Abstract
The host responds to lymphopenic environments by acute homeostatic proliferation of T lymphocytes, which acquire phenotypes similar to memory cells. Using T-cell knockout (KO) mice adoptively reconstituted with splenocytes from immunologically naive mice, we examined the immune responses of an immune system derived from homeostatically proliferating (HP) T cells. HP cells mounted relatively normal acute CD8 T-cell responses to lymphocytic choriomeningitis virus (LCMV), but with altered T-cell receptor (TCR) repertoires, and they became functional memory cells capable of recall responses. Although homeostatic proliferation does not normally fully restore T-cell numbers, the CD8+ T-cell pool was completely restored in T-cell KO mice after LCMV infection. CD4 T-cell responses were lower and not fully restored but seemed sufficient to allow for complete differentiation of CD8 memory T cells. The LCMV-immune HP mouse had an immune repertoire heavily biased with LCMV epitope-specific T cells with oligoclonal expansions. LCMV-immune HP mice had reduced cross-reactive and non–cross-reactive CD8 T-cell responses when challenged with a T cell–cross-reactive virus. Thus, whereas an HP immune system is capable of mounting relatively normal acute and memory CD8 T-cell responses, the narrowing of the T-cell repertoire may reduce immune responses to subsequently encountered pathogens.
Introduction
Acute homeostatic proliferation of naive CD8+ T cells occurs in hosts rendered lymphopenic by genetic deficiency, virus infection, radiotherapy, or chemotherapy.1,2 Homeostatically proliferating naive T cells require the interaction of self-antigens (Ags) presented by major histocompatibility class (MHC) class I molecules and growth factors, such as IL-7 and IL-15.3 When naive T cells are transferred into Rag−/−, CD3ϵ−/−, or irradiated mice, they respond by homeostatic proliferation, although their absolute numbers do not reach the same levels as seen in normal mice,4-6 and the reason for that is unknown. Homeostatic proliferation converts the phenotype of naive T cells to “memory-like” T cells, which express the memory marker CD44 and produce IFNγ on T-cell receptor (TCR) stimulation, but these memory-like cells have not gone through the differentiation process of bona fide foreign antigen-specific memory cells, and an important question is whether these memory-like homeostatically proliferating (HP) cells can respond to viral infections like naive cells or bona fide memory cells
Adding to this concern for generating a successful antiviral response is the phenomenon that only a subset of the lymphocyte population is successful at competing for homeostatic proliferation, and the TCR repertoire narrows as a consequence.7 Normally, the diversities in CD8 TCR repertoires allow for efficient immune responses against a wide variety of pathogens. Skewed oligoclonal TCR repertoires have been found in lymphopenia-associated human diseases, such as Wiskott-Aldrich syndrome, Omenn syndrome, complete DiGeorge syndrome, aplastic anemia, and ataxia telangiectasia.8-12 Narrow oligoclonal expansions of CD8 T cells are also found in aged healthy persons, some of whom bear high frequencies of cytomegalovirus-specific T-cell clones.13 This narrowing of the repertoire in aged persons may impair responses to influenza virus vaccines,14 and studies in mice have correlated decreased resistance to herpes simplex virus type 1 (HSV-1) with reduced complexity of the TCR repertoire.15 The T-cell repertoire in aged subjects contains bona fide memory cells, which had previously proliferated and differentiated in response to pathogens, and an uncertain proportion of memory-like cells that are the products of homeostatic proliferation. It is uncertain whether these HP cells function normally in response to pathogens and whether an HP repertoire would be sufficiently polyclonal to provide normal antiviral immunity with durability and flexibility to respond to additional pathogens.
Memory CD8+ T-cell populations may cross-react with new infectious agents, alloantigens, or self-antigens,16 and T-cell cross-reactivity between pathogens can result in heterologous immunity. In humans, cross-reactivities of epitope-specific CD8 T cells between Epstein-Barr virus and influenza virus, hepatitis C virus and influenza virus, papillomavirus type 16 and coronavirus, and HIV and influenza virus have been reported.17-20 Similar cross-reactivities are found in mouse model systems. Lymphocytic choriomeningitis virus (LCMV)–immune mice partially control Pichinde virus (PV) infection because of a cross-reactive CD8 T-cell response to partially homologous NP-205 epitopes, which share 6 of 8 amino acids.21 These cross-reactive responses become immunodominant with a narrow TCR repertoire and provide some protective immunity, in conjunction with enhanced immunopathology.22 How these cross-reactive oligoclonal T-cell responses would evolve in a host whose repertoire is reduced first by homeostatic proliferation and later by a virus infection is an additional unresolved question.
In this study, we show that the CD8+ T-cell numbers are completely restored in T-cell knockout (KO) mice after LCMV infection and that the generation of functional LCMV-specific acute and memory CD8 T cells is normal during the primary and secondary responses. However, LCMV-specific memory CD8 T cells have altered TCR repertoires and accumulate at high frequencies in the HP memory pool, resulting in a decreased T-cell repertoire available to combat other pathogens. This reduced repertoire is associated with reduced T-cell responses against a subsequently challenged cross-reactive virus.
Methods
Mice
C57BL/6 male mice (Ly5.2+) were purchased from The Jackson Laboratory (Bar Harbor, ME) and were used at 8 to 12 weeks of age. B6.SJL (Ly5.1+) mice, congenic to C57BL/6 mice except for Ly5, were from Taconic Farms (Germantown, NY) at 4 to 5 weeks of age. B6.129P2 TcrbtmlMomTcrdtmlMom (αβγδ KO) mice (T-cell KO) were bred within the Department of Animal Medicine at the University of Massachusetts Medical School (UMMS). All of these mice were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of UMMS.
Adoptive transfer of splenocytes
For reconstituting T-cell KO mice, splenocytes from naive Ly5.1 mice were harvested, suspended in HBSS at 3.5 × 107 cells/200 μL, and then adoptively transferred by intravenous injection into T-cell KO mice. Mice were considered reconstituted by 28 days after transfer. Donor cells included splenocytes from Ly5.1 LCMV-immune mice or from T-cell KO mice that had been reconstituted with naive splenocyte populations, or reconstituted naive T-cell KO mice that had been infected with LCMV and allowed to generate memory cells; 1.5 × 107 cells, 5 × 106 cells, or 3 × 106 cells were adoptively transferred into naive Ly5.2 mice.
Virus infection
LCMV (Armstrong strain) and PV (AN3739 strain) were propagated in BHK21 cells.23 Recombinant vaccinia virus expressing the LCMV glycoprotein (VV-GP) was propagated in LTK cells (L-929 cells lacking thymidine kinase).24 For primary infection, 28-day–reconstituted T-cell KO mice or Ly5.1 naive mice were infected intraperitoneally with 5 × 104 plaque-forming units (PFU) of LCMV or 2 × 107 PFU of PV. Mice were considered immune after 6 weeks. For recall responses, LCMV-immune mice were infected intraperitoneally with 2 × 106 PFU of LCMV or 106 PFU of VV-GP. In some cases, LCMV-immune splenocytes from either reconstituted T-cell KO or Ly5.1 mice were adoptively transferred into naive mice (Ly5.2), which were then inoculated intraperitoneally with 5 × 104 PFU of LCMV. For heterologous virus challenge, LCMV-immune mice were infected intraperitoneally with 2 × 107 PFU of PV.
