Abstract
The biochemical mechanisms controlling the diverse functional outcomes of human central memory (CM) and effector memory (EM) T-cell responses triggered through the T-cell receptor (TCR) remain poorly understood. We implemented reverse phase protein arrays to profile TCR signaling components in human CD8 and CD4 memory T-cell subsets isolated ex vivo. As compared with CD4 CM cells, EM cells express statistically significant increased amounts of SLP-76 and reduced levels of c-Cbl, Syk, Fyn, and LAT. Moreover, in EM cells reduced expression of negative regulator c-Cbl correlates with expression of c-Cbl kinases (Syk and Fyn), PI3K, and LAT. Importantly, consistent with reduced expression of c-Cbl, EM cells display a lower functional threshold than CM cells. Increasing c-Cbl content of EM cells to the same level as that of CM cells using cytosolic transduction, we impaired their proliferation and cytokine production. This regulatory mechanism depends primarily on c-Cbl E3 ubiquitin ligase activity as evidenced by the weaker impact of enzymatically deficient c-Cbl C381A mutant on EM cell functions. Our study reports c-Cbl as a critical regulator of the functional responses of memory T cell subsets and identifies for the first time in humans a mechanism controlling the functional heterogeneity of memory CD4 cells.
Introduction
Immunologic memory is a fundamental feature of the adaptive immune system characterized by a more rapid and vigorous response to pathogens that were previously encountered. Memory lymphocytes persist for a lifetime in the absence of antigen,1 confer immediate protection in peripheral tissues, and mount recall responses to antigens in secondary lymphoid organs upon antigen re-exposure.2 Memory T cells are heterogeneous; 2 broad, functionally and phenotypically distinct cell subsets, central memory (CM) and effector memory (EM) cells, have been delineated based on the expression of the phosphatase CD45 and the CC chemokine receptor CCR7.3 Both subsets lack CD45RA expression. Whereas CM cells express CCR7 and home to lymph nodes, EM cells are CCR7−, express receptors for migration to inflamed tissues, and display inflammatory and cytotoxic functions. CM cells have limited effector functions, but they proliferate and become EM cells in response to homeostatic cytokines or antigen stimulation.3-5 Human CM cells show a proliferation rate 3-fold slower than EM cells in vivo, indicating that EM cells constitute short-lived cell populations that require continuous replenishment in vivo.6 The lineage relationship between CM and EM populations in humans remains controversial.2
Understanding the molecular basis of human memory T-cell subset activation is of paramount significance in protective immunity and vaccine design. The importance of both CM and EM subsets has been reported for the control of human viral infections7-10 and the effectiveness of vaccines in murine models.11-13 Initial gene expression profiling of human memory CD4 T cells revealed few differences between CM and EM cells.14 More recently, CM cells (CD45RA−, CCR7+, CD27+) were reported to express lower levels of proapoptotic and cell cycle–related genes described as FOXO3a target genes, relative to their EM counterparts.15 The convergence of T-cell receptor (TCR) and cytokine-induced signals caused FOXO3a phosphorylation and seems to drive the survival of CM cells.15 Analysis of gene expression and cytokine signaling in human memory CD816 and CD415 T-cell subsets revealed a dichotomy between CM and EM cells with CM cells displaying a high basal and induced STAT5 phosphorylation in response to interleukin-2 (IL-2)–related cytokines. However, very few truly CM- and EM-specific genes were identified in CD8 cells, with CM cells displaying a gene transcription signature intermediate between naive and effector cells.16
The biochemical mechanisms controlling human or mouse memory T-cell responses triggered through the TCR have been poorly characterized. Indeed, efforts to correlate functional heterogeneity to the signaling mechanisms in memory cells are severely constrained by the low frequency of these cells and the limited amount of human specimens available for analysis. Furthermore, the role of many specific signaling molecules such as Lck, ZAP-70, SLP-76, LAT, and ERK in T-cell function in vivo has been difficult to establish due to the lack of peripheral T cells in the corresponding knockout (KO) mice.17-20 In humans, ligation of MHC class II and CD4 was reported to inhibit memory CD4 cells activation.21 CD4 effector cells generated in vitro, up-regulate the expression of FcRγ protein that belongs to an altered TCR/CD3 signaling complex lacking CD3ζ. Upon TCR triggering with anti-CD3 antibodies, this complex recruited and activated Syk instead of ZAP-70 kinase as observed in naive and memory cells.21-23
The multifunctional adaptor Cbl acts as a negative regulator of intracellular signaling induced by engagement of cell-surface receptors. The RING finger domain of Cbl has E3 ubiquitin ligase activity that catalyses ubiquitilation and targeting of critical signaling molecules to lysosomal or proteosomal degradation. Moreover, Cbl regulates endocytosis of receptor tyrosine kinase and activity of other signaling proteins through a proteolysis-independent mechanism that is not yet known.24-26 Two related members of the Cbl family, Cbl-b and c-Cbl, were reported to negatively regulate TCR signal transduction by distinct mechanisms.25 In thymocytes, c-Cbl modulates TCR expression, inhibits ZAP-70 activation by an unknown mechanism, and cooperates functionally with SLAP to target TCR/CD3ζ for ubiquitilation and degradation.27 The role of Cbl-b as key regulator of activation threshold and immunological tolerance was revealed by the study of Cbl-b KO mice that show hyperproliferation and increased production of IL-2 uncoupled from the requirement for CD28 costimulation.28-30 In naive T cells, Cbl-b interacts with PI3K and prevents hyper-phosphorylation of Vav-1 without affecting phospholipase Cγ1 (PLCγ1),28,29,31 while in preactivated T cells, Cbl-b binds to and inhibits PLCγ1 activation without further altering Vav-1 phosphorylation.32
This study is the first attempt to understand the biochemical signaling processes controlling the diverse functional outcomes of human memory T-cell subsets. To this end, we implemented reverse phase protein (RPP) arrays to profile the signaling components of human CD8 and CD4 memory T-cell subsets isolated ex vivo. Based on analysis of 8 to 13 healthy subjects, we showed that CD4 EM cells, relative to CM cells, expressed significantly lower levels of c-Cbl and c-Cbl kinases (Fyn and Syk) and specifically coregulated the expression of PI3Kinase, LAT, and SLP-76. Importantly, we demonstrate that the functional consequence of the decreased c-Cbl content includes an increased functional response of EM cells. Indeed, increasing c-Cbl content in EM cells to the same level as in CM cells using cytosolic transduction, we impaired EM cell proliferation and cytokine production. Of interest, transduction of C381A c-Cbl mutant, deficient in E3 ubiquitin ligase activity, only weakly impaired EM cell functional responses. Collectively, our data demonstrate the crucial role of c-Cbl in modulating the functional responses of EM cells through a mechanism mainly dependent on its E3 ubiquitin ligase activity. This study reports for the first time that differential regulation of TCR-mediated signal controls the functional heterogeneity of human memory CD4 T-cell subsets.
