Abstract
The C-type lectin receptor dectin-1 functions as a pattern recognition receptor for β-glucans and signals via Syk kinase but independently of the Toll-like receptor (TLR) pathway to regulate expression of innate response genes. Dectin-1 signaling can promote activation of dendritic cells (DCs), rendering them competent to prime Th1 and Th17 responses. Here we show that dectin-1–activated DCs can also prime cytotoxic T-lymphocyte (CTL) responses. DCs exposed to a dectin-1 agonist induced antigen-specific expansion of TCR transgenic CD8+ T cells and their differentiation into CTLs in vitro. Dectin-1 agonist also acted as an adjuvant for CTL crosspriming in vivo, eliciting potent CTL responses that protected mice from tumor challenge. In vitro but not in vivo, CTL crosspriming was dependent on IL-12 p70, which was produced by dectin-1–activated DCs in response to IFN-γ secreted by newly activated CD8+ T cells. The dectin-1/Syk pathway is thus able to couple innate immune recognition of β-glucans to all branches of the adaptive immune system, including CD4+ T-helper cells, B cells, and CD8+ cytotoxic T cells. These data highlight the ability of non-TLR receptors to bridge innate and adaptive immunity and suggest that dectin-1 agonists may constitute useful adjuvants for immunotherapy and vaccination.
Introduction
Primary immune responses to protein antigens are usually weak in the absence of adjuvant.1 Adjuvants act by enhancing the proliferation of newly activated T cells, by supporting their survival and by promoting their differentiation into effector and memory T cells. These properties of adjuvants are largely due to their effects on antigen presenting cells (APCs), in particular dendritic cells (DCs)1 Many adjuvants act by mimicking the presence of an infectious agent, which triggers pattern recognition receptors (PRRs) expressed on DCs and other cell types.1 The most renowned PRRs are the toll-like receptors (TLRs), transmembrane proteins that bind various microbe-derived “patterns” in the extracellular space or within endosomal compartments. Upon binding to their agonist ligands, TLRs signal via the adaptors MyD88 and/or TRIF and downstream factors such as TRAF6 and TRAF3, ultimately leading to the activation of transcription factors of the NF-κB, IRF, and AP-1 families.2 The concerted action of these transcription factors results in the expression of a panoply of costimulatory molecules and cytokines that, together, endow DCs with the capacity to prime T-cell responses.3,4 For example, TLR signaling in DCs causes the up-regulation of CD40, CD80, and CD86, as well as induction of IL-12 p70 and IFN-α/β, all of which act to drive Th1 and CD8+ T-cell responses.
Besides TLRs, other PRRs have been demonstrated to activate DCs and thereby link innate to adaptive immunity. These include the RIG-I–like and the NOD receptors, all of which are found in the cytoplasm where they sense viral nucleic acids or bacterial peptidoglycans.5 Recently, we have identified dectin-1 as an additional PRR that has the potential to activate DCs.6 Unlike the cytosolic PRRs but much like the TLRs, dectin-1 localizes to the plasma membrane and to endosomes, effectively surveying extracellular compartments. Dectin-1 is a C-type lectin that binds to β-1,3-glucans found on the cell walls of fungi and some bacteria.7 Dectin-1 engagement by agonistic β-glucans promotes signaling via the kinase Syk leading to production of reactive oxygen species and activation of MAP kinases, NF-κB, and NFAT.6,8-11 DCs activated selectively via the dectin-1/Syk pathway acquire effector functions similar to DCs stimulated by TLR agonists. They up-regulate costimulatory molecules, produce TNF-α, IL-6, IL-2, IL-10, and IL-23, and are able to prime CD4+ T cells and instruct their differentiation into IFN-γ and IL-17–producing T-helper cells.6,12 Adjuvants that stimulate the dectin-1 pathway also promote antibody responses in vivo.6 The dectin-1/Syk pathway is thus a novel TLR-independent pattern recognition pathway that couples pathogen sensing to CD4+ T-cell and B-cell responses.
Here we show that DCs activated by the selective dectin-1 agonist, curdlan, are also very efficient at priming cytotoxic T-cell responses. Curdlan-stimulated DCs promote the expansion and differentiation of cytotoxic T lymphocyte (CTL) precursors in vitro. This process is dependent on IL-12 p70, a cytokine found to be produced by curdlan-stimulated DCs in response to IFN-γ secreted from newly activated CD8+ T cells. Curdlan also acts as a potent adjuvant for CTL crosspriming in vivo, eliciting cytotoxic responses that can protect mice against tumor challenge. The dectin-1/Syk pathway is therefore able to activate all arms of the adaptive immune response, from CD4+ T cells and B cells to CD8+ T cells.
Methods
Mice
C57BL/6 mice were obtained from Charles River (Margate, United Kingdom). MyD88-deficient and TRIF-deficient mice were a gift from S. Akira (Osaka, Japan) and mice lacking both MyD88 and TRIF were generated by intercrossing the 2 strains. OT-I TCR transgenic mice on a rag1−/− background and B6.SJL mice were originally obtained from D. Kioussis (London, United Kingdom) and F. Powrie (Oxford, United Kingdom), respectively. IL-23 p19–deficient mice13 were provided by A. MacDonald (Edinburgh, United Kingdom) with kind permission from N. Ghilardi (Genentech, South San Francisco, CA). All mice were bred at Cancer Research UK in specific pathogen–free conditions. Dectin-1–deficient mice14 were bred at the University of Cape Town, South Africa. IL-12 p40–deficient and IL-12 p35–deficient mice were a kind gift from Annette Oxenius (Zürich, Switzerland) and were bred at the Swiss Federal Institute of Technology (ETH, Zurich, Switzerland). Radiation chimeras using fetal liver from syk−/− mice15 were generated as described.10 All animal experiments were performed in accordance with national and institutional guidelines for animal care.
Reagents and cell lines
Culture medium was RPMI 1640 (Invitrogen, Paisley, United Kingdom) supplemented with glutamine, penicillin, streptomycin, 2-mercaptoethanol, HEPES, nonessential amino acids, sodium pyruvate (all from Invitrogen), and 10% heat-inactivated fetal calf serum (Autogen, Bioclear, United Kingdom). Curdlan was obtained from Wako Chemicals (Neuss, Germany) and suspended in phosphate-buffered saline (PBS) at 10 mg/mL. Fluorescein-curdlan (CarboMer, San Diego, CA) was washed 5 times in PBS and suspended in PBS at 50 mg/mL. CpG oligonucleotide 1668 (CpG) was synthesized by Sigma-Aldrich (Poole, United Kingdom). PolyI:C was from GE Healthcare (Little Chalfont, United Kingdom). SIINFEKL peptide was synthesized and purified by high-performance liquid chromatography at Cancer Research UK. Ovalbumin protein (OVA) was from Merck (Nottingham, United Kingdom). Granulocyte macrophage–colony-stimulating factor (GM-CSF) was made by Cancer Research UK protein purification service and batches were titrated to give optimal growth conditions for bone marrow–derived DCs. Flt3L was purchased from R&D Systems (Abingdon, United Kingdom). Antibodies used for flow cytometric analysis experiments were from BD Pharmingen (Oxford, United Kingdom). Biotinylated 2A11 antibody16 followed by streptavidin-PE was used to stain for dectin-1. H-2Kb-SIINFEKL tetramers were purchased from Beckman Coulter (Wycombe, United Kingdom). Neutralizing antibodies against IL-12 p40 and IFN-γ as well as rat IgG1 and rat IgG2a isotype controls were from BD Pharmingen. Neutralizing antibody to IL-23 p19 was from eBioscience (San Diego, CA). Recombinant IFN-γ was from BioSource (Invitrogen). EL4 cells were obtained from CRUK Cell Culture Services (London, United Kingdom) and maintained in complete culture medium.