Tetramer and cell-surface staining of LCMV-specific CD8 T cells
Spleen or blood leukocytes were harvested from HP or wild-type (WT) mice, and cells were stained with MHC-class I peptide H2Db tetramers specific for LCMV GP-33-41, NP-396-404, or GP-276-286 or H2Kb tetramers specific for LCMV GP-34-41, LCMV NP-205-212, PV NP-38-45, or PV NP-205-212 for 30 minutes and then with monoclonal antibodies (mAbs) specific for CD8, CD44, CD122, CD62L, CD11a, CD27, CD49d, KLRG1, Ly6C, and CD127 added into the wells for another 30 minutes. All mAbs were purchased from BD PharMingen (San Diego, CA), except for mAbs specific for CD127, CD49d, and KLRG1, which were purchased from eBioscience (San Diego, CA) and Southern Biotechnology Associates (Birmingham, AL).
Intracellular staining of LCMV-specific T cells
Spleen leukocytes were incubated with 5 μM synthetic peptide, 10 U/mL human recombinant IL-2, and 0.2 μL GolgiPlug (BD PharMingen) for 5 hours at 37°C. After FcR blockade (anti-FcRII), the cells were stained with fluorescently labeled mAbs against CD8, CD44, and Ly5.1 for 30 minutes at 4°C. After staining, cells were permeabilized with Cytofix/Cytoperm for 20 minutes at 4°C and then stained with mAbs to IFNγ or Bcl-2 in perm/wash buffer (BD PharMingen) for 25 minutes at 4°C. Finally, cells were washed and resuspended in FACS buffer (phosphate-buffered saline with 2% fetal calf serum and 0.1% sodium azide) and analyzed by flow cytometry.
TCR Vβ analysis of LCMV-specific CD8 T cells
Splenocytes were harvested from reconstituted T-cell KO or Ly5.1 mice 8 days after LCMV primary infection or 7 days after infection with PV. Cells were stained with tetramers for 30 minutes, and mAbs specific for CD8, CD44, and FITC-labeled Vβ-specific mAbs (Vβ2-14; BD PharMingen Kit) were added into the wells for the other 30 minutes. Data represent the percentage of tetramer-positive cells staining with the Vβ mAb. The percentage for “other Vβ” was calculated by subtracting the sum of individual Vβ from 100%.
Results
Primary responses of homeostatically proliferating T cells
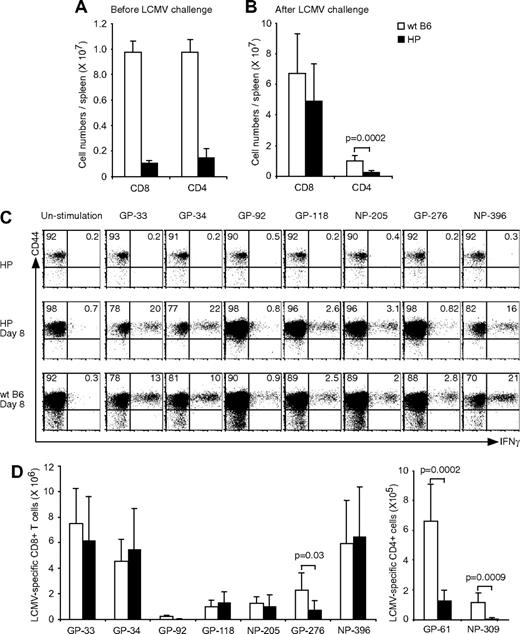
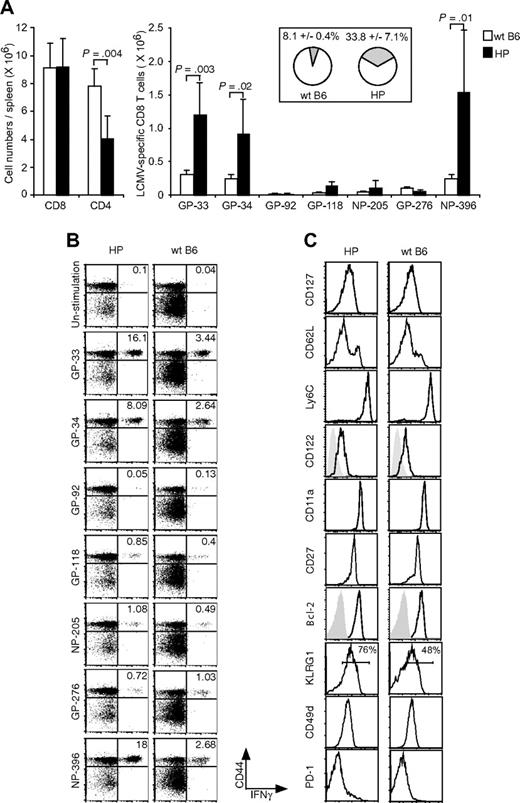
To investigate the primary T-cell response in an environment whose T-cell immune system is a consequence of homeostatic proliferation, splenocytes from immunologically naive mice were transferred into T-cell KO mice. After 4 to 5 weeks of reconstitution, the spleens contained 2% to 3% CD8 and CD4 T cells, and the absolute cell numbers are shown in Figure 1A. This result is consistent with previous studies showing that T-cell numbers after homeostatic reconstitution do not reach the levels seen in normal WT Ly5.1 B6 mice, in which CD8 and CD4 T cells are at levels of 10% to 20%.25 Despite these differences in starting populations, the numbers of CD8+ T cells between WT and HP mice were nearly equal by 8 days after LCMV challenge, although the CD4+ T-cell numbers in HP mice remained at a statistically significant lower level (P < .001; Figure 1B).
Primary T-cell response in HP mice. (A) Splenocytes were harvested, and CD8 or CD4 numbers (means ± SD) were counted from HP or WT naive mice. (B) Splenocytes were harvested, and CD8 or CD4 numbers were counted from LCMV-infected HP (n = 7) or WT naive mice (n = 5) at day 8. (C) Splenocytes were harvested and stimulated with LCMV-specific peptides for 5 hours. Dot plots show CD44+IFNγ+LCMV-specific CD8 T cells from a representative uninfected HP mouse, infected HP mouse, or infected WT mouse. Numbers refer to percentage of cells in designated quadrants. (D) Splenocytes from LCMV-infected mice were harvested and stimulated with LCMV-specific peptides for 5 hours. After stimulation the numbers of CD8+CD44+IFNγ+ (left; means ± SD) or CD4+CD44+IFNγ+ (right; means ± SD) cells recognizing each peptide were counted. This is a representative result from 3 repeated experiments with 2 to 7 mice per group. P value was analyzed by t test.
Primary T-cell response in HP mice. (A) Splenocytes were harvested, and CD8 or CD4 numbers (means ± SD) were counted from HP or WT naive mice. (B) Splenocytes were harvested, and CD8 or CD4 numbers were counted from LCMV-infected HP (n = 7) or WT naive mice (n = 5) at day 8. (C) Splenocytes were harvested and stimulated with LCMV-specific peptides for 5 hours. Dot plots show CD44+IFNγ+LCMV-specific CD8 T cells from a representative uninfected HP mouse, infected HP mouse, or infected WT mouse. Numbers refer to percentage of cells in designated quadrants. (D) Splenocytes from LCMV-infected mice were harvested and stimulated with LCMV-specific peptides for 5 hours. After stimulation the numbers of CD8+CD44+IFNγ+ (left; means ± SD) or CD4+CD44+IFNγ+ (right; means ± SD) cells recognizing each peptide were counted. This is a representative result from 3 repeated experiments with 2 to 7 mice per group. P value was analyzed by t test.