Methods
Antibodies and reagents
Anti-CD3 Ab (OKT3) was purified from hybridoma CRL-8001 (ATCC, Rockville, MD). Abs against CD3ϵ (M20), Lck (aa475–505), Cbl-b (C-20), PLCγ, and HRP-goat anti–mouse IgG1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Abs against ZAP-70 (99F2), p38 MAP Kinase, p44/42 MAP Kinase, and Syk were from Cell Signaling Technology (Danvers, MA). Anti-Lck (aa1-58) and anti-FcϵRI γ-subunit (aa80-86) Abs were purchased from Upstate Biotechnology (Lake Placid, NY). Antibodies against CD28 (CD28.2), CD3ζ (8D3), PI3 Kinase p85α subunit, (4) c-Cbl,(17) SLP76 (8) LAT, (45), Actin (C4), CD45RA-PE (HI100), INFγ-APC (B27), CCR7 (3D12), IL2-PE (MQ1-17H12), and HRP goat anti-rabbit were purchased from BD Biosciences (San Jose, CA). Anti–β2-microglobulin Ab was from BioVendor Laboratory Medicine (Modrice, Czech Republic). Rabbit anti–RNAse-2, anti-Fyn, and mouse anti-CD4 (B66-6) antibodies were provided by Drs J. Hofsteenge (Friedrich Miescher Institute, Basel, Switzerland), M. F. White (Harvard Medical School, Boston, MA), and D. Rimoldi, respectively. All chemicals unless indicated otherwise were from Sigma-Aldrich (St Louis, MO) and tissue culture media and reagents were from Gibco BRL (now Invitrogen, Carlsbad, CA). Tyramide amplification reagent was from Bio-Rad Laboratories (Hercules, CA) and human recombinant interleukin-2 (IL-2) from R&D Systems (Minneapolis, MN). Restriction enzymes were from Roche Applied Science (Indianapolis, IN) and ligation kit was from Takara Bio (Shiga, Japan). DNA purification kits were purchased from Macherey-Nagel (Duren, Germany) and PfuTurbo DNA-polymerase was from Stratagene-Agilent (La Jolla, CA).
T-cell isolation and lysis
Peripheral blood specimens from healthy donors were obtained from the local blood bank and lymphocytes were enriched by LymphoPrep (Axis-Shield, Dundee, United Kingdom) gradient centrifugation. After positive immunomagnetic selection (Miltenyi Biotec, Auburn, CA), CD4 or CD8 T cells were labeled with CD45RA and CCR7 and sorted with a FACSAria sorter (BD Biosciences) into N, CM, EM, and E populations (purity > 98%) and lysed (80 × 106/mL) on ice in 100 mM Na2HCO3, pH 8.0, 80 mM Octyl-β-D-glucopyranoside, 5 mM EDTA, 1% (v/v) glycerol, 2% (v/v) 2-mercaptoethanol, 1 mM Na3VO4, 15 mM NaF, and protease inhibitors. Lysates were heated for 10 minutes at 95°C and clarified by centrifugation. For functional assays, CD4 memory cells were isolated by CD4 negative immunomagnetic depletion (Invitrogen) supplemented with anti-CD45RA Ab (HI100).
The study received the approval of the Ethics Committee of the University of Lausanne and informed consent was obtained in accordance with the Declaration of Helsinki.
Lysate printing and detection of signaling molecules
Lysates were printed onto 16-pad 3D nitrocellulose Fast slides (Schleicher and Schuell/PerkinElmer, Waltham, MA) using the Omnigrid 300 contact-printing robotic microarrayer (Genomic Solutions, Ann Arbor, MI) equipped with SMP3 pins (TeleChem International, Sunnyvale, CA). The slides were dried, blocked for 2 hours at room temperature in phosphate-buffered saline 0.1%/Tween20 (PBST) containing 3% BioRad blocking solution, incubated with primary (14 hours, 4°C) and secondary HRP (60 minutes, room temperature) Abs in PBST containing 3% fish gelatin. Signals were amplified by biotin-tyramide and detected as described.33
Array analysis
Background-subtracted intensities of individual features were captured with GenePix Pro 6.0 software (Molecular Devices, Sunnyvale, CA) to determine the mean value of spots printed in triplicate. Mean values were then normalized to the mean values of corresponding actin signal. Data were considered when signal intensities were included in the linear range of detection of a lysate serial dilution present in the same pad.
Statistical analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA).
Functional assays
Cells were stimulated in 96-well plates coated with anti-TCR/CD3 (OKT3) Ab and indicated soluble antibodies at 1 μg/mL. IL-2 and INF-γ production were blocked with GlogiPlug transport inhibitor and monitored by flow cytometry as described.34 For proliferation assays, CFSE-labeled cells were surface-stained for CCR7 and sorted into CM and EM cell populations, and the percentage of proliferating cells determined by cytofluorimetry 6 days after stimulation. Alternatively, incorporation of H3 thymidine (MP Biomedicals, Illkirch, France) was measured over 24 hours 3 days after activation. Memory cells were labeled on ice with anti-CCR7, mouse anti-CD3, and CD28 Abs. Intracellular changes in calcium were monitored as described35 upon crosslinking with anti–mouse Abs.
Plasmids
CTP sequence and corresponding plasmids were provided by Dr Y.-S. Bae (Sungkyunkwan Univerity, Suwon, Korea). Complementary forward (5′-TATCGGATCCGCCACCATGTACGGACGCCGCGCACGCCGCCGCCGCCGCCGCGCTAGCTACCCTTATGATGTGCCAGATTATGC-3′) and reverse (5′-GGCCGCATAATCTGGCACATCATAAGGGTAGCTAGCGCGGCGGCGGCGGCGGCGTGCGCGCGTCCGTACATGGTGGCGGATCCGA-3′) primers coding for CTP-HA peptide were annealed and inserted into pET2a vector digested with NdeI/NotI (pET21a-CTP-HA). Wild-type (wt) and C381A mutant c-Cbl cDNA were amplified by polymerase chain reaction (PCR) from plasmids kindly provided by Dr S. Lipkowitz36 and Dr W. Langdon,37 and His-tagged GFP was obtained by PCR from pEGFP-CI vector (Clontech-Takara Bio, Shiga, Japan). Amplified fragments were inserted into NotI/XhoI sites of pET21a-CTP-HA vector and CTP constructs subcloned into pCR3 vector (Invitrogen). The sequences of all constructs were confirmed by DNA sequencing.