BMDC cultures
BMDCs were generated using GM-CSF as described17 and purified from bulk cultures with anti-CD11c microbeads before use (Miltenyi Biotec, Surrey, United Kingdom). BMDC purity was checked by FACS and was routinely more than 98% CD11c+ cells (data not shown). Alternatively, bone marrow cells from C57Bl/6 mice were cultured in the presence of 75 ng/mL Flt3L for 10 days to generate mixed populations of conventional and plasmacytoid DCs.18
Flow cytometry
Cell suspensions were stained in ice-cold PBS supplemented with 2 mM EDTA, 1% FCS, and 0.02% sodium azide. For intracellular cytokine staining, cells were stained with anti-CD8, fixed with Fix and Perm Reagent A (Invitrogen), and then resuspended in Fix and Perm Reagent B (Invitrogen) containing fluorochrome-labeled antibodies. Data were acquired on a FACSCalibur (BD Biosciences, Oxford, United Kingdom) and analyzed using FlowJo software (TreeStar, Ashland, OR).
In vitro T-cell differentiation
CD8+CD11c−CD44− T cells were sorted from spleen and lymph nodes of OT-I mice using a MoFlo (Dako UK, Ely, United Kingdom) or a FACSAria cell sorter (BD Biosciences). In some experiments, sorted OT-I T cells were labeled with 2 μM CFSE for 12 minutes at 37°C. Sorted OT-I T cells (5 × 104) were cocultured with 104 BMDCs and 10 pM to 100 pM SIINFEKL, as indicated, in the presence or absence of 50 μg/mL curdlan or 0.5 μg/mL CpG. In some experiments, neutralizing antibodies (10 μg/mL-20 μg/mL) were added. Cultures were restimulated on day 3 with 20 000 SIINFEKL-pulsed EL4 cells. IFN-γ in the supernatant was measured by enzyme-linked immunosorbent assay (ELISA) after 6 to 8 hours. Alternatively, brefeldin A (5 μg/mL) was added during the last 4 hours of restimulation and IFN-γ production was analyzed by intracellular staining.
For determination of IL-12 p70 production by DC–T-cell cocultures, C57BL/6 BMDCs (105 per well) and OT-I (3 × 105 per well) were cultured in 96-well U-bottom plates in the presence of GM-CSF. SIINFEKL peptide (100 pM), curdlan (100 μg/mL), CpG (0.5 μg/mL), IFN-γ (50 ng/mL), or IFN-γ neutralizing or control antibodies (10 μg/mL) were added in various combinations and IL-12 p70 levels in the supernatant were determined by ELISA after 24 hours.
Immunizations
Mice were immunized in both hind footpads with 50 μg OVA plus 200 μg curdlan or 10 μg poly I:C. In vivo CTL assays were performed 5 to 6 days later, as described.19 Briefly, splenocytes from B6.SJL mice (CD45.1+) were incubated with 20 nM, 200 nM, or 0 nM SIINFEKL peptide for 30 minutes and 0.1 μM, 0.5 μM, or 2.5 μM, respectively, of CFSE for an additional 10 minutes at 37°C. Labeled splenocytes were then injected intravenously (107/mouse) and the following day mice were killed, splenocytes were prepared, and the CD45.1+ population was analyzed for the presence of CFSE+ cells. Specific killing was calculated using the formula 100 × (1 −%CFSE peptide / %CFSE no peptide) as described.19 Splenocytes were also labeled with SIINFEKL-H2Kb tetramers, anti-CD8, and anti-Thy1.2 and analyzed for the percentage of tetramer+ cells among the Thy1.2+ CD8+ T-cell population.
For in vitro restimulation of CTLs, 106 splenocytes were cultured in the presence or absence of 1 μM SIINFEKL. IFN-γ in the supernatants was measured by ELISA after 2 days. In vitro killing assays were performed after 5 days of restimulation using a modification of a previously described protocol.20 Briefly, EL4 cells were incubated with 0.1 μM CFSE and 2 μM SIINFEKL or with 1 μM CFSE and no SIINFEKL, washed and used as targets. Targets (104) were mixed with different dilutions of effectors (100%, 30%, or 10% of restimulated splenocytes) and plated in a 96-well U-bottom plate. After incubation for 5 hours at 37°C, TO-PRO-3 was added to exclude dead cells and samples were analyzed by flow cytometry. The mean percentage survival in antigen-loaded targets was calculated relative to antigen-negative internal controls in each sample. Variation in the different target populations was assessed in wells without effectors and all data were adjusted accordingly using the formula: adjusted % survival = 100 × (% survival / % survival in absence of effectors). Finally, percentage of specific lysis was calculated using the equation: % specific lysis = 100 − adjusted % survival.
Tumor model
Naive C57BL/6 mice were injected in both hind footpads with 50 μg OVA plus 200 μg curdlan or 10 μg polyI:C. OVA-expressing B16 melanoma cells21 (5 × 105/mouse) were given intravenously 5 to 7 days later, and mice were killed 18 to 22 days after tumor challenge. Tumor burden was assessed by counting lung foci, and CTL responses were monitored as described in “Immunizations.”
Statistical methods
Statistical significance was determined by a 2-tailed unpaired t test using GraphPad Prism (GraphPad Software, La Jolla, CA).
Results
Curdlan induces CTL priming in vitro
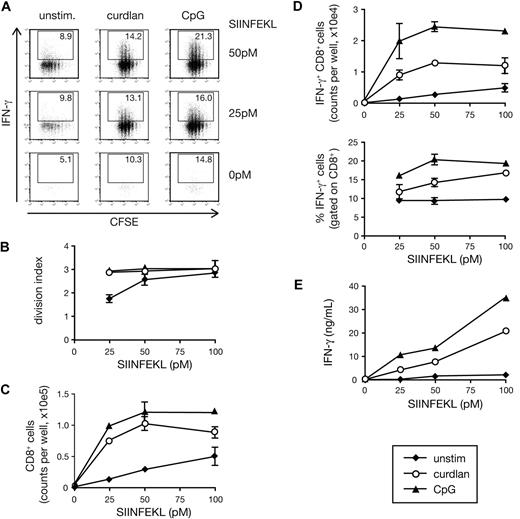
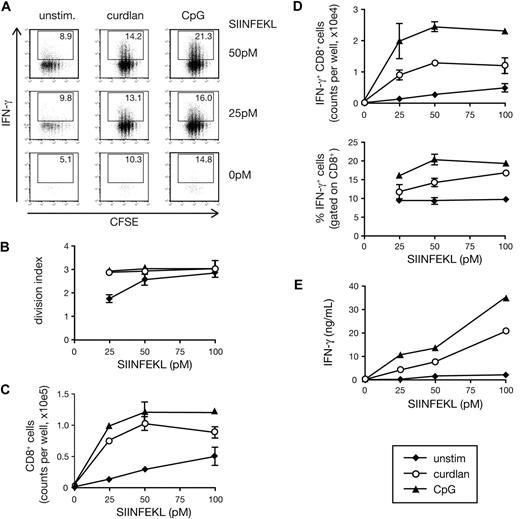
To evaluate the potential of dectin-1–activated DCs to prime CD8+ T cells, we cultured ovalbumin (OVA)–specific naive OT-I CD8+ T cells with BMDCs. Addition of OVA peptide (SIINFEKL) to the cultures was sufficient to induce OT-I T cell proliferation as assessed by CFSE dilution (Figure 1A,B). However, further addition of the dectin-1 agonist, curdlan, increased the T-cell division index (average number of divisions undergone by a cell present in the starting population) and allowed greater recovery of viable CD8+ T cells at the end of the culture period (Figure 1A-C). In addition, the presence of curdlan markedly increased OT-I differentiation into effector cells as measured by the ability to secrete IFN-γ upon restimulation (Figure 1A, D,E) and to become cytotoxic (data not shown). For all tested parameters, the effect of curdlan was weaker but comparable to that of a CpG-containing DNA oligonucleotide, a TLR9 agonist often used as an adjuvant for CTL priming22 (Figure 1A-E). Both CpG and curdlan acted in a dose-dependent manner when tested against a fixed dose of OVA peptide and also acted as adjuvants for crosspriming when using OVA protein as the antigen (data not shown).