P14 transgenic CD8+ T cells specific for an LCMV GP-33 peptide can recognize a self-peptide derived from dopamine-mono-oxygenase, an enzyme expressed in the adrenal medulla.26 Because homeostatic proliferation of T cells requires their interaction with self-peptides on MHC, we questioned whether significant numbers of HP cells would react with LCMV peptides before LCMV challenge in vivo. As shown in Figure 1C (top row), memory-like HP cells did not respond to in vitro stimulation with various LCMV-encoded peptides, including GP-33. Reconstituted T-cell KO mice were challenged with LCMV, and more than 90% of the CD8 (Figure 1C) or CD4 T cells (not shown) expressed high levels of the effector/memory marker CD44. The absolute LCMV-specific T-cell numbers were measured by IFNγ production after peptide stimulation (Figure 1C,D). The total numbers of LCMV-specific CD8+ T cells, with the exception of those specific for GP276, were similar in the reconstituted HP mice and WT mice at day 8 after infection. However, numbers of LCMV-specific CD4+ were significantly lower in the HP mice. Thus, the CD4 T cells were recruited into the LCMV-specific response much less effectively than the CD8 T cells.
The T-cell response was sufficient to clear virus in the HP mice, as plaque assays were run on liver, kidney, and spleen samples harvested at day 8 after infection, and no PFUs were found (data not shown).
TCR Vβ skewing in epitope-specific CD8+ HP cells
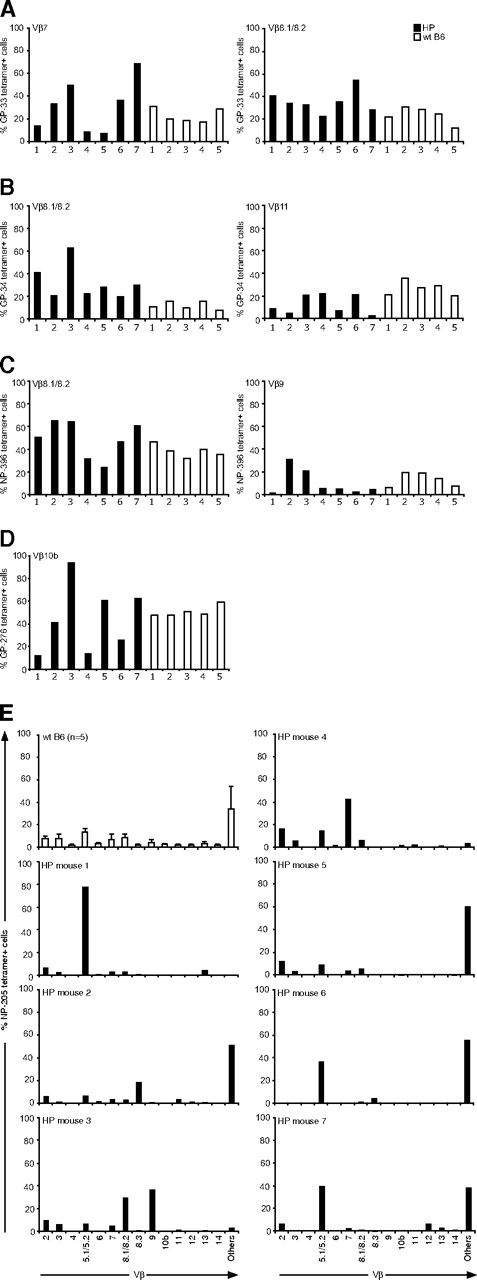
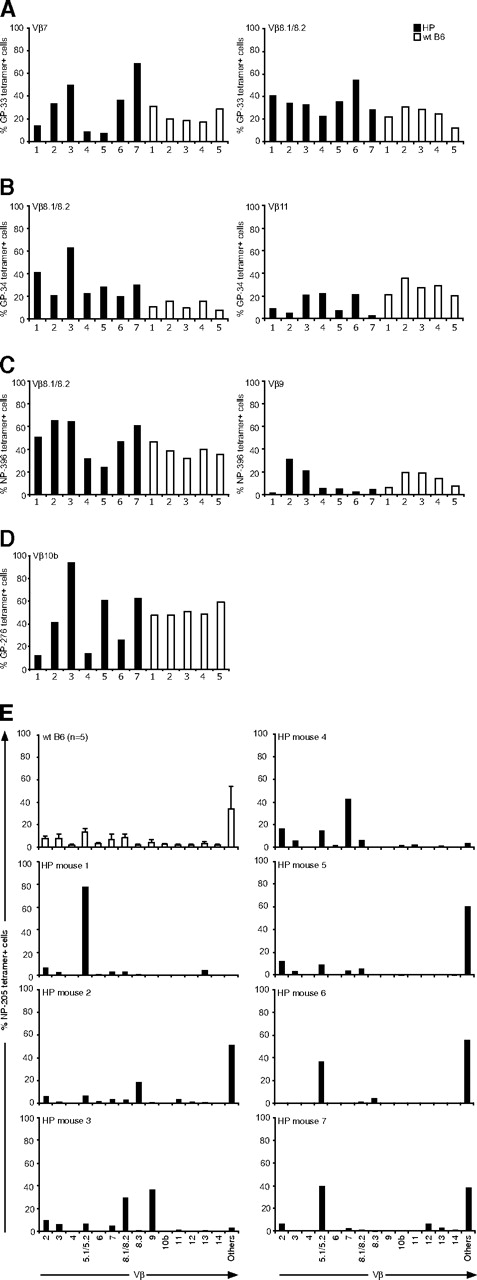
Although the number of epitope-specific CD8+ T cells in the HP mice was relatively normal in response to LCMV infection, except for those specific to GP-276, we questioned whether HP and WT virus-specific T cells differed in other characteristics. We first determined whether the LCMV-specific T-cell repertoire generated in HP mice would resemble the repertoire seen in WT mice. Distinct Vβ TCR usage correlates with T-cell responses to distinct LCMV-encoded peptides,27 and we focused our analyses on the Vβ TCR usage of T cells defined by epitope-specific tetramers at day 8 after infection. The percentages of epitope-specific T cells that stained with mAb specific for their predicted Vβ TCR are shown in Figure 2A-D. Although there was some variation, the proportion of epitope-specific T cells coexpressing a given Vβ TCR was similar among 5 WT mice (open bars) for 4 different epitopes. This contrasted with the repertoires from the HP mice, which had far more variation between individual animals (filled bars). This variation was seen for each epitope, but perhaps most clearly for GP-276–specific T cells, whose response was impaired overall (Figure 1). Figure 2D shows that, although approximately half of the GP-276–positive T cells from all WT mice stained with mAb to the normally dominant Vβ10b, the Vβ10b-expressing GP-276–specific HP cells ranged from 10% to 90%. Marked variations in Vβ TCR responses were also seen in HP mice for GP-33 (Vβ7, Vβ8.1/8.2), GP-34 (Vβ8.1/8.2, Vβ11), and NP-396 (Vβ8.1/8.2, Vβ9; Figure 2A-C). This indicates that an HP immune system uses altered TCR repertoires to mount an immune response.
TCR Vβ repertoires of LCMV-specific CD8 T cells. Splenocytes from individual LCMV-infected mice (7 T-cell KO HP mice, ■; 5 WT mice, □) at day 8 were harvested. Cells were stained with MHC class I tetramers specific for LCMV DbGP-33 (A), KbGP-34 (B), DbGP-276 (C), DbNP-396 (D), or for KbNP-205 (E) and then stained with surface makers and Vβ chains. The percentage of Vβ-positive cells was analyzed by gating on tetramer-positive cells.