Purification of CTP protein and cell transduction
Large-scale expression of CTP proteins in HEK293 cells was done at the Protein Expression Core Facility (EPFL; Lausanne, Switzerland). CTP-His tagged-proteins, either endogenous 7xHis (aa36-42) in c-Cbl or recombinant N terminal 6xHis in CTP-mock and -GFP, were purified from cell lysates by nickel-agarose chromatography according to the supplier's instructions. CTP-c-Cbls were enriched 100-fold and their sequences confirmed by nano liquid chromatography mass spectrometry. CTP proteins were transduced in T cells by incubation for 30 minutes at 37°C in RPMI containing 0.5% gelatin.
Results
RPP arrays to profile signaling proteins in human memory T cells
RPP arrays represent a multiplexed platform for profiling protein levels and their modifications at a scale that is beyond what traditional techniques can achieve. It was applied to monitor the response of living T cells to activation.33 We printed triplicate spots of 209 (± 15) μm in diameter containing 2 to 5 ng of protein from total cell lysates, which is equivalent to 20 to 50 T cells. Qualitative analysis of the spots indicated a good reproducibility in spot geometry with the circularity and diameter showing a coefficient of variation (CV) as defined by standard deviation/mean*100 in triplicate spots of 2.2% and 2.9%, respectively (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Analysis of 200 triplicate spots revealed a CV in spot intensity of 5.3% (data not shown). Specificity of the signal in the spots was assessed by measuring SLP-76 expression in peripheral blood mononuclear cells (PBMCs), where it is expressed, and in Caco-2 cells, where it is not expressed. Specificity was further demonstrated by detecting exogenously added RNase-2 in PBMC lysates (Figure 1A left). Following amplification with biotin-tyramide, signal linearity was demonstrated in spots containing 2 to 200 cell equivalent for most of the antibodies used in this report (Figure 1B and data not shown). An antibody was used for RPP arrays only if it detected by immunoblotting a single predominant band of the expected molecular weight in a biological sample similar to that analyzed on the arrays (Figure 1A right panels). Finally, we established that actin was a suitable protein for normalization of spot protein content, since the amount of actin per milligram of protein lysate was constant in the different T-cell subsets, while β2-microglobulin levels varied considerably (Figure 1C).
Characteristics of RPP microarrays and validation of β-actin as protein for normalization. (A) Mean fluorescence intensity (MFI) of SLP-76–specific signal detected in spots containing equal protein amount of PBMC or intestinal Caco-2 cell lysates. Alternatively, exogenous RNase-2 was added to PBMC lysate and specific RNase-2 signals were normalized to β-actin content (bar graphs). Immunodetection of Western blots (WB) with specific antibodies for SLP-76 and RNAse-2 in the corresponding lysates are shown in right panels (300 000 cells/lane). (B) Quantitative analysis of Lck detection in serially diluted PBMC lysates using specific antibodies. The bottom panel shows corresponding spots printed in triplicate. (C) β-Actin as protein for normalization. β-Actin and β2-microglobulin contents were measured in lysates of sorted N, CM, EM, and E cells following SDS-PAGE and immunoblotting with specific antibodies. Shown are representative immunodetection and quantitative measurements of β-actin signals from WB in 3 cumulated subjects.
Characteristics of RPP microarrays and validation of β-actin as protein for normalization. (A) Mean fluorescence intensity (MFI) of SLP-76–specific signal detected in spots containing equal protein amount of PBMC or intestinal Caco-2 cell lysates. Alternatively, exogenous RNase-2 was added to PBMC lysate and specific RNase-2 signals were normalized to β-actin content (bar graphs). Immunodetection of Western blots (WB) with specific antibodies for SLP-76 and RNAse-2 in the corresponding lysates are shown in right panels (300 000 cells/lane). (B) Quantitative analysis of Lck detection in serially diluted PBMC lysates using specific antibodies. The bottom panel shows corresponding spots printed in triplicate. (C) β-Actin as protein for normalization. β-Actin and β2-microglobulin contents were measured in lysates of sorted N, CM, EM, and E cells following SDS-PAGE and immunoblotting with specific antibodies. Shown are representative immunodetection and quantitative measurements of β-actin signals from WB in 3 cumulated subjects.
Expression of signaling components in human CD8 and CD4 T-cell subsets
A major advantage of RPP arrays is the requirement of minute amounts of protein extracts for accurate quantification of multiple signaling components. Thus, RPP arrays overcome the reduced number of highly purified cells obtained by sorting of low frequency memory T-cell subsets (0.3%-5% and 5%-12% of PBMC for CD8 and CD4 memory T-cell subsets, respectively) from limited amount of peripheral blood (100-250 × 106 PBMC per subject available for analysis). To understand how TCR triggering directs different functional outcomes in memory T-cell subsets, we first profiled 15 signaling components using RPP arrays. Highly purified (purity > 98%) CD8 and CD4 T-cell subsets were isolated by cytofluorimetry based on their surface expression of the phosphatase CD45RA and the CC chemokine receptor CCR7, molecules which define their distinct functional and homing capacities2 : naive (N, CD45RA+, CCR7+), central memory (CM, CD45RA−, CCR7+), effector memory (EM, CD45RA−, CCR7−), and terminally differentiated cells (E, CD45RA+, CCR7−). Cell lysates were printed in triplicate on a slide pad containing a serial dilution of CD4 T-cell lysate used as standard. Ratios of signaling components to actin were calculated by probing identical arrays on the same slide with actin-specific antibodies. We assessed the relative error in spot intensity ratio (ie, CV) for the 15 parameters under study in 200 distinct cell extracts from 10 subjects, each printed in triplicate. CV of the intensity ratio (normalized to actin) between triplicate spots ranged from 10.3% to 11.4% in the different T-cell subsets (Table 1) and represents the technical error in the quantification of the signaling components. Changes in the expression of a signaling component between 2 cell subsets were considered as significant when higher than 20% and calculated P value of 2-tailed paired t test was less than .05.