Curdlan-stimulated DCs prime CTLs in vitro. (A) CFSE-labeled OT-I T cells were cultured together with C57Bl/6 BMDCs in the presence of the indicated concentrations of SIINFEKL peptide and either curdlan, CpG, or no innate stimulus (unstim). Graphs show CFSE dilution versus intracellular IFN-γ on day 3 of culture. Numbers indicate percent of IFN-γ+ cells. (B) The extent of proliferation of the T cells from experiment shown in panel A calculated as division index. (C) CD8+ T-cell counts per well from the experiment shown in panel A. (D) IFN-γ+CD8+ T-cell counts per well (top) and percentage of IFN-γ+ cells (bottom) from the experiment shown in panel A. (E) Samples from the experiment shown in panel A were restimulated on day 3 with SIINFEKL-pulsed targets for 8 hours and IFN-γ levels in the supernatant were determined by ELISA. Data in panels B through E are mean plus or minus standard deviation (SD) of duplicate wells.
Curdlan-stimulated DCs prime CTLs in vitro. (A) CFSE-labeled OT-I T cells were cultured together with C57Bl/6 BMDCs in the presence of the indicated concentrations of SIINFEKL peptide and either curdlan, CpG, or no innate stimulus (unstim). Graphs show CFSE dilution versus intracellular IFN-γ on day 3 of culture. Numbers indicate percent of IFN-γ+ cells. (B) The extent of proliferation of the T cells from experiment shown in panel A calculated as division index. (C) CD8+ T-cell counts per well from the experiment shown in panel A. (D) IFN-γ+CD8+ T-cell counts per well (top) and percentage of IFN-γ+ cells (bottom) from the experiment shown in panel A. (E) Samples from the experiment shown in panel A were restimulated on day 3 with SIINFEKL-pulsed targets for 8 hours and IFN-γ levels in the supernatant were determined by ELISA. Data in panels B through E are mean plus or minus standard deviation (SD) of duplicate wells.
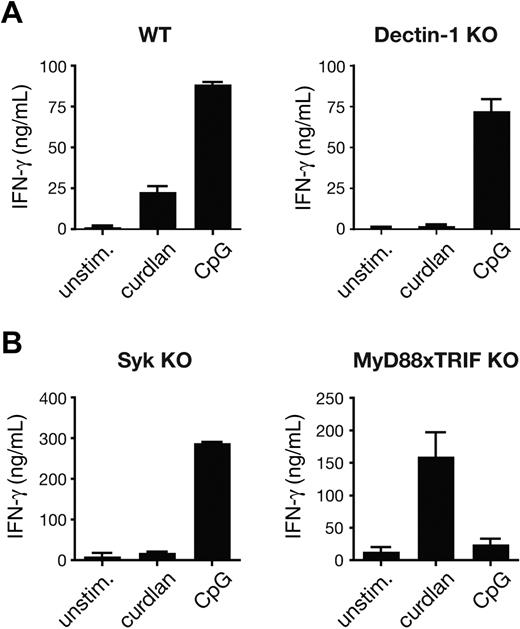
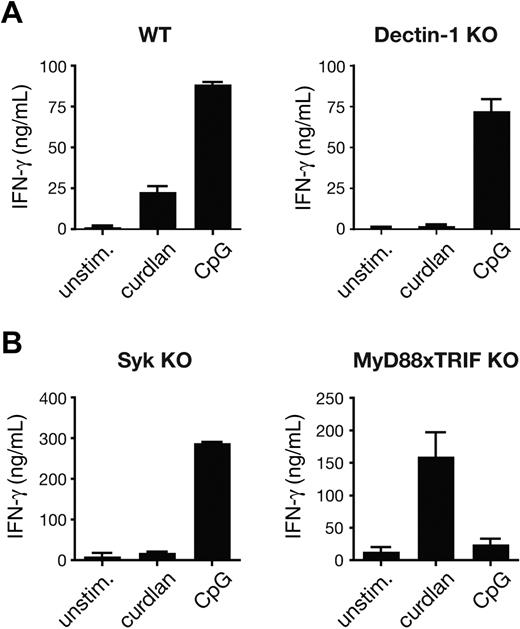
The adjuvant effect of curdlan was mediated by the DCs and not the T cells because it was abrogated by the use of BMDCs lacking dectin-1 (Figure 2A). Furthermore, it was dependent on signaling downstream of dectin-1, as demonstrated by the absence of OT-I priming in cultures with Syk-deficient BMDCs (Figure 2B). In contrast, MyD88 or TRIF was not required, confirming that the effects of curdlan are independent of TLR signaling (Figure 2B). The opposite was true for the CpG-mediated adjuvant effect, which was dectin-1– and Syk-independent and TLR signaling–dependent (Figure 2A,B). In summary, activation of the dectin-1/Syk pathway rendered BMDCs fully capable of supporting expansion of naive CD8+ T cells and instructing their differentiation into effector CTLs in vitro.
Curdlan-induced CTL priming by DC is dependent on the dectin-1 pathway. (A) Cocultures of OT-I T cells and either C57BL/6 (WT) or dectin-1–deficient BMDCs in the presence of 25 pM SIINFEKL peptide and no stimulus, curdlan, or CpG. Cultures were restimulated on day 3 with SIINFEKL-pulsed target T cells and IFN-γ levels in the supernatant were determined by ELISA. (B) As in panel A, but using Syk-deficient or MyD88 × TRIF doubly deficient BMDCs and 30 pM SIINFEKL peptide. Data in panels A and B are mean plus SD of duplicate wells.
Curdlan-induced CTL priming by DC is dependent on the dectin-1 pathway. (A) Cocultures of OT-I T cells and either C57BL/6 (WT) or dectin-1–deficient BMDCs in the presence of 25 pM SIINFEKL peptide and no stimulus, curdlan, or CpG. Cultures were restimulated on day 3 with SIINFEKL-pulsed target T cells and IFN-γ levels in the supernatant were determined by ELISA. (B) As in panel A, but using Syk-deficient or MyD88 × TRIF doubly deficient BMDCs and 30 pM SIINFEKL peptide. Data in panels A and B are mean plus SD of duplicate wells.
