Abstract
The lymphatic system plays pivotal roles in mediating tissue fluid homeostasis and immunity, and excessive lymphatic vessel formation is implicated in many pathological conditions, which include inflammation and tumor metastasis. However, the molecular mechanisms that regulate lymphatic vessel formation remain poorly characterized. Sphingosine-1-phosphate (S1P) is a potent bioactive lipid that is implicated in a variety of biologic processes such as inflammatory responses and angiogenesis. Here, we first report that S1P acts as a lymphangiogenic mediator. S1P induced migration, capillary-like tube formation, and intracellular Ca2+ mobilization, but not proliferation, in human lymphatic endothelial cells (HLECs) in vitro. Moreover, a Matrigel plug assay demonstrated that S1P promoted the outgrowth of new lymphatic vessels in vivo. HLECs expressed S1P1 and S1P3, and both RNA interference–mediated down-regulation of S1P1 and an S1P1 antagonist significantly blocked S1P-mediated lymphangiogenesis. Furthermore, pertussis toxin, U73122, and BAPTA-AM efficiently blocked S1P-induced in vitro lymphangiogenesis and intracellular Ca2+ mobilization of HLECs, indicating that S1P promotes lymphangiogenesis by stimulating S1P1/Gi/phospholipase C/Ca2+ signaling pathways. Our results suggest that S1P is the first lymphangiogenic bioactive lipid to be identified, and that S1P and its receptors might serve as new therapeutic targets against inflammatory diseases and lymphatic metastasis in tumors.
Introduction
Sphingosine-1-phosphate (S1P) has been implicated in a wide spectrum of biologic processes, including the promotion of cell growth and survival, migration and differentiation, platelet aggregation, inflammatory responses, and angiogenesis.1 S1P is generated by the phosphorylation of sphingosine through a process mediated by sphingosine kinase 1 (SphK1) and SphK2. S1P acts both intracellularly as a second messenger2 and extracellularly as a ligand for a family of G-protein–coupled S1P receptors.3 S1P1 couples stringently to the Gi protein family, whereas S1P2 and S1P3 couple to the Gi, Gq, and G12/13 protein families. Multiple interconnections of S1P signaling through S1P1 and S1P3 induce vascular endothelial cell proliferation, migration, morphogenesis, cytoskeletal reorganization, and adherens junction assembly, whereas signaling via S1P2 negatively regulates S1P-mediated multiple responses of vascular endothelial cells.4 The defective vascular maturation observed in S1P1-deficient mice highlights a fundamental role for S1P signaling on vasculogenesis.5 Neutralization of the action of extracellular S1P shows significant inhibition of angiogenesis, tumor growth, metastasis, and lymphocyte transmigration, indicating that S1P is an important pathological regulator of inflammation and angiogenesis.6-8
Lymphatic vessels play important roles in mediating tissue fluid homeostasis and immunity.9 Although lymphangiogenesis, the formation of new lymphatic vessels from preexisting vessels, occurs under physiological and pathological conditions, including chronic inflammation10 and tumor metastasis,11 the molecular mechanisms that regulate lymphatic vessel formation remain largely uncharacterized. Vascular endothelial growth factor C (VEGF-C), the first identified lymphangiogenic factor, is a secreted, proteolytically processed glycoprotein that activates VEGF receptors 2 and 3 on lymphatic endothelial cells.12,13 VEGF-C is overexpressed in many primary tumors and induces lymphangiogenesis and angiogenesis around tumor tissues, as well as promotes tumor metastasis via newly formed lymphatic or blood vessels.14,15 In addition, VEGF-C is up-regulated in inflammatory cells and so induces lymphangiogenesis and angiogenesis during chronic inflammation.10
VEGF-A, hepatocyte growth factor (HGF), angiopoietin-1, platelet-derived growth factor BB (PDGF-BB), and insulin-like growth factor 1/2 (IGF1/2), all of which are proangiogenic factors, can also give rise to in vivo lymphangiogenesis.16-19 However, relatively less interest has been focused on the involvement of bioactive lipid molecules compared with that of growth factors in lymphangiogenesis. Because S1P is a potent proangiogenic and inflammatory bioactive lipid molecule,20,21 we hypothesized that S1P could affect lymphangiogenesis. In this report, we show that S1P induced the migration and capillary-like tube formation of lymphatic endothelial cells in vitro and lymphangiogenesis in vivo. Real-time polymerase chain reaction (PCR) analysis revealed that human lymphatic endothelial cells (HLECs) expressed S1P1 and S1P3. S1P-induced lymphangiogenesis was significantly inhibited by pertussis toxin (PTX), RNA interference–mediated down-regulation of S1P1, and an S1P1 antagonist, indicating the involvement of Gi protein activation coupled with S1P1. In addition, the inhibition of S1P-induced in vitro lymphangiogenesis by U73122 and BAPTA-AM demonstrated that phospholipase C (PLC)/Ca2+ was an important signaling pathway. Moreover, we found that S1P induced intracellular Ca2+ mobilization and formation of actin stress fibers in a PTX/PLC-dependent manner in HLECs. Our findings strongly suggest that S1P is the first lymphangiogenic bioactive lipid and that S1P secreted from inflammatory cells or tumor cells may induce lymphangiogenesis during tumor growth, metastasis, and inflammation.