TCR Vβ repertoires of LCMV-specific CD8 T cells. Splenocytes from individual LCMV-infected mice (7 T-cell KO HP mice, ■; 5 WT mice, □) at day 8 were harvested. Cells were stained with MHC class I tetramers specific for LCMV DbGP-33 (A), KbGP-34 (B), DbGP-276 (C), DbNP-396 (D), or for KbNP-205 (E) and then stained with surface makers and Vβ chains. The percentage of Vβ-positive cells was analyzed by gating on tetramer-positive cells.
The LCMV NP-205 response is subdominant, and our molecular studies have shown that it is mostly associated with Vβ16+ T cells, against which there is no mAb available.23 Figure 2E shows, expectedly, that most of the NP-205–specific T cells from WT mice cannot be defined by the available antibodies and are listed as “others.” A repertoire analysis in 7 infected HP mice showed marked variations between mice, with several skewing toward non-Vβ16 TCR detectable by antibodies. The repertoire in mouse 1 was predominantly Vβ5.1/5.2, in mouse 3 it was Vβ8.1/8.2, Vβ9, in mouse 4 is was Vβ7, and in mice 6 and 7 Vβ5.1/5.2 and “others” seemed to be codominant. These studies collectively indicate a selection of different Vβ usage in LCMV-specific CD8+ T cells in HP mice.
Restoration of lymphocyte number and deposition of memory cells in immune mice
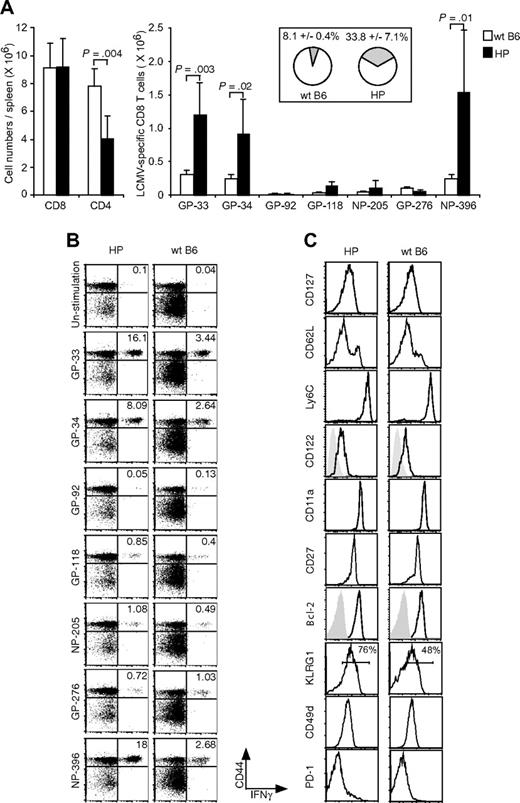
CD4+ T cells are involved in the generation and functional maintenance of conventional CD8+ memory T cells.28 Because total and LCMV-specific CD4+ T cells were at low levels at day 8 of LCMV acute infection in HP mice, we questioned whether these mice generated and maintained a normal pool of functional memory T cells after resolution of the acute infection. Our first observation was that, although the lymphocyte number in uninfected HP mice was well below normal WT mouse levels (Figure 1A,B), the total CD8 T-cell numbers were similar between the 2 types of mice in the resting immune state after resolution of the LCMV infection (Figure 3A). Thus, a virus infection can equilibrate CD8 T-cell numbers in a lymphopenic environment. However, the total number of CD4 T cells, although increased, was still approximately 50% lower in HP than in WT mice (P = .004).
Accumulation of memory LCMV-specific CD8 T cells in HP mice. (A) Splenocytes were harvested from HP and WT mice 6 months after infection and then stimulated with LCMV-specific peptides for 5 hours. After stimulation, CD8 and CD4 T cells (left; means ± SD) and CD8+CD44+IFNγ+ cells recognizing each peptide were counted (right; means ± SD). The total percentage of LCMV-specific memory CD8 T cells for GP-33, GP-92, GP-118, NP-205, GP-276, and NP-396 in the CD8 T-cell resting memory pool is shown in the right panel (means ± SD). This is a representative result from 3 repeated experiments with 2 to 5 mice per group. (B) Dot plots show LCMV-specific CD8 T cells from a representative LCMV-immune HP mouse and a WT mouse. Numbers refer to percentage of cells in designated quadrants. (C) The expression of surface markers on NP-396 tetramer-positive cells. Splenocytes were harvested from HP and WT mice 2 to 6 months after LCMV infection. Staining is shown for gated tetramer-positive cells (NP-205, GP-276, and NP-396), except for stains for CD27 and Bcl-2, in which CD8+ NP-396–specific IFNγ+ cells were gated. Similar expression patterns of surface markers and Bcl-2 on NP-396 CD8 T cells harvested from HP and WT mice 2 to 6 months after infection were seen. This is a representative result of NP-396–specific cells from 4 experiments with 1 to 2 mice per group.
Accumulation of memory LCMV-specific CD8 T cells in HP mice. (A) Splenocytes were harvested from HP and WT mice 6 months after infection and then stimulated with LCMV-specific peptides for 5 hours. After stimulation, CD8 and CD4 T cells (left; means ± SD) and CD8+CD44+IFNγ+ cells recognizing each peptide were counted (right; means ± SD). The total percentage of LCMV-specific memory CD8 T cells for GP-33, GP-92, GP-118, NP-205, GP-276, and NP-396 in the CD8 T-cell resting memory pool is shown in the right panel (means ± SD). This is a representative result from 3 repeated experiments with 2 to 5 mice per group. (B) Dot plots show LCMV-specific CD8 T cells from a representative LCMV-immune HP mouse and a WT mouse. Numbers refer to percentage of cells in designated quadrants. (C) The expression of surface markers on NP-396 tetramer-positive cells. Splenocytes were harvested from HP and WT mice 2 to 6 months after LCMV infection. Staining is shown for gated tetramer-positive cells (NP-205, GP-276, and NP-396), except for stains for CD27 and Bcl-2, in which CD8+ NP-396–specific IFNγ+ cells were gated. Similar expression patterns of surface markers and Bcl-2 on NP-396 CD8 T cells harvested from HP and WT mice 2 to 6 months after infection were seen. This is a representative result of NP-396–specific cells from 4 experiments with 1 to 2 mice per group.
Although the total numbers of CD8 T cells were similar in the resting memory state, the frequencies of CD8+ T cells specific to the immunodominant LCMV epitopes GP-33, GP-34, and NP-396 were 4- to 6-fold higher in the immune HP versus immune WT mice, as determined by peptide-induced intracellular IFNγ production (Figure 3A,B). By totaling the percentage of T cells specific for GP-33, GP-92, GP-118, NP-205, GP-276, and NP-396 (excluding GP-34, which is a subset of GP-33 in IFNγ assays), we showed that 33.8% (± 7.1%, n = 5) of the CD8 T cells were LCMV-specific in HP memory mice, whereas only 8.1% (± 0.4%, n = 5) were LCMV-specific CD8 T cell in WT memory mice. Figure 3B shows representative dot plots documenting the frequencies of LCMV-specific CD8 T cells from 1 LCMV-immune HP mouse and 1 immune WT mouse. These data suggest that an HP environment fills with virus-specific memory cells as an infection resolves.