Curdlan-induced CTL priming is dependent on IL-12 p70
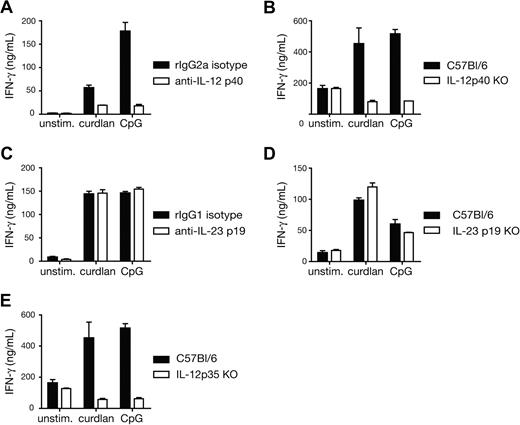
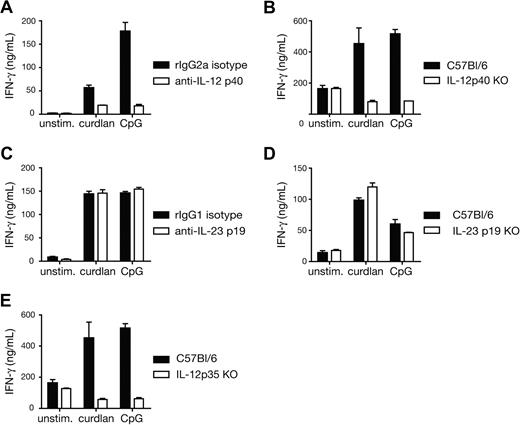
We next investigated the mechanism by which curdlan-stimulated BMDCs instructed the differentiation of CD8+ T cells into effectors. CD70 expression by DCs has been shown to enhance CTL priming in some instances, by triggering CD27 on T cells.23-25 We found that CD70 expression on DCs was up-regulated in response to curdlan stimulation (data not shown). However, blocking CD70-CD27 interactions with antibodies did not affect CTL priming in OT-I/BMDC/curdlan cultures (data not shown). Type I IFNs and IL-12 have also been shown to drive CD8+ T-cell effector differentiation in many cases.26-29 We have not detected reproducible induction of IFN-β or IFN-α upon curdlan stimulation of BMDCs (S.L.-L., M.J. Robinson, and C.R.S., unpublished results, July 2007) and, accordingly, CTL differentiation in OT-I and BMDC cocultures with curdlan was not affected by IFN-α/β neutralization (data not shown). In contrast, neutralizing antibodies against IL-12 p40 or the use of IL-12 p40–deficient BMDCs abolished CTL priming driven by curdlan (Figure 3A,B). IL-12 p40 is a common subunit for both IL-12 p70 and IL-23, and we have previously found that curdlan-stimulated BMDCs produce IL-23 but barely detectable IL-12 p70.6 Surprisingly, elimination of IL-23, either by blocking antibodies specific for the p19 subunit or by using p19-deficient BMDCs had no effect on the adjuvanticity of curdlan for CTL priming (Figure 3C,D). In contrast, priming was strongly reduced in cocultures with IL-12 p35–deficient BMDCs (Figure 3E). This indicates that IL-12 p70 is the major player in the priming of CTL responses by curdlan-stimulated BMDCs.
Curdlan-induced CTL priming by DCs is dependent on IL-12 p70. (A) Cocultures of OT-I T cells and C57BL/6 BMDCs in the presence of 30 pM SIINFEKL peptide and the indicated stimuli and either anti–IL-12 p40 blocking antibody or isotype-matched control antibody. (B) As in panel A but comparing C57BL/6 with IL-12 p40–deficient BMDCs in the absence of blocking antibodies. (C) As in panel A but with anti–IL-23 p19 blocking antibody or isotype-matched control antibody. (D) As in panel B, with either C57BL/6 or IL-23 p19–deficient BMDCs. (E) As in panel B, with either C57BL/6 or IL-12 p35–deficient BMDCs. IFN-γ levels were measured in day 3 culture supernatants (B-E) or after restimulation of total day 3 cultures with SIINFEKL-loaded targets (A). Similar results were obtained with and without restimulation (not shown). Data in all panels are mean plus SD of duplicate or triplicate wells.
Curdlan-induced CTL priming by DCs is dependent on IL-12 p70. (A) Cocultures of OT-I T cells and C57BL/6 BMDCs in the presence of 30 pM SIINFEKL peptide and the indicated stimuli and either anti–IL-12 p40 blocking antibody or isotype-matched control antibody. (B) As in panel A but comparing C57BL/6 with IL-12 p40–deficient BMDCs in the absence of blocking antibodies. (C) As in panel A but with anti–IL-23 p19 blocking antibody or isotype-matched control antibody. (D) As in panel B, with either C57BL/6 or IL-23 p19–deficient BMDCs. (E) As in panel B, with either C57BL/6 or IL-12 p35–deficient BMDCs. IFN-γ levels were measured in day 3 culture supernatants (B-E) or after restimulation of total day 3 cultures with SIINFEKL-loaded targets (A). Similar results were obtained with and without restimulation (not shown). Data in all panels are mean plus SD of duplicate or triplicate wells.