Methods
Materials
S1P, heparin, FITC-dextran (2000 kDa) and sulfinipyrazone were purchased from Sigma-Aldrich (St Louis, MO); S1P1 selective antagonist (R-isomer) and S1P1 control molecule (S-isomer) were acquired from Avanti Polar Lipids (Alabaster, AL); L-NAME, LY294002, and BAPTA-AM were obtained from BioMol Research Laboratories (Plymouth Meeting, PA); PTX was purchased from Calbiochem (La Jolla, CA); PD98059, SB203580, and U73122 were acquired from Alexis Biochemicals (San Diego, CA); recombinant human VEGF-C and basic fibroblast growth factor (bFGF) were obtained from R&D Systems (Minneapolis, MN); human fibronectin, growth factor–reduced (GFR)–Matrigel, and Matrigel were purchased from BD Biosciences (San Jose, CA); medium 199, fetal bovine serum (FBS), penicillin/streptomycin, human endothelial serum–free medium (HE-SFM), fura-2/AM, lipofectamine 2000, AlexaFluor594-streptavidin, AlexaFluor488 donkey anti–mouse and anti–rabbit, and AlexaFluor594 donkey anti–rat IgG antibodies were obtained from Invitrogen (Carlsbad, CA). Diff-Quick solution was purchased from Baxter Healthcare (McGraw Park, IL); One Step SYBR reverse-transcription (RT)–PCR kit was acquired from TaKaRa Bio (Tokyo, Japan); hamster antibody 8.1.1 was purchased from Developmental Studies Hybridoma Bank (Iowa City, IA); biotin-labeled anti–hamster IgG antibody, normal mouse, rat, rabbit IgGs were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit anti–Prox-1 antibody was acquired from RELIATech (Braunschweig, Germany); rat anti–lymphatic vascular endothelial hyaluronan receptor-1 (LYVE-1) antibody was kindly gifted by Dr Gou Young Koh (Korea Advanced Institute of Science and Technology, Daejeon, Republic of Korea); mouse anti–human podoplanin antibody (D2-40) was obtained from Signet Laboratories (Dedham, MA); rabbit anti–phospho-histone-H3 (PH3) antibody was obtained from Upstate Biotechnology (Lake Placid, NY); shRNAs in pRS plasmid against S1P1 and S1P3 were acquired from OriGene (Rockville, MD); HLECs and human dermal lymphatic microvascular endothelial cells (HMVECs-dLy) were purchased from ScienCell (San Diego, CA) and Lonza (Walkersville, MD), respectively.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated from freshly delivered umbilical cords as described previously22 and maintained in medium 199 supplemented with 20% FBS, 3 ng/mL bFGF, 5 U/mL heparin, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. HLECs were maintained in endothelial cell medium supplemented with 5% FBS, endothelial cell growth supplement (ScienCell), 100 U/mL penicillin, and 0.1 mg/mL streptomycin. HMVECs-dLy were cultured in EGM-2 MV medium (Lonza). Cells were maintained at 37°C in a humidified 5% CO2 atmosphere. Cells from 4 to 5 passages were used for the experiments in this study.