To determine whether the antigen-specific memory CD8 T cells from HP mice had properties similar to memory cells generated in WT mice, we analyzed their expression of memory cell markers. As shown in Figure 3C (right), conventional NP-396 tetramer-positive CD8 memory T cells are CD127hi, CD62hi/lo, Ly6Chi, CD122hi, CD11ahi, CD27hi, and Bcl-2hi. LCMV-specific CD8 memory T cells from reconstituted T-cell KO HP mice similarly expressed these markers (Figure 3B left). We also analyzed KLRG1, CD49d (integrin α4), and PD-1, which are associated with oligoclonal expansions of CD8+ T cells in aged or lymphopenic hosts,13,29,30 and found that tetramer-positive CD8+ T cells were CD49dhi and PD-1–negative in both WT and HP immune mice. KLRG1hi memory cells are reported to have poor proliferative ability, and the proportion of those cells declines over time.31 As shown in Figure 3C, 76% of tetramer-positive cells were KLRG1hi in the HP host, in comparison to 48% of the cells in the WT. The significance of this difference is unclear, but we questioned whether residual virus contributed to this difference and could not detect LCMV PFU in liver, kidney, and spleen at 5 months after LCMV infection. Taken together, the generation of CD8 memory T cells appeared either normal or enhanced in this low CD4+ T-cell HP environment.
Recall response in reconstituted T-cell KO host
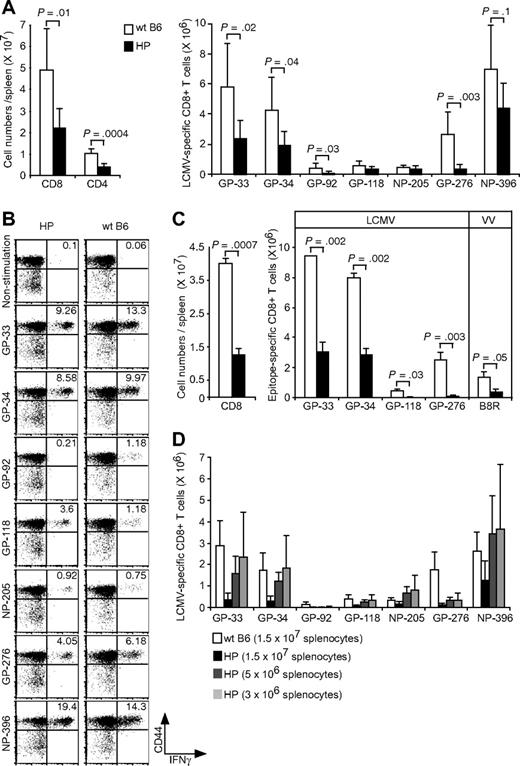
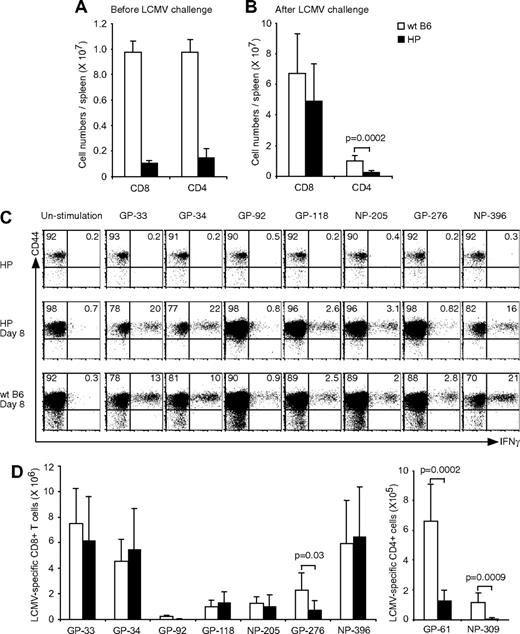
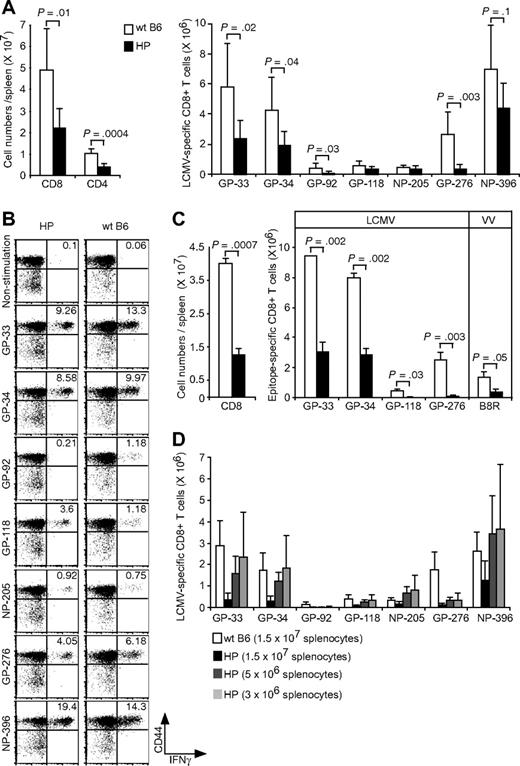
We questioned whether recall responses might be affected by the low number of CD4 T cells or the altered KLRG1 expression on CD8+ T cells or both. LCMV-immune hosts were challenged with a high dose (106 PFU) of LCMV and measured for LCMV-specific CD8+ T-cell numbers at day 6, when memory cell expansions are readily detected.32 Expansions of total CD8 or CD4 numbers were approximately 50% lower in the immune HP versus WT mice (Figure 4A,B). There also was a 30% to 80% reduction in the numbers of immunodominant LCMV-specific CD8+ T cells (GP-33, GP-34, GP-276, and NP-396) after rechallenge. Representative plots from one LCMV-immune HP and one WT control LCMV-immune mouse after LCMV rechallenge are shown in Figure 4B. This result suggested an impaired memory recall response in HP mice, but we questioned whether alternative possibilities may account for this apparent deficiency.
Relatively weak recall responses because of more LCMV-specific CD8 T cells in LCMV-immune HP mice. (A) LCMV-immune mice were rechallenged with LCMV. Cells were stimulated with LCMV-specific peptides, and the numbers of CD8+, CD4+ (left; means ± SD) or each LCMV-specific CD8+ T cells were counted (right; means ± SD). This is a representative result from 2 experiments with 2 to 7 mice per group. (B) Dot plots show LCMV-specific CD8 T cells from a representative rechallenged LCMV-immune HP or WT mouse. Numbers refer to percentage of cells in designated quadrants. (C) LCMV-immune mice were challenged with VV-GP, and their splenocytes were harvested at day 6. Cells were stimulated with LCMV-specific GP peptides, and then the cell numbers of total CD8+ (left; means ± SD) or of LCMV- and VV-specific CD8+ T cells were counted (right; means ± SD). This is a representative result from 2 repeated experiments with 2 mice per group. (D) 1.5 × 107 cells, 5 × 106 cells, or 3 × 106 cells either from Ly5.1 LCMV-immune HP or Ly5.1 WT mice were adoptively transferred into Ly5.2 naive B6 mice. The day after transfer, mice were infected with LCMV, and their splenocytes were harvested after 6 days. Cells were stimulated with LCMV-specific peptides and each LCMV-specific CD8+ T cell was counted (means ± SD). This is a representative result from 2 repeated experiments with 3 mice per group.