T-cell–derived IFN-γ promotes IL-12 p70 production from curdlan-stimulated DCs
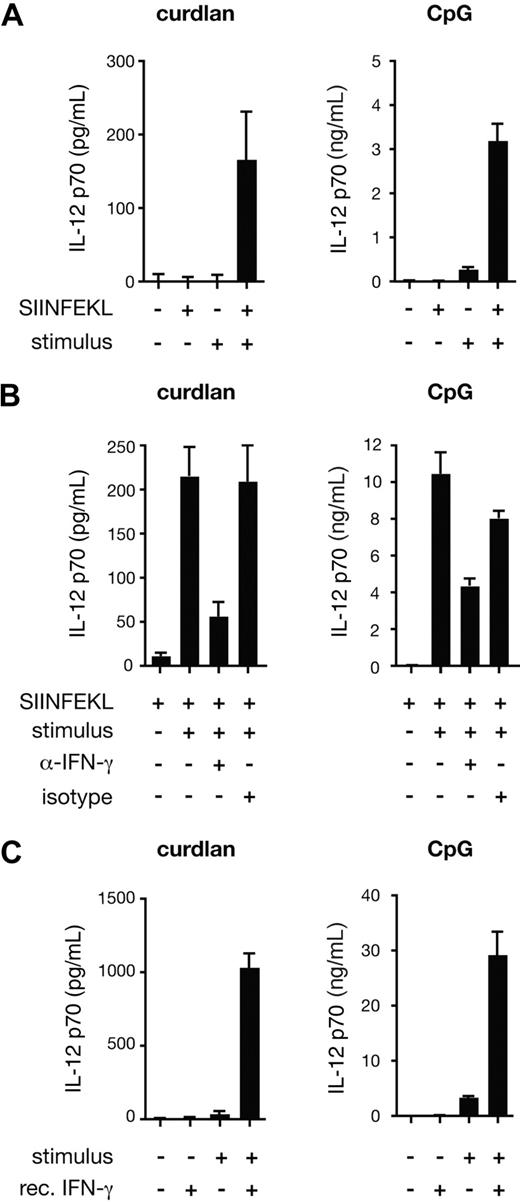
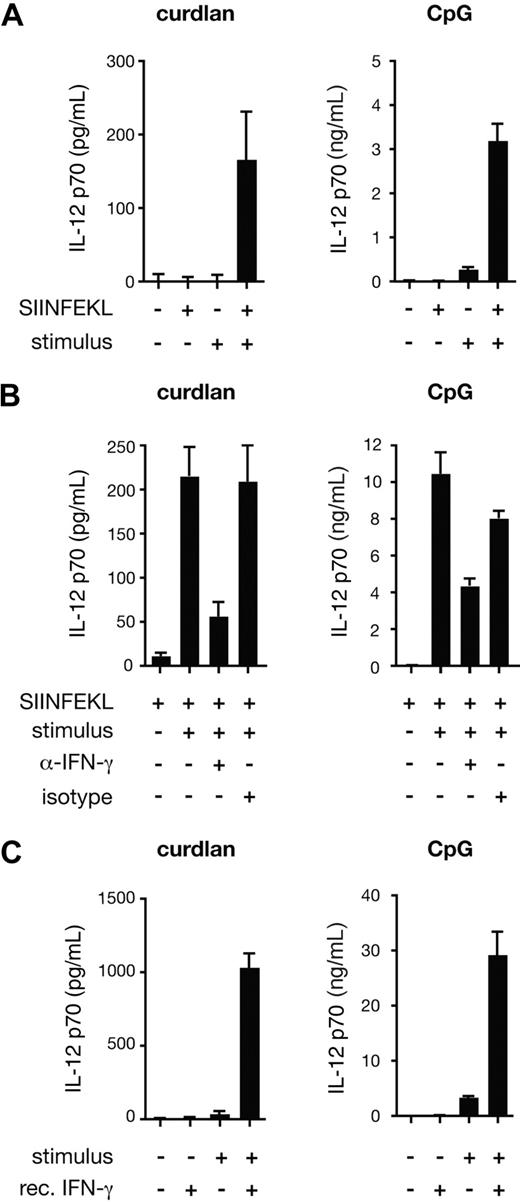
To reconcile the role of IL-12 p70 in curdlan-induced CTL priming with the fact that DCs produce only marginal amounts of the cytokine when stimulated through the dectin-1/Syk pathway,6 we compared IL-12 p70 production by BMDCs stimulated with curdlan in the presence or absence of CD8+ T cells. Confirming our earlier data,6 curdlan stimulation of BMDCs did not result in detectable IL-12 p70 production even when OT-I T cells were added to the culture provided no antigen was present (Figure 4A). However, when SIINFEKL peptide was included, IL-12 p70 was detected in curdlan-stimulated cultures but not in cultures lacking the innate stimulus (Figure 4A). A marked antigen- and OT-I–dependent increase in IL-12 p70 levels was also seen in cultures with CpG despite the fact that the latter stimulus by itself elicits substantial levels of the cytokine (Figure 4A; note difference in scale). The effect of the OT-I T cells was at least in part mediated by IFN-γ because IL-12 p70 production could be largely prevented by the addition of IFN-γ– specific neutralizing antibodies, but not by isotype-matched control antibodies (Figure 4B). Furthermore, recombinant IFN-γ could substitute for OT-I T cells plus antigen in eliciting IL-12 p70 production by curdlan-stimulated BMDCs (Figure 4C). We conclude that signaling through the dectin-1 pathway in BMDCs can lead to production of IL-12 p70 provided the cells are also exposed to IFN-γ, which can be produced by newly activated CD8+ T cells.
Curdlan-stimulated DCs produce IL-12 p70 in an IFN-γ–dependent manner. (A) C57BL/6 BMDCs and OT-I were cocultured in the presence or absence of SIINFEKL peptide, curdlan (left) or CpG (right) as indicated. (B) As in panel A, but with the addition of anti–IFN-γ blocking antibody or rat IgG1 isotype-matched control antibody. (C) C57BL/6 BMDCs were stimulated with curdlan or CpG and recombinant IFN-γ as indicated. Data in all panels are IL-12 p70 levels in the supernatant determined after 24 hours (mean plus or minus SD of triplicate wells).
Curdlan-stimulated DCs produce IL-12 p70 in an IFN-γ–dependent manner. (A) C57BL/6 BMDCs and OT-I were cocultured in the presence or absence of SIINFEKL peptide, curdlan (left) or CpG (right) as indicated. (B) As in panel A, but with the addition of anti–IFN-γ blocking antibody or rat IgG1 isotype-matched control antibody. (C) C57BL/6 BMDCs were stimulated with curdlan or CpG and recombinant IFN-γ as indicated. Data in all panels are IL-12 p70 levels in the supernatant determined after 24 hours (mean plus or minus SD of triplicate wells).
All conventional DC subsets respond to curdlan in vivo
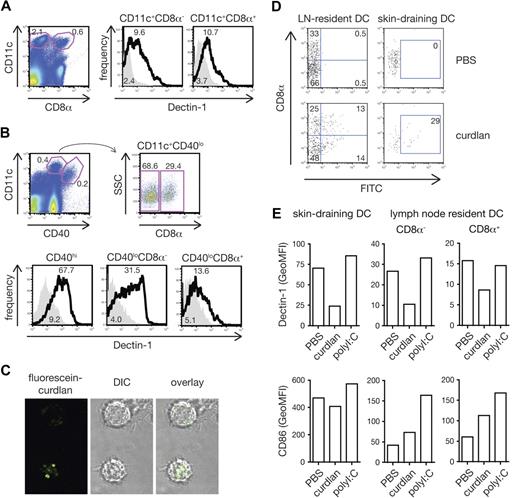
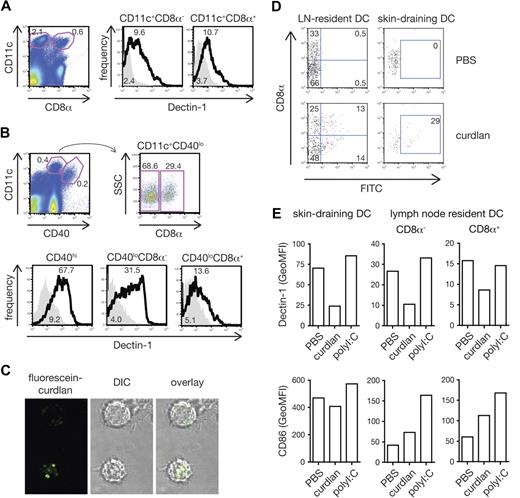
To assess whether dectin-1 agonists might also activate DCs to prime CTL in vivo, we first reexamined the expression of dectin-1 on DC subsets, which has been the subject of controversy.10,30-32 We found that dectin-1 was expressed uniformly and at similar levels on blood-derived resident CD8α+ and CD8α− DC subsets in both spleen and lymph nodes (Figure 5A,B). This is consistent with data previously reported by us and others10,30,32 but in contrast to one publication claiming dectin-1 expression only on CD8α− DCs.31 In addition, dectin-1 was expressed on skin-derived migratory DCs in lymph nodes draining the skin31,33 (Figure 5A,B). Like BMDCs, conventional DCs from spleen or from bone marrow cultures with Flt3L responded to curdlan stimulation with cytokine production (Figure S1A [available on the Blood website; see the Supplemental Materials link at the top of the online article] and data not shown). In contrast, we did not consistently observe cytokine production by spleen or Flt3L-induced plasmacytoid DCs (pDCs) in response to stimulation with curdlan (Figure S1A and data not shown), possibly due to the fact that these cells express lower levels of dectin-1 compared with conventional DCs (Figure S1B). Therefore, all conventional DC subtypes, including resident and migratory DCs, but probably not pDCs, can potentially be directly activated by dectin-1 agonists in vivo.