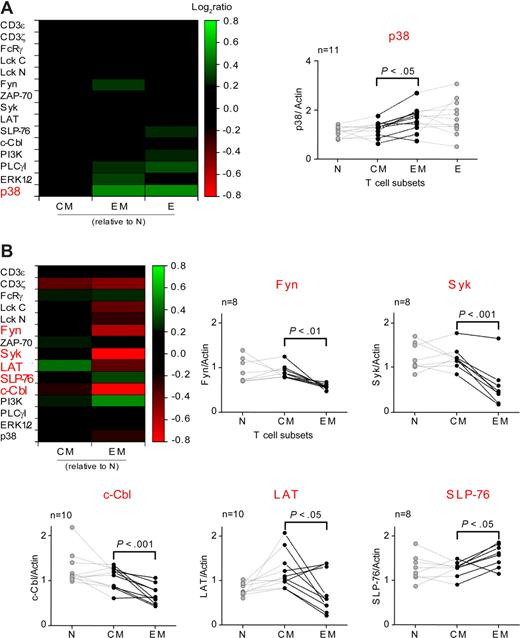
The examination of different CD8 T-cell subsets in 8 to 13 healthy subjects revealed a comparable and homogenous composition of the signaling machinery with EM and E cells, relatively to CM cells, expressing significantly increased amounts of p38MAP (P < .05) but not of the functionally related ERK1/2 (P > .05, Figure 2A). Although PLCγ and Fyn expression increased consistently form CM to EM cell subsets (P < .05), the amplitudes of these increases were close to 20% (19.5%-20.1%) and therefore, based on our criteria, not further investigated (Figure 2A left and data not shown). Taken together, our data showed that CM cells were significantly more closely related to N cells than EM or E cells (Figure 2A), in agreement with their gene expression and cytokine signaling profiles.16
Analysis of the signaling components in CD8 and CD4 T-cell subsets. Signaling components were quantified by RPP arrays and normalized to β-actin in the CD8 (A) and CD4 (B) T-cell subsets. The colors indicate the mean log2 ratios of signaling components relative to N cells from 8 to 13 subjects. Signaling components showing significant differences (2-tailed paired t test; P < .05, shown in red) between CM and EM cells are represented as row ratios on individual graphs. CM and EM compartments are shown in black. Each set of linked dots corresponds to a distinct subject; n indicates the total number of subjects examined. Lck was detected using specific antibodies recognizing epitopes located at the N- and C-terminal ends of the protein (Lck N and Lck C, respectively) to ensure fully denaturing conditions.
Analysis of the signaling components in CD8 and CD4 T-cell subsets. Signaling components were quantified by RPP arrays and normalized to β-actin in the CD8 (A) and CD4 (B) T-cell subsets. The colors indicate the mean log2 ratios of signaling components relative to N cells from 8 to 13 subjects. Signaling components showing significant differences (2-tailed paired t test; P < .05, shown in red) between CM and EM cells are represented as row ratios on individual graphs. CM and EM compartments are shown in black. Each set of linked dots corresponds to a distinct subject; n indicates the total number of subjects examined. Lck was detected using specific antibodies recognizing epitopes located at the N- and C-terminal ends of the protein (Lck N and Lck C, respectively) to ensure fully denaturing conditions.
In contrast, CD4 memory T-cell subsets showed more profound changes in the expression levels of specific signaling components. Indeed EM cells expressed significantly increased amount of SLP-76 (P < .05) and reduced levels of c-Cbl (P < .001), Syk (P < .001), Fyn (P < .01), and LAT (P < .05) as compared with CM cells (Figure 2B). Importantly, 3 out of the 5 proteins showing significant changes in their expression levels are adaptor molecules: c-Cbl, SLP-76 and LAT. The largest differences were observed for Syk (−46%) and these adaptor molecules (−33% to −35%, Figure 2B). Finally, the changes in the expression of Lck and PI3K were lower than 20% and not significant, respectively.
Heterogeneity in the expression of the signaling components among subjects in CD4 and CD8 T-cell subsets
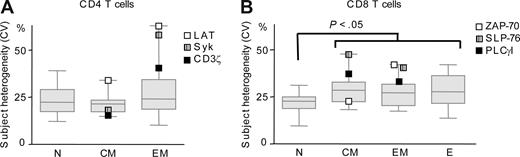
We observed that the variations in the expression of signaling components among the subjects were higher than the technical error (Figure 3, median 21.5%-28.5% > technical error 11%) and are thus likely to represent intersubject heterogeneity. Furthermore, a limited number of signaling components showed drastically high intersubject heterogeneity (up to 63%): LAT, Syk, and CD3ζ in memory CD4 T cells (Figure 3A); ZAP-70, SLP-76, and PLCγI in memory CD8 T cells (Figure 3B). These variations were independent of the signaling parameter examined and, moreover, restricted to specific T-cell subsets such as observed for CD3ζ and Syk in EM, but not CM CD4 T cells (Figure 3A). In memory CD4 cells, subject heterogeneity increased from CM to EM cells along CD3ζ/Syk/LAT pathway (Figure 3A). In CD8 T cells, overall subject heterogeneity increased with cell differentiation with signaling components being significantly (P < .05) more homogenous in N than in memory and effector cells (see median lines in Figure 3B). The expression of SLP-76 and PLCγ among the subjects was highly heterogenous in both memory CD8 T-cell subsets while heterogeneity in ZAP-70 expression increased from CM to EM cells (Figure 3B). Finally, Lck was the most homogeneously expressed protein among subjects in all CD4 and CD8 T-cell subsets (data not shown).
Subject heterogeneity in the expression of signaling components in CD4 and CD8 T-cell subsets. Subject heterogeneity as expressed by CV of the 15 signaling components (n = 15) under study in 8 to 13 cumulated subjects in CD4 (A) and CD8 (B) T-cell subsets. Box plots were automatically generated using GraphPad, the box represents values between 25th and 75th percentile with a line at the median (50th percentile). The whiskers extend above and below the box to show the highest and the lowest values. Shown are signaling components with highest subject heterogeneity in CM or EM cells.
Subject heterogeneity in the expression of signaling components in CD4 and CD8 T-cell subsets. Subject heterogeneity as expressed by CV of the 15 signaling components (n = 15) under study in 8 to 13 cumulated subjects in CD4 (A) and CD8 (B) T-cell subsets. Box plots were automatically generated using GraphPad, the box represents values between 25th and 75th percentile with a line at the median (50th percentile). The whiskers extend above and below the box to show the highest and the lowest values. Shown are signaling components with highest subject heterogeneity in CM or EM cells.