Relatively weak recall responses because of more LCMV-specific CD8 T cells in LCMV-immune HP mice. (A) LCMV-immune mice were rechallenged with LCMV. Cells were stimulated with LCMV-specific peptides, and the numbers of CD8+, CD4+ (left; means ± SD) or each LCMV-specific CD8+ T cells were counted (right; means ± SD). This is a representative result from 2 experiments with 2 to 7 mice per group. (B) Dot plots show LCMV-specific CD8 T cells from a representative rechallenged LCMV-immune HP or WT mouse. Numbers refer to percentage of cells in designated quadrants. (C) LCMV-immune mice were challenged with VV-GP, and their splenocytes were harvested at day 6. Cells were stimulated with LCMV-specific GP peptides, and then the cell numbers of total CD8+ (left; means ± SD) or of LCMV- and VV-specific CD8+ T cells were counted (right; means ± SD). This is a representative result from 2 repeated experiments with 2 mice per group. (D) 1.5 × 107 cells, 5 × 106 cells, or 3 × 106 cells either from Ly5.1 LCMV-immune HP or Ly5.1 WT mice were adoptively transferred into Ly5.2 naive B6 mice. The day after transfer, mice were infected with LCMV, and their splenocytes were harvested after 6 days. Cells were stimulated with LCMV-specific peptides and each LCMV-specific CD8+ T cell was counted (means ± SD). This is a representative result from 2 repeated experiments with 3 mice per group.
The presence of neutralizing antibodies to LCMV or of more LCMV-specific memory CD8+ T cells might rapidly clear the virus and impair the recall responses. To rule out a role for neutralizing antibodies, memory responses were examined in mice inoculated with 106-PFU of VV-GP, which does not express LCMV GP on the virion surface.33 A 68% reduction in total CD8+ cells and 65% to 95% reductions of LCMV-specific CD8+ T cells in HP host versus WT hosts are shown in Figure 4C. This suggests that neutralizing antibodies, which would not bind to the VV virion, would not account for the deficient recall response.
We next questioned whether the 4- to 6-fold higher frequencies of memory cells present in the HP host (Figure 3A) may have impaired the induction of a recall response, because their higher number may have quickly restricted replication of the challenge virus. We adoptively transferred splenocytes from immune mice (Ly5.1) into naive B6 mice (Ly5.2) and tested for donor cell expansion 6 days after LCMV challenge. When equal numbers of splenocytes (1.5 × 107) from the 2 types of immune mice were transferred, the response from the WT B6 immune population was substantially higher than that from the HP population (Figure 4D). This experiment would appear to suggest an impaired memory response in the HP host. However, when donor cell populations were diluted 3- or 5-fold, such that the input number of virus-specific CD8 T cells was comparable between the WT and HP groups, the HP responses were similar to those of WT mice. This indicates that the HP environment cannot only generate an acute and memory T-cell response, but it can also mount a relatively normal recall response, despite the low frequency of CD4 T cells.
Altered T-cell response to PV in LCMV-immune HP hosts
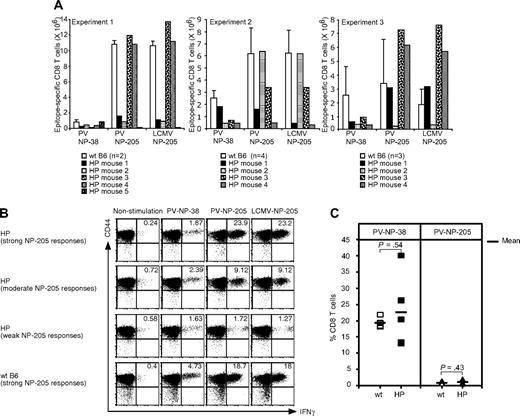
Our studies showed that the cellular response to LCMV infection filled that deficient HP environment with a high frequency of LCMV-specific T cells, and we questioned whether a sufficient repertoire remained to respond to a subsequent infection with a different virus. In addition, the HP immune system generated altered repertoires to LCMV epitopes, and there was narrowed and varied oligoclonal expansion of T cells specific for LCMV NP-205 in the HP versus WT mice. We thus challenged the immune mice with PV, which has an immunodominant NP-38 epitope not cross-reactive with LCMV and a subdominant NP-205 epitope (YTVKFPNM), which shares 6 of 8 amino acids and cross-reacts with LCMV NP-205 (YTVKYPNL). This cross-reactivity provides some heterologous immunity between LCMV and PV, and we questioned whether the altered repertoire in the HP mice may be associated with an altered heterologous immune response.
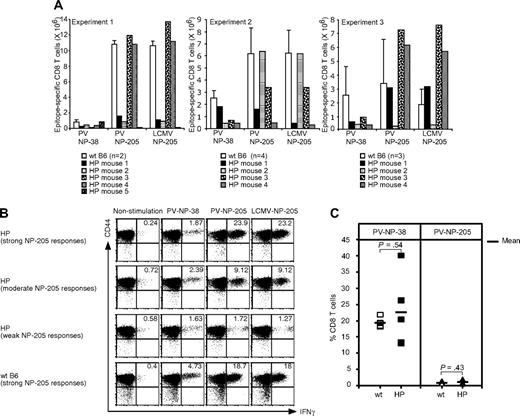
The frequencies of T cells responding to the immunodominant (NP-38) and cross-reactive (NP-205) epitopes in directly PV-challenged LCMV-immune HP and WT mice are shown in Figure 5A,B. There was a relatively weak response to the immunodominant NP-38 epitope in the immune HP mice, in comparison to immune WT controls. This contrasted greatly with the high levels (approximately 20%) of NP38-specific CD8 T-cell responses in nonimmune HP mice acutely infected with PV (Figure 5C). This weak NP38 response in the LCMV-immune HP mice could either have been due to the limited TCR repertoire available or because the NP-38 response had been immunodominated by an overwhelming cross-reactive NP-205 response. This latter explanation seems unlikely, because only 7 of 13 LCMV-immune HP mice challenged with PV had strong PV NP-205 responses. This variation in cross-reactive heterologous responses to the NP205 epitope was in contrast to the strong responses shown here consistently with the PV-challenged LCMV-immune WT mice (Figure 5A) and with virtually all LCMV-immune mice that we have previously tested.23 Thus, responses to a non–cross-reactive epitope seemed depressed in all LCMV-immune HP mice, and heterologous immunity in the form of cross-reactive T-cell responses was deficient in approximately half of the LCMV-immune HP mice (Figure 5A,B). In a total of 4 experiments, 21 mice were examined for correlations between viral PFU in visceral fat compared with the percentage of NP205-specific CD8 T cells in their spleens at day 4. A Spearman rank test34 revealed a statistically significant correlation between reduced viral titers and elevated NP205 responses (P < .05), suggesting that protective immunity was compromised by diminished cross-reactive immune responses.
T-cell responses to heterologous or primary PV infection in LCMV-immune or naive HP mice. (A) LCMV-immune mice were challenged with PV, and splenocytes were harvested at day 6 (experiment 1) or day 7 (experiments 2 and 3). Cells were stimulated with PV-specific peptides NP-38 or NP-205 or with the LCMV NP-205 peptide for 5 hours. The numbers of NP-38–specific CD8 T cells or cross-reactive LCMV/PV NP-205 CD8 T cells were counted. (B) Dot plots show the Ag-specific CD8 T cells from experiment 2 in panel A. The magnitude of the NP-205 responses is based on the numbers of NP-205–specific CD8 T cells in WT B6, whereby a strong HP response shown here is 103% of WT, a moderate response is 55% of WT, and a weak response is 7% of WT. Numbers refer to percentage of cells in designated quadrants. (C) Blood was collected from HP (n = 5) or WT (n = 5) mice day 8 after PV infection, and cells were stained with either KbPV NP-38 or KbPV NP-205 tetramers. The percentage of tetramer-positive cells of total CD8 T cells is shown. The horizontal bar represents the mean value.