All conventional DC subsets respond to curdlan in vivo. (A) Expression of dectin-1 on spleen CD11c+CD8α− and CD11c+CD8α+ cells. Splenocytes were stained with anti–dectin-1 (thick line) or rat IgG2b (solid gray). DC subtypes were defined by gating as shown on the dot plot (left panel). Numbers indicate percentages of events in gate (dot plot) or mean fluorescence intensity (GeoMFI; histograms). (B) Expression of dectin-1 on lymph node DC subsets. Cell suspensions from peripheral lymph nodes were stained as in panel A. Blood-derived resident CD11c+CD40loCD8α− or CD11c+CD40loCD8α+ cells and skin-derived migratory CD11c+CD40hi subsets were defined by gating as shown on the dot plots (upper panels). (C) BMDCs were incubated with fluorescein-curdlan and analyzed by confocal microscopy. Note the fluorescein signal within the cells. (D) Mice were injected in both hind footpads with 1 mg fluorescein-curdlan or PBS 6 hours before popliteal lymph nodes were removed and analyzed by flow cytometry. Dot plots show fluorescein signal on resident DCs (CD11c+CD40lo, left) and migratory DCs (CD11c+CD40hi, right) plotted against expression of CD8α. (E) Mice were injected in both hind footpads with 200 μg curdlan, 10 μg polyI:C or PBS 6 hours before popliteal lymph nodes were removed and analyzed. Data are GeoMFI of dectin-1 (top) and CD86 staining (bottom) on skin-draining DCs (CD11c+CD40hi, left), and CD8α− and CD8α+ lymph-node resident DC subsets (CD11c+CD40lo, middle and right).
All conventional DC subsets respond to curdlan in vivo. (A) Expression of dectin-1 on spleen CD11c+CD8α− and CD11c+CD8α+ cells. Splenocytes were stained with anti–dectin-1 (thick line) or rat IgG2b (solid gray). DC subtypes were defined by gating as shown on the dot plot (left panel). Numbers indicate percentages of events in gate (dot plot) or mean fluorescence intensity (GeoMFI; histograms). (B) Expression of dectin-1 on lymph node DC subsets. Cell suspensions from peripheral lymph nodes were stained as in panel A. Blood-derived resident CD11c+CD40loCD8α− or CD11c+CD40loCD8α+ cells and skin-derived migratory CD11c+CD40hi subsets were defined by gating as shown on the dot plots (upper panels). (C) BMDCs were incubated with fluorescein-curdlan and analyzed by confocal microscopy. Note the fluorescein signal within the cells. (D) Mice were injected in both hind footpads with 1 mg fluorescein-curdlan or PBS 6 hours before popliteal lymph nodes were removed and analyzed by flow cytometry. Dot plots show fluorescein signal on resident DCs (CD11c+CD40lo, left) and migratory DCs (CD11c+CD40hi, right) plotted against expression of CD8α. (E) Mice were injected in both hind footpads with 200 μg curdlan, 10 μg polyI:C or PBS 6 hours before popliteal lymph nodes were removed and analyzed. Data are GeoMFI of dectin-1 (top) and CD86 staining (bottom) on skin-draining DCs (CD11c+CD40hi, left), and CD8α− and CD8α+ lymph-node resident DC subsets (CD11c+CD40lo, middle and right).
Curdlan is largely insoluble and its stimulatory activity resides in the particulate fraction, which consists of large aggregates of material (data not shown). In vitro, DCs cluster around these aggregates and internalize small fragments of curdlan that have been shed or were scavenged from the larger particles (Figure 5C). To determine whether such curdlan fragments are also captured by DCs in vivo, we injected fluorescent curdlan into mice and analyzed DCs isolated from the draining lymph nodes 6 hours later. As shown in Figure 5D, fluorescent signal was found in both the lymph node resident CD8α+ and CD8α− DC populations, as well as in skin-derived DCs. Notably, surface dectin-1 expression on each of the DC subsets was significantly down-regulated in mice receiving curdlan compared with mice injected with PBS or with other innate stimuli such as poly I:C, a TLR3 and MDA5 agonist (Figure 5E). These observations suggest that resident and migratory DC subsets in lymph nodes draining the sites of curdlan injection come into direct contact with the stimulus. Consistent with that notion, curdlan led to up-regulation of CD86 by lymph node DCs although this was less noticeable on the skin-draining subset, which constitutively expresses higher levels of the costimulatory molecule (Figure 5E).
Curdlan acts as an adjuvant for CTL priming in vivo
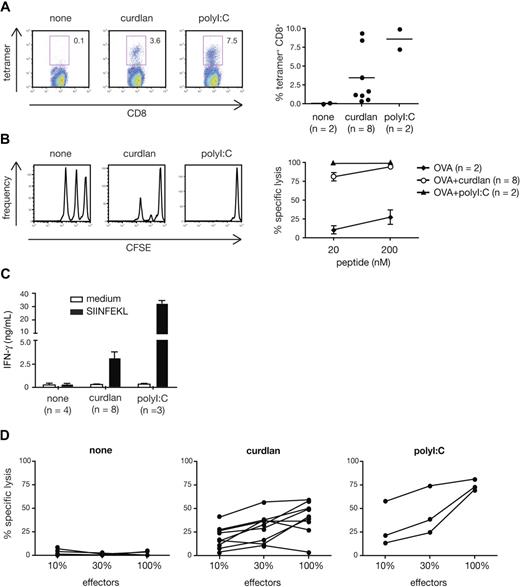
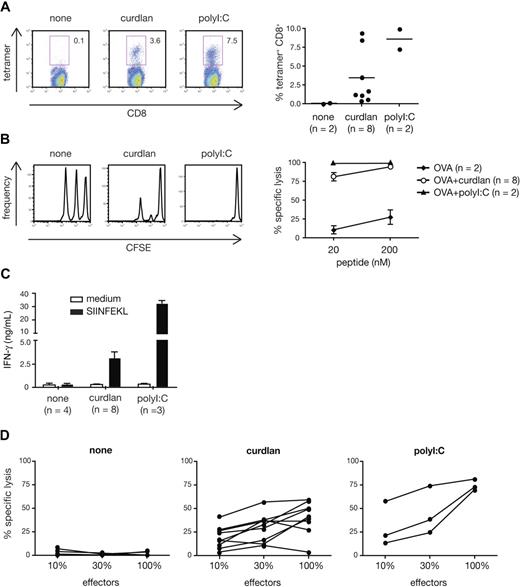
The fact that all conventional DCs, including the CD8α+ subset specialized in crosspriming,34 can respond to curdlan suggested that the latter might be useful for promoting CTL responses to exogenous antigens in vivo. We therefore tested curdlan as an adjuvant for CTL crosspriming to OVA protein. Endogenous OVA-specific CD8+ T cells expanded strongly in mice immunized with OVA mixed with curdlan, but not with OVA alone (Figure 6A). The T-cell expansion was evident in both spleen and peripheral blood (Figure 6A and data not shown) and was comparable to that observed in mice immunized with OVA + polyI:C (Figure 6A) or OVA + CpG (data not shown). The expanded T cells acquired cytolytic effector functions as demonstrated by their capacity to eliminate SIINFEKL peptide-pulsed target cells but not antigen-negative control cells injected 5 days after immunization (Figure 6B). Restimulation with SIINFEKL peptide in vitro resulted in secondary CTL expansion, which was accompanied by IFN-γ production and specific killing activity (Figure 6C,D and data not shown). Importantly, the response induced by OVA plus curdlan in vivo seemed independent of the TLR pathway and of IL-1 and IL-18 receptor signaling because CTL priming was not affected by MyD88 deficiency, whether measured at the level of OVA-specific CD8+ T-cell expansion or in vivo cytotoxic activity (Figure S2A,B). In contrast, CTL priming in response to CpG was abolished in MyD88-deficient mice, as expected (data not shown). We also investigated the role of IL-12 p70 as a critical mediator for CTL priming in vivo. In contrast to the in vitro system, IL-12 p70 was redundant for the adjuvanticity of curdlan in vivo as mice deficient in IL-12 p35 showed normal expansion and killing activity of OVA-specific CD8+ T cells in vivo (Figure S3A,B). We conclude that activation of the dectin-1 pathway is sufficient for inducing potent CTL responses in vivo and that curdlan, a selective agonist for this pathway, is a potent adjuvant for CTL priming.