Migration assay
A migration assay was performed in a 48-well microchemotaxis chamber (Neuro Probe, Cabin John, MD) as described previously.23 Polycarbonate membranes with 12-μm pores (Neuro Probe) were coated with 1 μg/mL human fibronectin in double-distilled water and then dried for 1 hour. Cells were harvested and resuspended in HE-SFM containing medium only or with 100 ng/mL PTX, 10 μM LY294002, 1 mM L-NAME, 10 μM PD98059, 25 μM SB203580, 30 μM BAPTA-AM, 5 μM U73122, S1P1 antagonist, or S1P1 control molecule. The bottom chamber was loaded with 3 × 104 cells, and the filter membrane was laid over the cells. The microchamber was then inverted and incubated at 37°C for 2 hours. After reinverting the chamber to its upright position, the upper wells were then loaded with HE-SFM containing the indicated concentration of S1P or 100 ng/mL S1P with 100 ng/mL PTX, 10 μM LY294002, 1 mM L-NAME, 10 μM PD98059, 25 μM SB203580, 30 μM BAPTA-AM, 5 μM U73122, S1P1 antagonist, or S1P1 control molecule. The chamber was then reincubated at 37°C for 4 hours, and the filter membrane was fixed and stained using Diff-Quick solution. The cells that migrated through the filter membrane were quantified by counting 3 random fields of each well using a Nikon Eclipse TS100 microscope (Nikon, Tokyo, Japan) equipped with a Plan Fluor 20×/0.50 DIC M/N2 objective lens and COOLPIX 995 digital camera (Nikon). Experiments were carried out in triplicate and repeated independently 3 times.
Proliferation assay
HLECs and HMVECs-dLy were plated at 2.5 × 104 cells/well on a fibronectin-coated 48-well plate. After starvation in HE-SFM for 6 hours, the cells were incubated with S1P or VEGF-C for 24 hours. The cells were treated with 0.5 μCi (0.0185 MBq) [3H]-thymidine per well and further incubated for 12 hours. The radioactivity of incorporated [3H]-thymidine was determined in a liquid scintillation counter. Experiments were carried out in triplicate and repeated 3 times.
Capillary-like tube formation assay
The tube formation assay was performed as described previously.23 Cells (2 × 104 cells/well) in 0.4 mL HE-SFM with the indicated concentration of S1P, 500 ng/mL VEGF-C, or 100 ng/mL S1P with 100 ng/mL PTX, 10 μM LY294002, 1 mM L-NAME, 10 μM PD98059, 25 μM SB203580, 30 μM BAPTA-AM, 5 μM U73122, S1P1 antagonist, or S1P1 control molecule were plated on a GFR Matrigel-coated 24-well plate. After a 6-hour incubation, the cells were fixed with Diff-Quick solution, and 2 randomly chosen fields per well were visualized and acquired using a Nikon Eclipse TS100 microscope equipped with a 10×/0.25 Ph1 ADL objective lens and COOLPIX 995 digital camera, and processed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). Total tube area was analyzed using Scion Image software (Frederick, MD). Analyses of the test samples were performed in duplicate and independent experiments were repeated 3 times.
Immunocytochemistry
HLECs and HMVECs-dLy were fixed with 4% paraformaldehyde and incubated with anti–Prox-1, anti–LYVE-1, or anti-podoplanin antibody. For immunostaining of Prox-1, fixed cells were permeabilized with 0.2% Triton X-100. Isotype-matched control IgGs were used for negative staining. After treatment of AlexaFluor-conjugated secondary antibodies, cells were counterstained with Hoechst. Five randomly chosen fields were visualized using AxioSkop2 Plus Fluorescent microscope (Carl Zeiss, Jena, Germany) equipped with an Achroplan 20×/0.45 objective lens and images were acquired using an AxioVision 4.0 software (Carl Zeiss) and processed using Adobe Photoshop 7.0.
Real-time RT-PCR
PCR primers for human S1P1, S1P2, S1P3, and β-actin were designed using the Primer3 program (Whitehead Institute, http://biotools.umassmed.edu/bioapps/primer3_www.cgi). The primers used are shown in Table 1. After phosphate-buffered saline washing, subconfluent HLECs were trypsinized and centrifuged. Cell pellets were used for isolation of total RNA via RNease Mini Kit (QIAGEN, Valencia, CA). For real-time RT-PCR, total RNA (100 ng) was amplified with a One Step SYBR RT-PCR kit using a LightCycler 2.0 PCR system (Roche Diagnostics, Mannheim, Germany). This experiment was repeated twice.