In CD4 EM T cells, reduced expression of c-Cbl correlates with LAT, SLP-76, and PI3K content
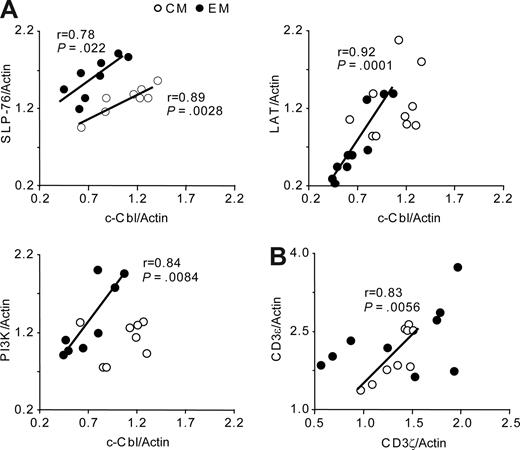
Examination of c-Cbl expression levels in 10 subjects by RPP arrays revealed a 34% reduction in EM as compared with CM CD4 T cells (P < .001, Figures 2B,4A). c-Cbl immunoblots of T-cell lysates from 4 different subjects confirmed the result obtained by RPP arrays (Figure 4A,C). Of note, 300 000 cells are required for the quantification of c-Cbl by immunodetection of Western blot while these amounts of cells are sufficient to print more than 4000 spots. Importantly, in contrast to c-Cbl, CM and EM cells showed similar levels of Cbl-b expression (Figure 4D). We next compared the expression levels of c-Cbl to other signaling proteins and observed a linear correlation with the adaptors SLP-76 and LAT (Pearson r = 0.78, P = .022 and r = 0.92, P < .001, respectively) and PI3K (r = 0.84, P = .008), suggesting that these proteins are coregulated (Figure 5A). Interestingly, these correlations were specific to EM cells and restricted to c-Cbl and SLP-76 in CM cells (r = 0.89, P < .003, Figure 5A first graph). Among the signaling parameters examined, only one additional significant correlation was observed between CD3ϵ and ζ in CM (r = 0.83, P < .006, Figure 5B) and N cells (r = 0.95, P < .001, data not shown), consistent with their dimerization in the TCR complex. This was not the case in EM cells (Figure 5B) that, as has been reported for effector cells,38 may regulate their ζ-chain independently.
c-Cbl, but not Cbl-b, expression is reduced in EM CD4 T cells. (A) Quantification of c-Cbl expression level in CM and EM cells by RPP arrays and immunodetection of Western blots (WB) with antibodies specific for c-Cbl and actin. Shown are c-Cbl to actin ratios in 10 (RPP arrays, 30 cells equivalent/spot) or 4 (WB, 300 000 cells equivalent/lane) distinct subjects. Examples of detected spots (B) and quantified immunodetections (C) are shown. (D) Quantification of Cbl-b by RPP arrays in 9 subjects as described for panel A.
c-Cbl, but not Cbl-b, expression is reduced in EM CD4 T cells. (A) Quantification of c-Cbl expression level in CM and EM cells by RPP arrays and immunodetection of Western blots (WB) with antibodies specific for c-Cbl and actin. Shown are c-Cbl to actin ratios in 10 (RPP arrays, 30 cells equivalent/spot) or 4 (WB, 300 000 cells equivalent/lane) distinct subjects. Examples of detected spots (B) and quantified immunodetections (C) are shown. (D) Quantification of Cbl-b by RPP arrays in 9 subjects as described for panel A.
In EM cells, SLP-76, LAT, PI3K, and c-Cbl expression are coregulated. (A) Correlation of the expression of SLP-76, LAT, PI3K with c-Cbl in 8 to 10 distinct subjects. The significance of their linear correlation is shown (Pearson r and P < .05). (B) Correlation of the expression of TCR/CD3 ϵ and ζ components as described for panel A.
In EM cells, SLP-76, LAT, PI3K, and c-Cbl expression are coregulated. (A) Correlation of the expression of SLP-76, LAT, PI3K with c-Cbl in 8 to 10 distinct subjects. The significance of their linear correlation is shown (Pearson r and P < .05). (B) Correlation of the expression of TCR/CD3 ϵ and ζ components as described for panel A.
EM CD4 T cells show a lower functional threshold and differentially regulate TCR-mediated signals as compared with CM cells
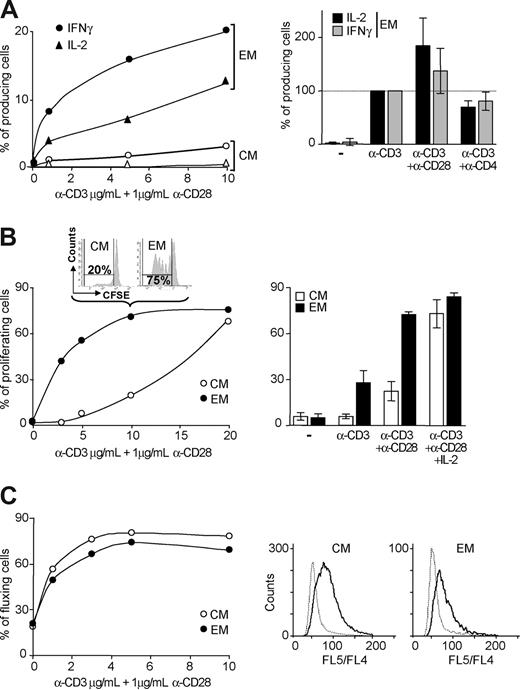
EM CD4 T cells display immediate effector functions3 and produce IL-2 and interferon-γ (IFN-γ) in response to polyclonal stimulation with TCR/CD3 and CD28-specific antibodies. In contrast, CM cells lack immediate effector functions and produce limited amounts of these cytokines, detectable only at high levels of TCR triggering (Figure 6A left). In EM T cells, CD28 costimulation enhanced significantly IL-2, but not IFN-γ production, while CD4 cross-linking had little effect (Figure 6A right). Highly purified memory CD4 T-cell subsets (purity > 98%) were analyzed for their ability to proliferate following 6 days of polyclonal stimulation. EM cells were consistently more responsive and showed a lower threshold for proliferation than CM cells (Figure 6B left). High level of TCR triggering (10μg/mL of anti-CD3 antibodies) in the presence of co-stimulation (1μg/mL of anti-CD28 antibodies) induced proliferation of 20% of CM cells after 6 days (Figure 6B left) consistent with low but detectable number of IL-2 producing cells following 1 night of stimulation (Figure 6A left). Under these conditions of stimulation (10μg/mL anti-CD3 and 1 μg/mL anti-CD28 antibodies), roughly 4 times more EM cells were proliferating and producing IL-2 (Figure 6A,B left). Proliferation of both cell subsets was highly dependent on CD28 costimulation. However, CD28 costimulation and IL-2 impacted significantly stronger CM than EM cells (Figure 6B right). At lower level of TCR triggering, this effect was even stronger with IL-2 inducing a 9.3- and 1.8-fold increase in CM and EM cell proliferation, respectively (data not shown). In contrast, the analysis of 5 subjects revealed no significantly different intracellular calcium mobilization in CM versus EM cells (Figure 6C) upon TCR and CD28 cross-linking (CM, 47.8% ± 11.5% and EM, 38.8%±9.5%, n = 5, P = .102).