Curdlan acts as an adjuvant for CTL crosspriming in vivo. C57Bl/6 mice were immunized in both hind footpads with OVA protein alone (none) or together with curdlan or polyI:C as indicated. Target cells were injected 6 days later and mice were analyzed on day 7. (A) H-2Kb-SIINFEKL tetramer staining of splenocytes. Left: representative dot plots of tetramer staining vs CD8 in gated Thy1.2+ T cells. Numbers indicate percent of tetramer+ cells. Right: frequency of tetramer+ CD8+ T cells (gated on scatter and Thy1.2+ cells). Individual mice from 2 pooled experiments are shown. (B) In vivo CTL activity measured by target cell elimination. Histograms show target cell frequency in representative mice from each group (CFSElo, 20 nM peptide, CFSEint, 200 nM peptide, CFSEhi, no peptide). Graph shows mean plus or minus SD of percent specific lysis from 2 pooled experiments. (C) In vitro restimulation with SIINFEKL peptide or in medium alone. IFN-γ content in supernatants at the end of the 2-day culture. Restimulations and ELISA measurements were done in triplicates. Data are the mean plus SD of the indicated number of mice in 2 pooled experiments. (D) Specific CTL activity of cells restimulated for 5 days in vitro. Effectors were used at different dilutions as indicated. Individual mice from 2 pooled experiments are shown.
Curdlan acts as an adjuvant for CTL crosspriming in vivo. C57Bl/6 mice were immunized in both hind footpads with OVA protein alone (none) or together with curdlan or polyI:C as indicated. Target cells were injected 6 days later and mice were analyzed on day 7. (A) H-2Kb-SIINFEKL tetramer staining of splenocytes. Left: representative dot plots of tetramer staining vs CD8 in gated Thy1.2+ T cells. Numbers indicate percent of tetramer+ cells. Right: frequency of tetramer+ CD8+ T cells (gated on scatter and Thy1.2+ cells). Individual mice from 2 pooled experiments are shown. (B) In vivo CTL activity measured by target cell elimination. Histograms show target cell frequency in representative mice from each group (CFSElo, 20 nM peptide, CFSEint, 200 nM peptide, CFSEhi, no peptide). Graph shows mean plus or minus SD of percent specific lysis from 2 pooled experiments. (C) In vitro restimulation with SIINFEKL peptide or in medium alone. IFN-γ content in supernatants at the end of the 2-day culture. Restimulations and ELISA measurements were done in triplicates. Data are the mean plus SD of the indicated number of mice in 2 pooled experiments. (D) Specific CTL activity of cells restimulated for 5 days in vitro. Effectors were used at different dilutions as indicated. Individual mice from 2 pooled experiments are shown.
Use of curdlan as an adjuvant promotes tumor immunity
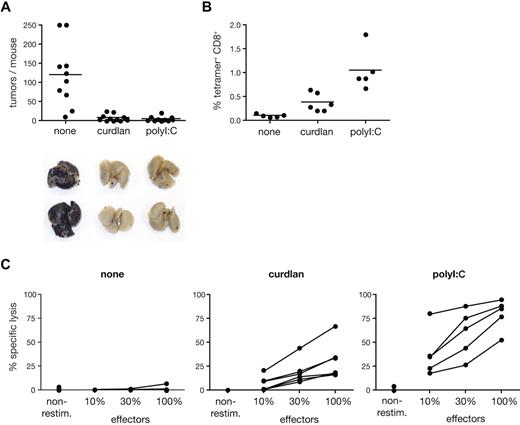
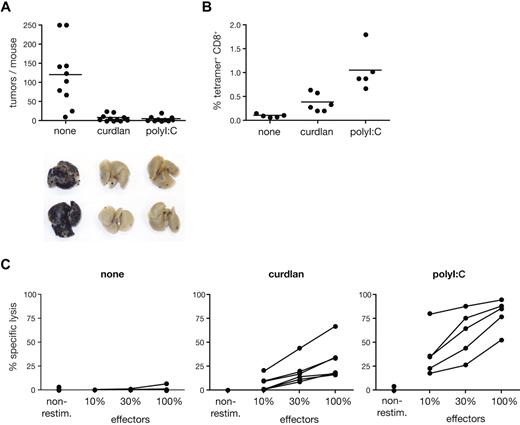
To assess the potential of curdlan as an adjuvant for immunotherapy, we tested it in a tumor prevention model. We immunized mice with a single dose of ovalbumin alone or mixed with curdlan or poly I:C and challenged them with ovalbumin-expressing B16 melanoma cells. The number of lung pseudo-metastases determined 3 weeks later was close to background in the antigen plus curdlan or poly I:C groups, whereas no protection from tumor growth was observed in mice immunized with antigen alone (Figure 7A). Tumor immunity correlated with expansion of OVA-specific CD8+ T cells and with OVA-specific lytic activity in the spleen of each mouse (Figure 7B,C). Thus, curdlan promotes a long-lasting CTL response that is sufficient to protect against tumor challenge.
Curdlan-dependent CTL crosspriming protects mice from tumor challenge. C57Bl/6 mice were immunized in both hind footpads with OVA protein alone (none) or together with curdlan or polyI:C as indicated. Melanoma cells were injected intravenously 5 to 7 days after immunization and tumor growth was analyzed 18 to 22 days thereafter. (A) Data represent tumor counts in each mouse from 2 pooled experiment (n = 10-11 mice/group). Lungs of 2 representative mice from each group are shown below the graph. (B) Frequency of SIINFEKL-H2Kb tetramer+ CD8+ cells in spleens from mice of 1 of 2 experiments shown in panel A. (C) Splenocytes from individual mice in 1 of 2 experiments shown in panel A were restimulated in vitro with SIINFEKL peptide. Data show CTL activity after restimulation as in Figure 6D.