Matrigel plug assay, immunostaining, and lymphangiography
C57BL/6 (male, 5 weeks old, 5 mice per group) mice were subcutaneously injected with 0.5 mL Matrigel containing 0.4 μg S1P or 1 μg VEGF-C. The mice were killed at 10 days after implantation, and the Matrigel was removed, fixed in 4% paraformaldehyde, and embedded in paraffin. Cross-sections of paraffin-embedded Matrigel were stained with hematoxylin and eosin (H&E) and images were visualized using an Olympus BX51 microscope (Olympus, Tokyo, Japan) equipped with an UPlanFL N 100×/1.30 oil objective lens and acquired using analysis LS Research software (Soft Imaging System, Munster, Germany). Lymphatic vessels were immunostained with anti–Prox-1, anti–LYVE-1, or anti-podoplanin antibody. Proliferating nuclei in lymphatic vessels were immunostained with rabbit anti-PH3 antibody. For lymphangiography, at 10 days after implantation, FITC-dextran (2000 kDa, 10 mg/mL) was injected intradermally apart from Matrigel, and lymphatic vessel networks were analyzed. All images were visualized using an FV1000 Olympus Confocal microscope (Olympus) equipped with an UPlanSApo 20×/0.75 objective lens and acquired using FV1000-ASW 1.5 software (Olympus). This experiment was independently repeated twice. Animal study protocols were approved by the Institutional Animal Care and Use Committee at Pohang University of Science and Technology.
RNA interference–mediated down-regulation of S1P1 and S1P3
The shRNA retroviral pRS plasmids for S1P1 and S1P3 were transfected into mouse packaging PT67 cells (Clontech, Palo Alto, CA) using lipofectamine 2000 and cultured with puromycin (Clontech). For negative control, empty pRS plasmid was used. After infection with retrovirus, HLECs were cultured with puromycin. The shRNA sequences against S1P1 and S1P3 are as follows: S1P1, 5′-GTACTTCCTGGTGTTAGCTGTGCTCAACT-3′; S1P3, 5′-TCACCACCGTGCTCTTCTTGGTCATCTGC-3′.
Measurement of intracellular free calcium mobilization
Intracellular Ca2+ mobilization in HLECs was determined with the fluorescent Ca2+ indicator fura-2/AM as described previously.24 Briefly, HLECs were incubated with fura-2/AM at a final concentration of 3 μM in HE-SFM at 37°C for 30 minutes. After loading, the cells were washed twice with Ca2+-free Locke solution (158.4 mM NaCl, 5.6 mM KCl, 1.2 mM MgCl2, 0.2 mM EGTA, 5 mM HEPES, and 10 mM glucose, pH 7.3) to remove extracellular dye. Sulfinipyrazone was added to both the loading medium and the washing solution to a final concentration of 250 μM to prevent dye leakage. Approximately 1.5 × 105 cells of the cell suspension were transferred to a quartz cuvette and placed in a thermostatically controlled cell holder at 37°C, in which the cell suspension was continuously stirred, and exposed to 100 ng/mL S1P with 100 ng/mL PTX, 10 μM LY294002, 1 mM L-NAME, 10 μM PD98059, 25 μM SB203580, 30 μM BAPTA-AM, or 5 μM U73122. LY294002, L-NAME, PD98059, SB203580, BAPTA-AM, and U73122 were preincubated with the cells for 30 minutes prior to exposure to S1P, and PTX was preincubated for 6 hours. Fluorescence ratios were taken by dual excitation at 340 and 380 nm, and emission at 500 nm by the alternative wavelength time scanning method. Experiments were repeated 3 times.
Statistical analyses
Student t test was used to calculate P values based on comparisons with the appropriate control samples tested at the same time.
Results
S1P induced in vitro lymphangiogenesis
Lymphangiogenesis is a complex cellular process that occurs via proliferation, migration, and differentiation of lymphatic endothelial cells. To investigate whether S1P had in vitro lymphangiogenic activity, we performed migration, proliferation, and capillary-like tube formation assays in HLECs, lymphatic endothelial cells derived from lymph nodes. S1P significantly induced the migration of HLECs in a dose-dependent manner over the migration in the presence of medium alone (Figure 1A), whereas the presence of S1P at concentrations up to 200 ng/mL did not show any effect on the proliferation of HLECs (Figure 1B). VEGF-C, a potent lymphangiogenic factor, also induced the migration and proliferation of HLECs (Figure 1A,B). Although S1P had no mitogenic activity on HLECs, the migratory activity of S1P (100 ng/mL) was much higher than that of VEGF-C (500 ng/mL). We next examined the ability of S1P to promote the capillary-like tube formation of HLECs on GFR Matrigel. In HLECs, the presence of S1P at 20 ng/mL, 100 ng/mL, and 200 ng/mL caused 1.7 plus or minus 0.3-fold, 2.1 plus or minus 0.2-fold, and 2.2 plus or minus 0.1-fold increases in tube area, respectively, compared with the negative control containing only the medium (Figure 1C). VEGF-C (500 ng/mL) also stimulated tube formation (2.4 ± 0.3-fold increase).
