of KN62 is 7.2 g/L) (n = 8) or equal volume of dimethyl sulfoxide (n = 8) 30 minutes before intraperitoneal administration with 10 mg/kg body weight of LPS. Serum samples were obtained at 1.5 hours after LPS injection. Serum IL-6 (A), TNF-α (B), and IFN-β (C) were quantified by ELISA. Data represent mean plus or minus SD. *P < .05. Three experiments were performed with similar results. (D) Survival curve of mice (n = 8 per group) treated as described in panels A to C. The survival of the LPS-challenged mice was monitored for 7 days. *P < .05. Similar results were obtained in 3 independent experiments.
Discussion
As an intracellular second messenger, Ca2+ can be triggered by many extracellular stimuli.7 Here we show that TLR4, 9, and 3 ligands can induce Ca2+ fluxes in macrophages, consistent with other observations that LPS can induce Ca2+ fluxes in murine macrophages,16,17 human monocytes.36 In addition, TLR2 ligand was reported to induce Ca2+ release in mouse macrophages and human airway epithelial cell lines.21 The accumulating evidence indicates that Ca2+ triggered by various TLRs may serve as a common and universal second messenger in TLR signaling. However, the source of the released Ca2+, extracellular space or intracellular stores, remains controversial, which may be attributed to the different cell types and the magnitude of stimulation used. Ca2+ fluxes was shown to play an important role in LPS-induced TNF-α production of murine macrophages.17,18 Our findings also demonstrate that LPS or poly(I:C)–induced TNF-α, IL-6, and IFN-β production was significantly decreased in BAPTA-AM–treated macrophages (data not shown). So intracellular release of Ca2+ is required for TLR-activated inflammatory cytokine response in macrophages. CaMKII was also reported to be involved in the enhanced TNF-α or IL-12p40 production in LPS-stimulated human monocytic cells, whereas the underlying mechanisms are not fully understood.19,36 On the basis of this experimental clue, we further found that downstream the major effector of Ca2+ flux, CaMKII-α, becomes active in TLR signaling and showed that the interaction of activated CaMKII-α with TAK1 and IRF3 promotes both MyD88- and TRIF-triggered efficient production of proinflammatory cytokine and type I IFN in macrophages.
Many proteins have been shown to be the substrates of CaMKII, and such proteins can be divided into 2 types: one group with an RXXS/T motif and the other group with no definitive motif except a preference of negative-charged residue at the +2 position.12 We for the first time, have identified TAK1 and IRF3 as new substrates of CaMKII-α. We determined S386 of IRF3 as the targeted phosphorylation site of CaMKII-α. The IRF3 sequence surrounding S386, GASSLEN, is consistent with the second type of motif, which confirms our results, whereas the targeted site(s) of CaMKII-α in TAK1 remains unclear. The sequence surrounding the known phosphorylation sites of TAK1, Thr184, Thr187, and Ser19237 is not consistent with the recognition motif of CaMKII, which implies that other target(s) of phosphorylation by CaMKII may exist.
TBK1 can directly phosphorylate IRF3 and the group 2 residues (serines 396, 398, 402, and 405 and threonine 404) are the primary targets of the phosphorylation.31 However, it has been reported that the phosphorylation of IRF3 by TBK1 was not sufficient to impart transcriptional activity to IRF3, although it was sufficient for IRF3 dimerization and nuclear translocation.5 Full activation of IRF3 required further phosphorylation by the PI3K pathway, presumably of other specific Ser/Thr residue(s).5 Our data also support this model of 2-step phosphorylation of IRF3: after TLR3 or TLR4 ligation, TBK1 phosphorylates IRF3 on S396 and other group 2 residues, leading to IRF3 dimerization and nuclear translocation; then CaMKII-α directly binds and further phosphorylates IRF3 on S386 other than group 2 residues, leading to the enhanced phosphorylation (S396), nuclear translocation, transcriptional activity of IRF3, and the increased IRF3-activated IFN-β expression. So we can conclude that CaMKII, similar to PI3K, is required for the full activation of IRF3.
Dysregulated Ca2+ responses have been shown to be associated with the pathophysiologic processes in inflammation and autoimmune diseases. During sepsis, elevated cytosolic calcium has been demonstrated to be an early event, which contributes to increased cellular injury in veins and multiple organs.38 Calcium antagonists can improve survival in the endotoxin model,39 which is consistent with our observation that in vivo blockade of CaMKII activation by CaMKII inhibitor KN62 can improve the survival of lethal LPS-challenged mice, suggesting that the outcomes of elevated Ca2+ are primarily mediated by the subsequent activation of CaMKII. The significantly decreased production of proinflammatory cytokines and IFN-β by KN62 may be the predominant reason for the increased survival in the mice. Increased Ca2+ responses may be involved in the breakdown of B-cell tolerance and autoimmunity in systemic lupus erythematosus.40,41 In addition, the disturbance of Ca2+ signaling is related to the generation of autoreactive T cells, which play important roles in the development of rheumatoid arthritis, multiple sclerosis, and type I diabetes.40,42 CaMKII, as a major and important downstream effector of Ca2+, may be also involved in the pathophysiologic processes of these autoimmune diseases. Considering that TLR activation by endogenous ligands has an important role in the development of autoimmune disease,43 whether the enhanced TLR activation by CaMKII is another mechanism for the pathogenesis of autoimmune diseases needs further investigation. So, the involvement of dysregulated Ca2+/CaMKII signaling in the pathogenesis of inflammatory and autoimmune disorders suggests that interference with Ca2+/CaMKII signaling may be a useful approach to treat inflammatory disorders and autoimmune diseases.
In conclusion, our study provides insight into the roles of Ca2+ and CaMKII in TLR signaling. CaMKII, which is activated by Ca2+ fluxes triggered by TLR ligands, can directly bind and activate TAK1 and IRF3, then in turn promote the activation of MyD88- and TRIF-dependent pathways leading to the enhanced production of proinflammatory cytokines and type I IFN in macrophages. Therefore, cross-talk with the Ca2+/CaMKII pathway is needed for full activation of both MyD88- and TRIF-dependent TLR signaling in macrophages.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kunihiro Matsumoto for providing HA-tagged TAK1 plasmid, Dr Takashi Fujita for kindly providing IRF3 reporter plasmid, Yanan Wu, Qian Zhang, Mei Jin, and Yan Li for their technical assistance, and Dr Huazhang An and Dr Taoyong Chen for their helpful discussion.
This work was supported by the National Natural Science Foundation of China (grants 30490240, 30721091) and the National Key Basic Research Program of China (grant 2007CB512403).
Authorship
Contribution: X.C. and X.L. designed research; X.L., M.Y., C.W., N.L., and Y.Z. performed experiments; and X.L. and X.C. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xuetao Cao, Institute of Immunology and National Key Laboratory of Medical Immunology, Second Military Medical University, 800 Xiangyin Road, Shanghai 200433, China; e-mail: caoxt@public3.sta.net.cn.