Functional threshold in CM and EM CD4 T cells. (A) Detection by FACS of intracellular production of IFN-γ and IL-2 in memory cells exposed for 1 night to graduated concentrations of immobilized anti-CD3 and 1 μg/mL soluble anti-CD28 antibodies. Shown is 1 representative response of 4 (left). Cumulated data of EM cells from 4 subjects in response to 5 μg/mL immobilized anti-CD3 antibody supplemented with 1 μg/mL soluble anti-CD28 or -CD4 antibodies are shown (right). (B) Proliferative potential of memory T cells measured by CFSE dilution in sorted CM and EM cells stimulated for 6 days as described for panel A (left). Shown are cumulated data of 4 subjects in response to 5 μg/mL of immobilized anti-CD3 antibody supplemented with 1 μg/mL soluble anti-CD28 antibody in the presence or absence of IL-2 (right). (C) Intracellular calcium mobilization in CM and EM cells in response to graduated concentrations of soluble mouse anti-CD3 and 1 μg/mL soluble anti-CD28 antibodies (left). The percentage of cell showing changes in intracellular calcium concentration was assessed by measuring variations of FL5/FL4 ratio (right).
Functional threshold in CM and EM CD4 T cells. (A) Detection by FACS of intracellular production of IFN-γ and IL-2 in memory cells exposed for 1 night to graduated concentrations of immobilized anti-CD3 and 1 μg/mL soluble anti-CD28 antibodies. Shown is 1 representative response of 4 (left). Cumulated data of EM cells from 4 subjects in response to 5 μg/mL immobilized anti-CD3 antibody supplemented with 1 μg/mL soluble anti-CD28 or -CD4 antibodies are shown (right). (B) Proliferative potential of memory T cells measured by CFSE dilution in sorted CM and EM cells stimulated for 6 days as described for panel A (left). Shown are cumulated data of 4 subjects in response to 5 μg/mL of immobilized anti-CD3 antibody supplemented with 1 μg/mL soluble anti-CD28 antibody in the presence or absence of IL-2 (right). (C) Intracellular calcium mobilization in CM and EM cells in response to graduated concentrations of soluble mouse anti-CD3 and 1 μg/mL soluble anti-CD28 antibodies (left). The percentage of cell showing changes in intracellular calcium concentration was assessed by measuring variations of FL5/FL4 ratio (right).
Finally, in marked contrast to double positive thymocytes27,37 and consistent with observations in peripheral CD4 T cells from c-Cbl KO mice,37 the expression of TCR/CD3, CD4, or CD28 at the surface of EM cells was not increased (Figure S1). These observations indicate that CM and EM cells differentially regulate signaling pathways downstream of the TCR.
c-Cbl expression controls the functional response of EM CD4 T cells
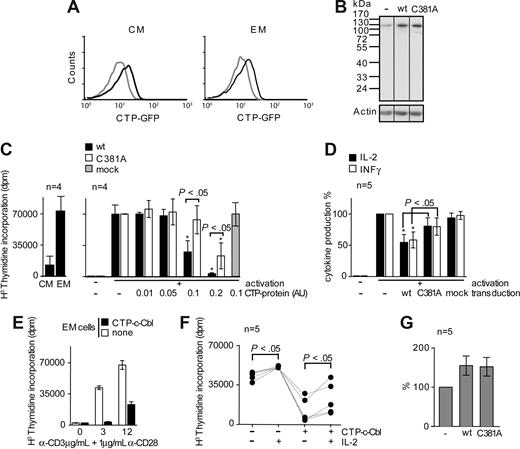
Consistent with their reduced expression of the negative regulator c-Cbl (Figures 2B,4A), EM cells show a lower threshold for activation (Figure 6A,B left) relative to CM cells. These observations suggest that c-Cbl expression may control the functional response of memory cells. In order to verify this hypothesis, we increased c-Cbl content of EM cells and measured their functional responses. Ex vivo isolated T cells are refractory to transfection and modulation of gene expression through viral infection remains suboptimal. Furthermore, the strong and sustained cell activation required for viral infection interferes with memory T-cell differentiation and is incompatible with our study. To circumvent these limitations, we took advantage of the recently described cytosolic transduction peptide (CTP:YGRRARRRRRR) that delivers CTP-fused protein to the cytosol.39 Recombinant CTP-GFP, CTP-c-Cbl (wt and C381A mutant) and a control CTP-fusion protein (CTP-mock) were expressed in Hek293 cells and purified by affinity chromatography. The C381A substitution in the C3HC4 RING domain of c-Cbl abolishes its interaction with E2 conjugating enzymes, thus preventing c-Cbl from functioning as an E3 ubiquitin ligase without affecting its association with signaling molecules.40 Both memory T-cell subsets were transduced with comparable efficiency as assessed by transduction of low amounts of CTP-GFP (1.25- and 1.3-fold increase, respectively; Figure 7A) or CTP-c-Cbl constructs (data not shown). To examine the impact of c-Cbl on the functional responses of EM cells, memory cells were transduced with wt and C381A mutant CTP-c-Cbls and stimulated with anti-CD3 and -CD28 antibodies at concentrations (5 μg/mL anti-CD3 and 1μg/mL anti-CD28) inducing EM but only limited CM cell functional responses (Figures 6A,B,7C). Transduced wt and C381A mutant CTP-c-Cbls remained intact over the duration of T-cell activation (Figure 7B). Furthermore, while in EM cells the expression levels of Syk, LAT, SLP-76, and PI3K was not altered by CTP-c-Cbl transduction, TCR and CD28 triggering increased SLP-76 expression by 45% (Figure S2).
Transduction of CTP-c-Cbl in EM cells results in lower functional response. (A) Limited amounts of CTP-GFP were transduced in memory cells and the transduction efficiency in CM and EM cells assessed by FACS. Thin and bold lines depict non-transduced and transduced cells, respectively. (B) Detection of c-Cbl following SDS-PAGE and immunoblotting of lysates of memory CD4 T cells previously transduced with wt or C381A CTP-c-Cbls and activated overnight with anti-CD3 and -CD28 antibodies. Vertical lines have been inserted to indicate repositioned gel lanes. (C) Proliferative capacities of CM and EM cells as measured by H3 thymidine incorporation (left). Memory cells were transduced with increasing amount of wt and C381A CTP-c-Cbls fusion proteins (AU: arbitrary units), stimulated for 16 hours with anti-CD3 (5 μg/mL) and -CD28 (1 μg/mL) antibodies and their proliferation measured after 3 days. CTP-mock transduction was used as negative control ( ). Shown are data from 4 cumulated subjects (right). (D) Effect of CTP-c-Cbls transduction on memory T-cell cytokine production in response to the stimulation described for panel C. Cytokine production was normalized to non-transduced cells and CTP-mock transduction used as negative control. Significant changes (P < .05) in the functions of transduced cells relative to control and mock-transduced cells are indicated (★) as well as P values for significant differences between wt and C381A mutant c-Cbls. (E) Effect of CTP-c-Cbl on the proliferation threshold of purified EM cells stimulated as described for panel C. One representative example out of 3 is shown. (F) Impact of CTP-c-Cbl transduction on the proliferation of EM cells in response to IL-2, anti-CD3 (3 μg/mL) and -CD28 (1 μg/mL) antibodies. Each set of linked dots correspond to a distinct subject and significant changes (P < .05) between IL-2–treated and untreated cells are shown. (G) Quantification of the amounts of CTP-c-Cbls transduced in memory T cells relative to endogenous expression of c-Cbl following 16 hours of activation.