T-cell responses to heterologous or primary PV infection in LCMV-immune or naive HP mice. (A) LCMV-immune mice were challenged with PV, and splenocytes were harvested at day 6 (experiment 1) or day 7 (experiments 2 and 3). Cells were stimulated with PV-specific peptides NP-38 or NP-205 or with the LCMV NP-205 peptide for 5 hours. The numbers of NP-38–specific CD8 T cells or cross-reactive LCMV/PV NP-205 CD8 T cells were counted. (B) Dot plots show the Ag-specific CD8 T cells from experiment 2 in panel A. The magnitude of the NP-205 responses is based on the numbers of NP-205–specific CD8 T cells in WT B6, whereby a strong HP response shown here is 103% of WT, a moderate response is 55% of WT, and a weak response is 7% of WT. Numbers refer to percentage of cells in designated quadrants. (C) Blood was collected from HP (n = 5) or WT (n = 5) mice day 8 after PV infection, and cells were stained with either KbPV NP-38 or KbPV NP-205 tetramers. The percentage of tetramer-positive cells of total CD8 T cells is shown. The horizontal bar represents the mean value.
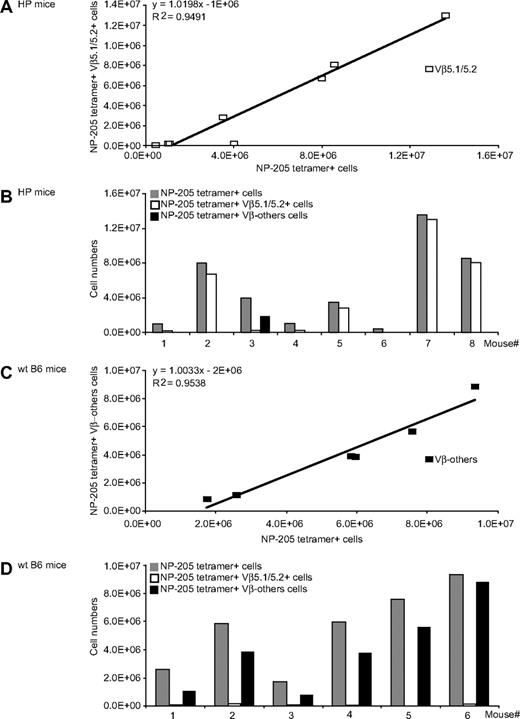
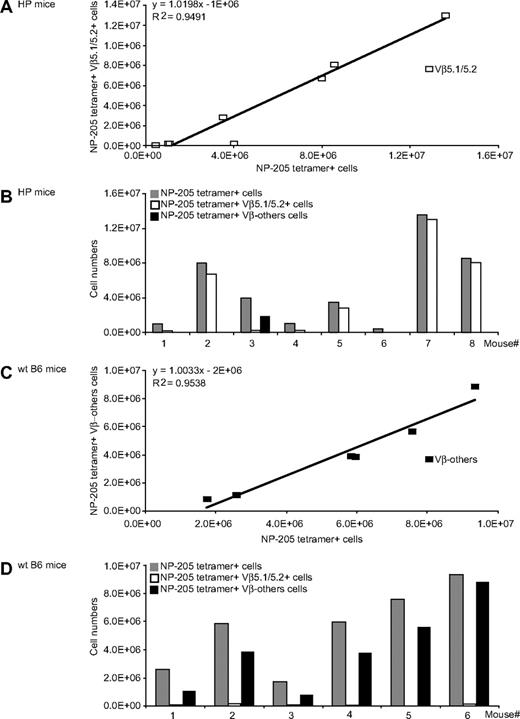
The immune HP mice that generated NP-205 responses after PV challenge had altered TCR usage. Nearly all the strong responders stained with antibody to Vβ5.1/5.2, with a strong correlation between the magnitude of the tetramer-defined NP-205 response and the number of Vβ5.1/5.2+ cells (Figure 6A,B). Our previous work using molecular approaches showed that NP-205 responses in WT LCMV-immune mice challenged with PV were often Vβ16+, against which there is no antibody, and Figures 6C and 6D shows that NP205+ T cells in WT control mice, as expected, did not react with antibody to Vβ5.1/5.2 or to any of the other tested Vβ antibodies. Thus, the HP environment results in altered TCR repertoires on heterologous virus challenge.
Altered Vβ usage of heterologous immune T-cell responses in LCMV-immune HP mice. LCMV-immune HP or WT mice were challenged with PV, and 7 days later splenocytes were stained with NP-205 tetramer and with the entire panel (Vβ2-14) of mAb to Vβs. (A) A linear regression analysis of the number of NP-205+Vβ5.1/5.2+ costaining cells (y-axis) versus the number of NP-205+ cells (x-axis). (B) The NP-205 responses in individual HP mice ( ) and the proportions of those cells staining with mAb to Vβ5.1/5.2 (
) and the proportions of those cells staining with mAb to Vβ5.1/5.2 ( ) or to no tested Vβ (Vβ-others; ▬). (C) A linear regression analysis of WT mice NP-205 responses to Vβ-others, and (D) the responses of individual WT mouse to Vβ5.1/5.2 and Vβ-others, as in panel B. These data are a composite of 2 different experiments.
) or to no tested Vβ (Vβ-others; ▬). (C) A linear regression analysis of WT mice NP-205 responses to Vβ-others, and (D) the responses of individual WT mouse to Vβ5.1/5.2 and Vβ-others, as in panel B. These data are a composite of 2 different experiments.
Altered Vβ usage of heterologous immune T-cell responses in LCMV-immune HP mice. LCMV-immune HP or WT mice were challenged with PV, and 7 days later splenocytes were stained with NP-205 tetramer and with the entire panel (Vβ2-14) of mAb to Vβs. (A) A linear regression analysis of the number of NP-205+Vβ5.1/5.2+ costaining cells (y-axis) versus the number of NP-205+ cells (x-axis). (B) The NP-205 responses in individual HP mice ( ) and the proportions of those cells staining with mAb to Vβ5.1/5.2 (
) and the proportions of those cells staining with mAb to Vβ5.1/5.2 ( ) or to no tested Vβ (Vβ-others; ▬). (C) A linear regression analysis of WT mice NP-205 responses to Vβ-others, and (D) the responses of individual WT mouse to Vβ5.1/5.2 and Vβ-others, as in panel B. These data are a composite of 2 different experiments.
) or to no tested Vβ (Vβ-others; ▬). (C) A linear regression analysis of WT mice NP-205 responses to Vβ-others, and (D) the responses of individual WT mouse to Vβ5.1/5.2 and Vβ-others, as in panel B. These data are a composite of 2 different experiments.
Discussion
Lymphopenia is induced under a variety of conditions, including irradiation therapy, immunosuppressive drug treatment, viral infection, and genetic deficiency.1,2 To compensate for this lymphopenia, host T cells undergo acute homeostatic proliferation, whereby a subset of the T cells acquires some properties of effector/memory cells and undergoes 8 or more divisions. The host is left with an immune system whose T cells have undergone a differentiation process different from the normal generation of memory cells and whose repertoire has extensively narrowed. We questioned here whether an immune system virtually totally composed of a T-cell repertoire that was the product of acute homeostatic proliferation was capable of mounting a normal T-cell response to viral infection. We show here that, even though the CD4 T-cell counts were low, this HP immune system could mount a relatively normal acute, memory and recall CD8 T-cell response to a virus. This observation is consistent with a recent report by Hamilton et al,35 indicating that ovalbumin-specific OT-1 transgenic T cells conditioned in an irradiation-induced lymphopenic environment can, on adoptive transfer into other hosts, generate a protective memory CD8 T-cell response to ovalbumin-expressing Listeria monocytogenes infection. Our data evaluate the effects of homeostatic proliferation on a polyclonal antiviral response and show that, although the HP host is capable of making a fully functional T-cell response to infection, the host has altered immune responses to a second pathogen, perhaps because of the narrowed T-cell repertoire.