Curdlan-dependent CTL crosspriming protects mice from tumor challenge. C57Bl/6 mice were immunized in both hind footpads with OVA protein alone (none) or together with curdlan or polyI:C as indicated. Melanoma cells were injected intravenously 5 to 7 days after immunization and tumor growth was analyzed 18 to 22 days thereafter. (A) Data represent tumor counts in each mouse from 2 pooled experiment (n = 10-11 mice/group). Lungs of 2 representative mice from each group are shown below the graph. (B) Frequency of SIINFEKL-H2Kb tetramer+ CD8+ cells in spleens from mice of 1 of 2 experiments shown in panel A. (C) Splenocytes from individual mice in 1 of 2 experiments shown in panel A were restimulated in vitro with SIINFEKL peptide. Data show CTL activity after restimulation as in Figure 6D.
Discussion
Little is known about the ability of non-TLR innate immune receptors to couple pathogen recognition to the induction of adaptive immune responses. Dectin-1 is the only transmembrane PRR shown to date to possess the capacity to induce innate gene transcription independently of TLRs.6 In DCs, dectin-1 signaling via the kinase Syk and the downstream adaptor CARD9 leads to maturation and production of cytokines and allows priming of CD4+ T-cell responses characterized by the presence of Th17 cells.6 Here we show that dectin-1–activated DCs are able to prime CTLs and that dectin-1 agonists can be used as adjuvants for the induction of cytotoxic T cells in vivo. These data extend our earlier observations that dectin-1 agonists act as adjuvants for CD4+ T-helper cell priming and antibody responses and indicate that the dectin-1/Syk-pathway can couple innate immune recognition to all branches of the adaptive immune system.
Our data indicate that curdlan enhances CTL priming in part by activating antigen-presenting DCs to produce IL-12 p70, which acts as a key polarizing signal to sustain differentiation of naive CD8+ T cells into effectors.26,29 We have previously shown that curdlan-stimulated DCs produce IL-2, IL-10, IL-6, TNF-α, and IL-23, but not IL-12 p70.6 The absence of IL-12 p70 production was due to the lack of expression of the IL-12 p35 subunit because dectin-1 signaling leads to production of high levels of IL-12 p40.6 In contrast, we now provide evidence that IL-12 p70 can be produced by curdlan-stimulated DCs in some instances such as when the innate stimulus is accompanied by secondary signals delivered by newly activated CD8+ T cells. This is reminiscent of the observation that TLR-induced IL-12 p70 production is strongly enhanced by CD4+ T-cell–derived feedback factors that regulate production of the limiting IL-12 p35 subunit.35,36 Such feedback factors can include CD40L, GM-CSF, IL-4, or IFN-γ35,37-41 but, in the case of CD8+ T cells, our data indicate that the latter cytokine acts as the dominant signal. This is consistent with the fact that naive CD8+ T cells can produce IFN-γ shortly after antigen stimulation.42 Consistent with that observation, we find the presence of the cytokine in culture supernatants at 24 hours but not at 72 hours of OT-I antigen stimulation in the absence of innate stimuli, suggesting a burst of production followed by consumption (data not shown). The importance of IFN-γ as a CD8+ T-cell feedback signal for IL-12 p70 production by DCs has been noted previously in the human system.42 The situation for curdlan and TLR agonists is thus comparable in that both classes of innate stimulus induce abundant IL-12 p40 but limited IL-12 p35 synthesis until subsequent CD4+ or CD8+ T-cell feedback.35 The main difference between the 2 types of stimulus is that whereas IL-12 p70 levels induced by CpG alone lie between 1 ng/mL and 5 ng/mL (ie, easily detectable by ELISA measurements), the levels induced by curdlan alone are below detection limits. These results emphasize that DC cytokine measurements should be analyzed in the appropriate context (eg, in DC–T-cell cocultures) to account for amplification provided by feedback signals received from T cells during priming.
In contrast to the results obtained in vitro, IL-12 p70 was not essential for CTL crosspriming in vivo when curdlan was used as an adjuvant. Therefore, it is likely that no one single factor is the critical mediator of curdlan-induced augmentation of CTL priming in vivo but that several factors (eg, IL-12, CD70, type I IFNs) can act in redundant fashion.43 This is reminiscent of CTL priming experiments using TLR agonists as adjuvants where different mediators are involved and can substitute for one another.43 The redundancy seen in vivo yet not in vitro is also likely to reflect the involvement of multiple DC subsets in the former setting. Indeed, although crosspresentation is generally thought to be a property of CD8α+ DCs, recent studies indicate that for yeast-expressed antigens crosspriming is carried out preferentially by CD8α− DCs, partly through a mechanism involving dectin-1.30 As multiple DC subsets in vivo directly respond to curdlan (this study) and CD8α+ and CD8α− DC subsets can differentially use IL-12 p70 and CD70 for the induction of T-cell responses in vivo,44 further studies will be necessary to dissect the contribution of distinct DC subtypes and their effector molecules to crosspriming in our model.
Dectin-1 is essential for host defense against fungal pathogens.14,45 Whereas its expression on neutrophils and macrophages is mainly involved in triggering early and direct antifungal mechanisms, dectin-1 activation in DCs can lead to the activation of T cells. The protective role of CD4+ T cells in host defense from fungal infections is well established46 but the role of antifungal CTLs is less clear. Two groups have reported that CD8+ T cells are primed in response to recombinant OVA-expressing Saccharomyces cerevisiae and that this is, at least in part, mediated by dectin-1.30,47 Moreover, CD8+ T cells are activated during fungal infections in human and mouse and, in some instances, play a protective role.48-51 Independently of the physiologic role of dectin-1 in antifungal adaptive immune responses, our data show that dectin-1 agonists act as potent adjuvants for priming of CTLs with the potential to destroy tumors. Notably, β-glucans are used clinically in some countries as cancer therapeutics.52 Our demonstration that dectin-1 can couple innate sensing of β-glucans to CTL priming may perhaps provide a mechanistic basis to explain some of the antitumor activity of those polymers. Added to the fact that the endocytic properties of dectin-1 also make this receptor useful for antigen targeting to APCs in vivo,31 our data therefore support further exploitation of dectin-1 agonists in immunotherapeutic applications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Shizuo Akira and Nico Ghilardi for provision of genetically modified mice; Edina Schweighoffer and Victor Tybulewicz for fetal liver from syk−/− embryos; Annette Oxenius for IL-12 p40–deficient and IL-12 p35–deficient mice; the FACS Service and Animal Unit from the London Research Institute for technical support; Michele Weber for help with acquisition of confocal images; and members of the Immunobiology Laboratory, Cancer Research UK, for advice and discussions.
This work was funded by Cancer Research UK. S.L.-L. was supported by a European Molecular Biology Organization long-term fellowship. G.D.B. was funded by CANSA South Africa.
Authorship
Contribution: S.L.-L. performed the research; S.L-L. and C.R.S. planned the research, analyzed and interpreted data, and wrote the manuscript; F.O. carried out the experiments with pDCs; and G.D.B. contributed a vital reagent.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Caetano Reis e Sousa, Immunobiology Laboratory, Cancer Research UK, London Research Institute, Lincoln's Inn Fields Laboratories, 44 Lincoln's Inn Fields, London WC2A 3PX, United Kingdom; e-mail: caetano@cancer.org.uk.