). Shown are data from 4 cumulated subjects (right). (D) Effect of CTP-c-Cbls transduction on memory T-cell cytokine production in response to the stimulation described for panel C. Cytokine production was normalized to non-transduced cells and CTP-mock transduction used as negative control. Significant changes (P < .05) in the functions of transduced cells relative to control and mock-transduced cells are indicated (★) as well as P values for significant differences between wt and C381A mutant c-Cbls. (E) Effect of CTP-c-Cbl on the proliferation threshold of purified EM cells stimulated as described for panel C. One representative example out of 3 is shown. (F) Impact of CTP-c-Cbl transduction on the proliferation of EM cells in response to IL-2, anti-CD3 (3 μg/mL) and -CD28 (1 μg/mL) antibodies. Each set of linked dots correspond to a distinct subject and significant changes (P < .05) between IL-2–treated and untreated cells are shown. (G) Quantification of the amounts of CTP-c-Cbls transduced in memory T cells relative to endogenous expression of c-Cbl following 16 hours of activation.
Transduction of CTP-c-Cbl in EM cells results in lower functional response. (A) Limited amounts of CTP-GFP were transduced in memory cells and the transduction efficiency in CM and EM cells assessed by FACS. Thin and bold lines depict non-transduced and transduced cells, respectively. (B) Detection of c-Cbl following SDS-PAGE and immunoblotting of lysates of memory CD4 T cells previously transduced with wt or C381A CTP-c-Cbls and activated overnight with anti-CD3 and -CD28 antibodies. Vertical lines have been inserted to indicate repositioned gel lanes. (C) Proliferative capacities of CM and EM cells as measured by H3 thymidine incorporation (left). Memory cells were transduced with increasing amount of wt and C381A CTP-c-Cbls fusion proteins (AU: arbitrary units), stimulated for 16 hours with anti-CD3 (5 μg/mL) and -CD28 (1 μg/mL) antibodies and their proliferation measured after 3 days. CTP-mock transduction was used as negative control ( ). Shown are data from 4 cumulated subjects (right). (D) Effect of CTP-c-Cbls transduction on memory T-cell cytokine production in response to the stimulation described for panel C. Cytokine production was normalized to non-transduced cells and CTP-mock transduction used as negative control. Significant changes (P < .05) in the functions of transduced cells relative to control and mock-transduced cells are indicated (★) as well as P values for significant differences between wt and C381A mutant c-Cbls. (E) Effect of CTP-c-Cbl on the proliferation threshold of purified EM cells stimulated as described for panel C. One representative example out of 3 is shown. (F) Impact of CTP-c-Cbl transduction on the proliferation of EM cells in response to IL-2, anti-CD3 (3 μg/mL) and -CD28 (1 μg/mL) antibodies. Each set of linked dots correspond to a distinct subject and significant changes (P < .05) between IL-2–treated and untreated cells are shown. (G) Quantification of the amounts of CTP-c-Cbls transduced in memory T cells relative to endogenous expression of c-Cbl following 16 hours of activation.
). Shown are data from 4 cumulated subjects (right). (D) Effect of CTP-c-Cbls transduction on memory T-cell cytokine production in response to the stimulation described for panel C. Cytokine production was normalized to non-transduced cells and CTP-mock transduction used as negative control. Significant changes (P < .05) in the functions of transduced cells relative to control and mock-transduced cells are indicated (★) as well as P values for significant differences between wt and C381A mutant c-Cbls. (E) Effect of CTP-c-Cbl on the proliferation threshold of purified EM cells stimulated as described for panel C. One representative example out of 3 is shown. (F) Impact of CTP-c-Cbl transduction on the proliferation of EM cells in response to IL-2, anti-CD3 (3 μg/mL) and -CD28 (1 μg/mL) antibodies. Each set of linked dots correspond to a distinct subject and significant changes (P < .05) between IL-2–treated and untreated cells are shown. (G) Quantification of the amounts of CTP-c-Cbls transduced in memory T cells relative to endogenous expression of c-Cbl following 16 hours of activation.
Transduction of EM cells with CTP-c-Cbls impaired significantly their proliferation in a dose dependent manner whereas CTP-mock had no effect (Figure 7C). However, wt CTP-c-Cbl was 3-fold more potent (P < .05) than C381A mutant (Figure 7C). Importantly, transduction of EM cells with CTP-c-Cbl increased their threshold for proliferation (Figure 7E) and their sensitivity to IL-2 (Figure 7F) thus restoring, at least partially, the functional phenotype of CM cells (Figure 6B right).
In addition, wt CTP-c-Cbl specifically and significantly impaired IFN-γ and IL-2 production by 46% and 40%, respectively while C381A mutant was significantly less efficient (P < .05; Figure 7D). In contrast to cell proliferation, cytokine production was not further lowered by increasing the amount of wt CTP-c-Cbl transduced in memory cells (data not shown). Remarkably, these effects were obtained by increasing c-Cbl content of EM cells using CTP-c-Cbl transduction (+55% in wt or C381A mutant CTP-c-Cbl, Figure 7G) to that of CM cells (+34% average increase in CM cells as compared with EM cells, Figures 2B,4A) thus matching c-Cbl content in CM and transduced EM cells (data not shown). These data demonstrate that c-Cbl expression controls the functional phenotype of memory T cells through a mechanism mainly dependent of its E3 ubiquitin ligase activity, as evidenced by the weaker impact of enzymatically deficient c-Cbl C381A on EM cell functions.
