Abstract
The clinical outcomes of 169 patients enrolled in the first clinical trial of thalidomide for advanced or refractory myeloma are updated. Seventeen patients remain alive and 10 are event-free, with a median follow-up of 9.2 years. According to multivariate analysis of pretreatment variables, cytogenetic abnormalities, present in 47% of patients within 3 months of enrollment, and λ light chain isotype both affected overall survival and event-free survival adversely. Forty percent of the 58 patients lacking these 2 unfavorable features, one-half of whom had no disease recurrence, survived at least 6 years, in contrast to fewer than 5% among those with 1 or 2 risk features (P < .001). Patients who had received cumulative thalidomide doses in excess of 42 g in the first 3 months enjoyed superior overall and event-free survival. The poor outcome associated with λ-type myeloma may relate to its overrepresentation in molecularly defined high-risk disease gleaned from studies in newly diagnosed myeloma.
Introduction
The prognosis for patients with multiple myeloma (MM) was radically improved when the melphalan dose-response effect could be safely exploited by applying autografts. Thus, the frequency of complete response (CR) was raised from less than 5% with standard melphalan-prednisone1 (MP) to 40% and higher when tandem transplants were applied.2 During the past 10 years, many new, mainly nongenotoxic, agents have been investigated. Thalidomide was the first since the introduction of melphalan and glucocorticoids to exhibit single-agent activity in MM that had become refractory to MP and even high-dose melphalan regimens.3,4 The recognition of thalidomide's activity in MM, long considered an “orphan disease” because of its relatively low incidence, ushered in an entirely new era of concerted clinical investigations of new agents, leading to US Food and Drug Administration approval of the first-in-class proteasome inhibitor bortezomib5 and the immunomodulatory agent lenalidomide,6 which are now being applied in the primary management of patients with MM.7-11
Given the landmark observation of thalidomide's activity in end-stage MM, we now report on the long-term outcome of the 169 patients enrolled in the original phase 1/2 trial conducted at the University of Arkansas for Medical Sciences and in accordance with the Declaration of Helsinki.
Methods
We examined the long-term clinical outcomes of the original 169 patients who were enrolled on our UARK 98-003 protocol that called for administra-tion of thalidomide in a dose-escalated fashion for all patients according to tolerance, starting with 200 mg/d and allowing for dose increases every 2 weeks by 200 mg to a maximum dose of 800 mg/d.1,2 The protocol had been approved by the institutional review board of the University of Arkansas for Medical Sciences. All subjects had signed a written informed consent delineating the investigational nature of the trial, in line with institutional and Food and Drug Administration guidelines. We scrutinized the initial baseline characteristics and now provide follow-up information on event-free survival (EFS) and overall survival (OS) from start of protocol therapy, accounting for risk factors before therapy. Seventeen patients remain alive with a median follow-up of 9.2 years.
The presence of metaphase-based cytogenetic abnormalities (CAs) was identified in terms of CA subtypes, as previously published.12 Briefly, clinical outcomes were examined with regard to the presence of any CA and of the following subtypes: hypodiploidy/deletion 13, chromosome 1q amplification, chromosome 1p deletion, complete or partial deletion of chromosome 17, specific translocations, and the presence of myelodysplasia-associated abnormalities within a MM-typical CA.12
Results and discussion
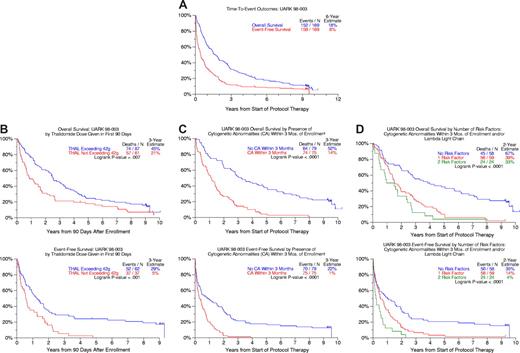
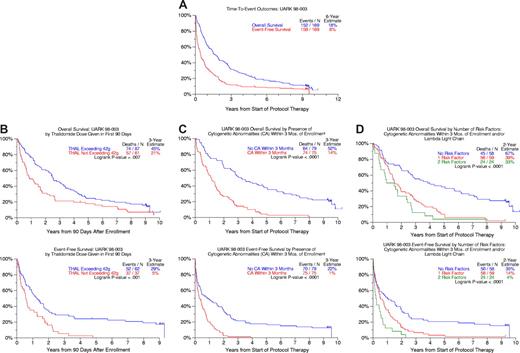
As of March 24, 2008 (median follow-up, 9.2 years), 10 patients remain event-free and 17 alive (Figure 1A). Twenty-five percent of patients remained event-free 2 years after discontinuing the study drug. The median time to next therapy was 3 months for the 53 patients receiving salvage regimens, which comprised further high-dose melphalan regimens in 29% and bortezomib in only 7%.
Survival outcomes after starting thalidomide therapy. (A) Overall and event-free survival for all 169 patients. At 6 years, 18% of the original 169 enrolled were alive and 8% event-free. (B) Overall and event-free survival according to cumulative thalidomide dose consumed within the first 3 months from protocol start. Patients whose cumulative thalidomide dose exceeded 42 g within 90 days of enrollment had significantly longer OS and EFS. (C) Overall and event-free survival comparisons according to the presence of cytogenetic abnormalities detected within 3 months before protocol enrollment. Patients without cytogenetic abnormalities (CA) enjoyed superior overall and event-free survival. (D) Overall and event-free survival according to the number of independent adverse parameters present before protocol enrollment (cytogenetic abnormalities (CA), λ light chain). Overall survival and event-free survival were superior in patients lacking cytogenetic abnormalities and exhibiting κ light chain isotypes.