HP mice had fewer CD8 cells than did WT mice before infection, but LCMV induced a marked CD8 T-cell expansion such that by day 8 the HP CD8 T-cell numbers were nearly identical to WT, thereby showing that an HP cell population could respond well to acute infection. The proportions of epitope-specific responses were similar between the 2 types of mice for all the tested CD8 epitopes except for GP-276, whose responses were lower in the HP mice. An analysis of TCR usage showed some skewing, suggestive of oligoclonality in the epitope-specific responses of HP mice, and this was particularly noted in that residual GP276-specific population. This probably reflected the reduced TCR repertoire in the HP mice rather than any impaired function of the cell population. CD4 T-cell numbers in the HP mice, however, did not approach that of the WT mice at the peak of the acute response, and the percentage of CD4 T cells specific for the 2 class II–restricted LCMV epitopes was quite low. The reason for this is unclear, but other studies have shown that a subset of CD4 T cells responds more poorly to conditions of acute homeostatic expansion.36,37
Previous studies had shown that naive CD8 T cells transferred into lymphopenic hosts do not fill up the peripheral T-cell pools to levels comparable with the full T-cell pool in normal mice.4-6 The LCMV infection, however, induced a T-cell response that restored the CD8 T-cell numbers to normal levels even in the resting immune state after resolution of infection. It is likely that most of the cells restoring this pool were specific for LCMV, because 7 LCMV peptides could account for 34% of the CD8 T cells in the HP memory pool after infection (Figure 3A). Some epitope-specific T cells would likely be of too low affinity to react in these assays, and another 19 LCMV epitopes have been recently defined but were not tested.38 It is unclear why homeostatic proliferation does not spontaneously fill up this pool by itself, but the viral infection, perhaps by liberating cytokines such as IL-2 and IL-15, acted as strong stimulus to do so. This is consistent with the observation that the percentage of melanoma-specific CD8+ T cells increases after vaccination of reconstituted lymphopenic mice.39 The LCMV-specific HP CD8 T cells may have fewer competing entities, such as new thymic emigrants, to reduce their proportions. These high proportions of LCMV-specific T cells would probably leave a restricted T-cell repertoire available for responses against other pathogens.
The presence of CD4 T cells is important at the time of initial antigenic challenge for the conditioning of CD8 T cells to become fully functioning memory cells, having the capacity to mount recall responses on secondary challenge.28 A recent report that used a transgenic model for homeostatic proliferation confirmed the requirement of CD4 T cells for the development of memory transgenic CD8 HP cells.35 Although the CD4+ T-cell pool was only partially restored after LCMV infection in the reconstituted T-cell KO HP mice (Figure 3A), the functions and properties of the LCMV-specific CD8 T cells were normal, suggesting that this number of HP CD4 T cells was sufficient to provide help for the CD8 T cells.
As the TCR repertoire narrows, it would be expected that the ability of a host to respond to viral infections might become impaired. We thus questioned the capacity of the HP host to mount a response to a second virus, whereby heterologous immunity influences the outcome of infection. The NP-205 epitopes are mostly homologous subdominant cross-reactive epitopes encoded by LCMV and PV, but they become dominant after mice immune to one of these viruses are challenged with the other. The cross-reactive T cells that grow out under conditions of heterologous virus challenge are a small subset of the original NP-205–specific repertoire and normally show a narrowed oligoclonal TCR usage.23 Our repertoire analysis of NP-205–specific T cells in the LCMV-immune HP host showed evidence of oligoclonality that differed between mice (Figure 2E), suggesting that the repertoire was narrow after a single infection. To test whether there were altered heterologous immune responses, these LCMV-immune HP mice were challenged with PV and tested for recall of the cross-reactive population. In contrast to WT immune mice, in which there always is a good NP-205 recall response, 6 of 13 LCMV-immune HP mice did not generate a cross-reactive PV NP-205 response on challenge with PV (Figure 5A). This suggests that those HP mice lost cross-reactive PV NP-205 responses because of the narrowed LCMV NP-205–specific repertoire elicited during the primary LCMV infection. Those heterologous responses that were found in the PV-challenged HP mice had different TCR repertoires than did WT mice in that the HP responses were mostly Vβ5.1/5.2 (Figure 6). This indicates a switch in immunodominance of Vβ usage in an HP environment and might reflect the possibility that homeostatic proliferation is more selective for Vβ5.1/5.2+ than Vβ16 T cells. Importantly, none of the LCMV-immune HP mice generated strong responses to the normally immunodominant but not cross-reactive PV NP-38 epitope (see day 7 values in Figure 5A experiments 2 and 3). In contrast, acute PV infection of nonimmune HP mice generated strong NP38-specific responses (Figure 5C). This weak NP38 response in PV-challenged LCMV-immune mice was unlikely due to suppression by a strong protective cross-reactive NP-205 response, because the NP-38 response was just as low in mice showing no response to NP-205 and was low whether the mice rapidly cleared the PV infection (data not shown). This suggests that the limitations in available T-cell repertoire may contribute to this weak response.
HP-conditioned T cells can nevertheless mount an effective CD8 T-cell response against a primary virus infection and differentiate into fully functioning memory T cells able to mount recall responses. Under those conditions the narrowed HP T-cell repertoire was sufficiently diverse to mount responses against all tested LCMV-encoded class I epitopes. The diversity of the repertoire, however, could presumably be adjusted upward or downward by transfer of larger or smaller numbers of T cells into the T-cell KO host.40 One could predict that the diversity of the repertoire in humans after lymphopenia-induced homeostatic proliferation could be highly variable and depend in part on the numbers of thymic emigrants to restore a complex repertoire. As persons age, the thymus involutes, with greatly reduced thymic output. This would result in a less diverse repertoire after homeostatic proliferation. Indeed, narrowed oligoclonal CD4 or CD8 T-cell populations are observed in elderly patients with lymphopenic rheumatoid arthritis with reduced thymic output.41-43 We thus conclude that the effect of a narrowed immune repertoire occurring as a consequence of homeostatic proliferation, particularly in older persons in whom the thymus is functioning poorly, may be of greater significance as the host experiences infections with additional viruses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Keith Daniels and Keisha Mathurin for technical support and Drs Liisa K. Selin and Michael A. Brehm for helpful discussion.
This work was supported by National Institutes of Health (NIH) grants AI17672, AR35506, and AI-073871.
The contents of this study represent the opinions of the authors and do not necessarily reflect the official views of NIH.
National Institutes of Health
Authorship
Contribution: S.-J.L. designed and performed experiments, analyzed data, and cowrote the manuscript; A.T.C. assisted in experiments and did viral assays; and R.M.W. guided experimental design and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raymond M. Welsh, Department of Pathology, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655; e-mail: raymond.welsh@umassmed.edu.


 ) and the proportions of those cells staining with mAb to Vβ5.1/5.2 (
) and the proportions of those cells staining with mAb to Vβ5.1/5.2 ( ) or to no tested Vβ (Vβ-others; ▬). (C) A linear regression analysis of WT mice NP-205 responses to Vβ-others, and (D) the responses of individual WT mouse to Vβ5.1/5.2 and Vβ-others, as in panel B. These data are a composite of 2 different experiments.
) or to no tested Vβ (Vβ-others; ▬). (C) A linear regression analysis of WT mice NP-205 responses to Vβ-others, and (D) the responses of individual WT mouse to Vβ5.1/5.2 and Vβ-others, as in panel B. These data are a composite of 2 different experiments.