Discussion
This is the first study reporting accurate ex vivo quantification of signaling components in human memory T-cell subsets and demonstrating that the functional response of these subsets depends on specific variations in the expression levels of signaling proteins downstream of the TCR. This has been possible due to the implementation of RPP array approach for protein profiling in ex vivo isolated T cells. Furthermore, cytosolic transduction ofT cells represents an attractive alternative strategy to virus-mediated alteration of gene expression yet inadequate to the study of memory T-cell activation. Combined use of RPP arrays and cytosolic T-cell transduction, shown here for the first time, represents a powerful strategy to quantitatively analyze and functionally assess that reduced expression of c-Cbl in EM CD4 T cells critically lowers the threshold of their functional responses. Furthermore, in EM cells, reduced expression of c-Cbl is significantly associated with lower expression of c-Cbl kinases (Fyn and Syk) and co-regulated expression of PI3K, SLP-76 and LAT (Figures 2,Figure 3,Figure 4–5). Of interest, the expression levels of the related kinases Lck and ZAP70, that do not phosphorylate c-Cbl, remained unchanged. Importantly, inactivation of c-Cbl was reported to alter the functions of PI3K,32 SLP-76,41 and LAT41,42 and consequently to interfere with T-cell development and activation. The regulation of the expression of these proteins is likely to occur posttranscriptionally since the transcription of the corresponding genes was not reported as significantly altered in CD4 memory T-cell subsets.14,15
Moreover, the observed subject heterogeneity along CD3ζ/Syk/LAT pathway (Figure 3A) is likely to reflect inaccurate cell subset delineation based on partial phenotypical and functional correlates. The inclusion of CCR7 as a subset marker is justified due to the very clear correlation with cytokine secretion.3-5 However, more recently CD4 cells were shown to be distributed among 6 major compartments with EM cells (CD28+, CCR7−, CD45RA−) having the greatest capacity to produce IL-2, IFN-γ and TNF-α in response to staphylococcal enterotoxin B (SEB) stimulation and showing proliferation when cocultured with autologous monocytes and exposed to PHA.43 In keeping with previous studies,3,5 our data show that in response to CD3 and CD28 stimulation, EM cells have a higher capacity for cytokine production and a lower threshold for proliferation than CM cells (Figure 6). CM cells produced IL-2 in response to EBV or CMV antigens34,43 or when triggered with anti-CD3 antibodies and PHA,3 but not in response to anti-CD3 and anti-CD28 antibodies (Figure 6). These results indicate that IL-2 production in CM cells requires additional triggering to TCR and CD28.
Our results are in marked contrast with CD4 peripheral T cells from c-Cbl KO mice, which show a higher functional threshold and are likely to arise from altered thymic selection and to reflect unbalanced c-Cbl and Cbl-b expression.26 While c-Cbl and Cbl-b have been reported to be functionally redundant,26 their specific substrates are cell type–dependent and their regulatory effects controlled by their relative abundance.24 Indeed, the functional phenotype of c-Cbl KO thymocytes and peripheral cells differed dramatically, consistent with high expression of c-Cbl in thymocytes and preferential expression of Cbl-b in mature CD4 T cells. c-Cbl KO thymocytes exhibit hyperphosphorylation of ZAP-70 and ERK1/2, and TCR signaling uncoupled from CD4 co-stimulation requirements leading to increased proliferation,44 intracellular calcium mobilization40 and TCR/CD3ζ degradation.27 Finally, positive selection is enhanced in CD4 c-Cbl KO thymocytes that express higher levels of TCR, CD3, CD4, CD5 and CD69 at their surface.40,44 We report similar expression of Cbl-b in CM and EM cells and reduced expression of c-Cbl in EM cells, leading to lower proliferation threshold, increased cytokine production but comparable intracellular calcium mobilization and expression of TCR/CD3, CD4 and CD28 (Figures 4,6,S1). Consistent with our data, cells from double Cbl KO mice show phenotype and functions of EM cells,26 suggesting that the balance between c-Cbl and Cbl-b expression might control the functional phenotype of human memory cells. Importantly, we report that IL-2 impacted more strongly the proliferation of CM cells than EM cells (Figure 6B) and that IL-2 treatment reversed the proliferation defect of EM cells induced by CTP-c-Cbl transduction (Figures 6B,7F). These data underline the functional plasticity of memory cells and indicate for the first time that c-Cbl regulates the functional phenotype of these subsets. Finally, the weaker impact of enzymatically deficient CTP-c-Cbl C381A mutant on EM cell proliferation and cytokine production (Figure 7) is supportive of a mechanism mainly mediated by the RING finger domain and likely to involve multiple targets. Transduction of larger amounts of c-Cbl in memory cells failed to further reduce their cytokine production, suggesting that besides Cbl, TCR engagement triggers additional negative regulatory pathways for the fine tuning of TCR signaling.
The functional significance of the decreased Syk expression in EM cells (Figure 2) remains to be determined. Increased expression of Syk in human CD4 effector cells differentiated in vitro was found to be associated with TCR signaling through FcRγ/Syk complex in place of CD3ζ/ZAP-70 in naive cells.22 Along the same lines, ZAP-70 deficient patiens expressing high level of Syk show impaired CD3-mediated proliferation and IL-2 secretion.42 In EM cells, reduced expression of Syk and Fyn (Figure 2) which coordinately bind and phosphorylate c-Cbl45 may impair c-Cbl phosphorylation and its E3 ligase activity.
In conclusion, this work demonstrates for the first time the pivotal role of c-Cbl in the control of the heterogeneity and the plasticity of the functional phenotype of human memory CD4 T-cell subsets. In addition, regulating c-Cbl expression in memory cells may be a key control point for attenuating TCR-mediated signals and promoting long term survival of CM cells. Since inactivating c-Cbl mutations observed in acute myeloid leukemia were associated to increased proliferation46,47 and reduced expression of Cbl occurred in HIV-positive patients,48 better understanding of Cbl expression and action in memory cells might contribute to the development of novel therapeutic approaches to control lymphocyte functions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Isabelle Cohen-Salmon and Jérome Thomas for technical assistance, Dr Kai Johnsson (EPFL, Lausanne, Switzerland) for use of Axon scanner, and Dr Patrice Waridel (Center for Integrative Genomics, Lausanne, Switzerland) for mass spectrometry analysis. We are grateful to Dr Jan Hofsteenge (Friedrich Miescher Institute, Basel, Switzerland) for providing us with anti–RNAse-2/RNase-2. We acknowledge Dr P. Schneider (University of Lausanne, Switzerland), Dr W. Langdon (School of Surgery and Pathology, University of Western Australia, Crawley, Australia), Dr S. Lipkowitz (Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD), and Dr Y.-S. Bae (Sungkyunkwan Univerity, Suwon, Korea) for pET21a, Cbls, and CTP plasmids, respectively.
This work was supported by grants from the Swiss National Foundation (M.-A.D., project 31.00AO-105702) and the Novartis Foundation (M.Q.).
Authorship
M.-A.D., N.C.B., and D.R. conceived and designed the experiments; N.C.B., M.-A.D., and J.W. performed the experiments; M.-A.D., N.C.B., M.Q., C.R., and K.H. analyzed and interpreted the data; S.P., F.S., and G.P. performed statistical analysis; K.H., G.P., and M.Q. contributed reagents/materials/analysis tools; and M.-A.D. and N.C.B. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie-Agnès Doucey, Protein Analysis Facility, Center for Integrative Genomics, Le Génopode, CH1015 Lausanne, Switzerland; e-mail: Marie-Agnes.Doucey@unil.ch.