Survival outcomes after starting thalidomide therapy. (A) Overall and event-free survival for all 169 patients. At 6 years, 18% of the original 169 enrolled were alive and 8% event-free. (B) Overall and event-free survival according to cumulative thalidomide dose consumed within the first 3 months from protocol start. Patients whose cumulative thalidomide dose exceeded 42 g within 90 days of enrollment had significantly longer OS and EFS. (C) Overall and event-free survival comparisons according to the presence of cytogenetic abnormalities detected within 3 months before protocol enrollment. Patients without cytogenetic abnormalities (CA) enjoyed superior overall and event-free survival. (D) Overall and event-free survival according to the number of independent adverse parameters present before protocol enrollment (cytogenetic abnormalities (CA), λ light chain). Overall survival and event-free survival were superior in patients lacking cytogenetic abnormalities and exhibiting κ light chain isotypes.
Of the 140 patients with well-documented dosing information beyond 90 days of trial participation, 58% proceeded to the planned 800-mg target dose; 68% reached the 600-mg and 87% the 400-mg level, whereas 13% of patients did not proceed to doses beyond 200 mg. The median duration of maintaining the highest thalidomide dose level was 49 days for the 800-mg dose, 20 days for both 600-mg and 400-mg doses, and 13 days for the 200-mg dose level. As expected, those failing to proceed beyond the 400-mg dose level comprised significantly higher proportions of patients with high-risk features such as β2-microglobulin more than or equal to 3.5 mg/L (59% vs 31%, P = .002) and hypodiploidy (35% vs 11%, P = .026).
This phase 1/2 trial was not designed to define the optimal thalidomide dose but to enable maximum dose escalation, where feasible, in the absence of information on a thalidomide dose-response effect. Using a 90-day landmark, both OS and EFS were superior among subjects who had taken at least 42 g of study drug (median total dose; range, 0.5-963.5 g) during this time frame (Figure 1B).
The presence of CAs was associated with inferior 3-year OS and EFS rates of 14% and 1% compared with 52% and 22% in patients with normal karyotypes (Figure 1C). Previously not appreciated was the inferior outcome associated with λ versus κ light chain myeloma which, along with the presence of CAs, imparted inferior OS and EFS in multivariate analysis (Table 1). When specific CA subgroups were considered, deletion 13, along with renal insufficiency, dominated both OS and EFS models (Table 1). Among 141 patients with complete information, the 58 (41%) lacking CAs and presenting with κ-type myeloma constituted a favorable group whose 5-yr OS and EFS rates were 45% and 20%, respectively, compared with 5% and 2% for the remainder (Figure 1D).
The listed toxicities have to be viewed with some caution because of possible underreporting by patients for fear of having to discontinue thalidomide, which was then the only available salvage option. The most common grade 3 or higher toxicities recorded included sensory/motor neuropathy and weakness in 20%, somnolence in 14%, and constipation in 12%; 7% experienced tremor and 5% complained of ataxia.
In this long-term follow-up of a quintessential trial of the first novel agent with antimyeloma efficacy in 3 decades, we note that 17 patients are still alive; 10 of the 17 have had no recurrence since trial initiation more than 9 years ago. Consistent with the initial report, the presence of CAs was the dominant feature linked to both inferior OS and EFS; deletion 13 was the only significant adverse genetic feature when individual CA subgroups were considered. A novel hitherto unrecognized favorable feature was the presence of a κ rather than λ light chain isotype. The poor outcome of patients with λ-type myeloma may be due to its association with high-risk molecular subtypes of myeloma.16 Gene expression profiling studies as part of Total Therapy 2 and 3 trials17,18 revealed an even distribution of κ and λ light chain myeloma (frequency ratio, 1.1) in 3 high-risk entities (MAF/MAFB, FGFR3/MMSET, and proliferation) compared with a strong κ light chain predominance (frequency ratio 2.3) in the remaining molecular subgroups with a superior prognosis (P < .001).
Thalidomide has since been effectively combined with dexamethasone,7 standard melphalan in MPT (melphalan, prednisone, thalidomide),9 and bortezomib in VTD (bortezomib, thalidomide, dexamethasone),11 resulting in remarkably high partial and complete response rates and promising survival duration. When used as part of a tandem transplant trial in Total Therapy 2, randomization to thalidomide resulted in superior CR rates and EFS17 and, according to a recent update, also in extension of OS, which was however limited to the CA subgroup,19 contradicting the findings reported in this salvage setting.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Cancer Institute (P01 grant CA55819) and Celgene (Summit, NJ).
National Institutes of Health
Authorship
Contribution: F.v.R., J.D.S., J.Z., and B.B. designed the study and wrote the manuscript; M.D. wrote the original protocol; F.v.R., E.A., D.S., S.S., J.M., S.J., and B.B. enrolled and cared for patients; B.J. collected data; and A.H. and J.C. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bart Barlogie, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, 4301 West Markham, Little Rock, AR 72205; e-mail: barlogiebart@uams.edu.