S1P induced migration and the formation of capillary-like tube structure of human lymphatic endothelial cells. (A) After 4 hours of incubation with S1P or VEGF-C, migrated HLECs were stained and counted in 3 random fields. (B) S1P or VEGF-C was added to serum-starved HLECs for 24 hours, followed by additional incubation for 12 hours with 0.5 μCi (0.0185 MBq) [3H]-thymidine in HE-SFM. Results are expressed as the percentage [3H]-thymidine incorporation of the control versus S1P- or VEGF-C–treated HLECs. (C) HLECs were laid on a 24-well, GFR Matrigel-coated plate and incubated with S1P or VEGF-C for 6 hours. Two randomly chosen fields per well were photographed and the total tube area was analyzed using Scion Image. (D,E) Effect of S1P on the migration (D) and capillary-like tube formation (E) of HMVECs-dLy, lymphatic endothelial cells derived from dermal skins, was examined as described in panels A and C, respectively. (F) Cultured HLECs and HMVECs-dLy were fixed, and immunostained using antibodies against Prox-1 (green), LYVE-1 (red), and podoplanin (green). The nuclei were counterstained by Hoechst (blue). Scale bars represent 50 μm. Note that HLECs and HMVECs-dLy were not immunolabeled by isotype-matched control IgG antibodies (data not shown). All values are expressed as means (± SD). Data are representative of 3 independent experiments with similar results. * and ** indicate statistically significant differences (P < .05 and P < .01, respectively).
S1P induced migration and the formation of capillary-like tube structure of human lymphatic endothelial cells. (A) After 4 hours of incubation with S1P or VEGF-C, migrated HLECs were stained and counted in 3 random fields. (B) S1P or VEGF-C was added to serum-starved HLECs for 24 hours, followed by additional incubation for 12 hours with 0.5 μCi (0.0185 MBq) [3H]-thymidine in HE-SFM. Results are expressed as the percentage [3H]-thymidine incorporation of the control versus S1P- or VEGF-C–treated HLECs. (C) HLECs were laid on a 24-well, GFR Matrigel-coated plate and incubated with S1P or VEGF-C for 6 hours. Two randomly chosen fields per well were photographed and the total tube area was analyzed using Scion Image. (D,E) Effect of S1P on the migration (D) and capillary-like tube formation (E) of HMVECs-dLy, lymphatic endothelial cells derived from dermal skins, was examined as described in panels A and C, respectively. (F) Cultured HLECs and HMVECs-dLy were fixed, and immunostained using antibodies against Prox-1 (green), LYVE-1 (red), and podoplanin (green). The nuclei were counterstained by Hoechst (blue). Scale bars represent 50 μm. Note that HLECs and HMVECs-dLy were not immunolabeled by isotype-matched control IgG antibodies (data not shown). All values are expressed as means (± SD). Data are representative of 3 independent experiments with similar results. * and ** indicate statistically significant differences (P < .05 and P < .01, respectively).
To further confirm that S1P has in vitro lymphangiogenic activity, we examined the ability of S1P to promote the migration and capillary-like tube formation of HMVECs-dLy, lymphatic endothelial cells derived from dermal skins. S1P significantly promoted the migration and tube formation of HMVECs-dLy in a dose-dependent manner (Figure 1D,E), whereas the presence of S1P at concentrations up to 200 ng/mL did not show any effect on the proliferation of HMVECs-dLy (data not shown). We next characterized HLECs and HMVECs-dLy with lymphatic endothelial cell–specific marker proteins by immunocytochemistry. More than 95% of HLECs and HMVECs-dLy expressed Prox-1, LYVE-1, and podoplanin (Figure 1F). These results suggest that S1P exerts in vitro lymphangiogenic activity by promoting migration and differentiation, but not proliferation, of human lymphatic endothelial cells.
S1P promoted in vivo lymphangiogenesis
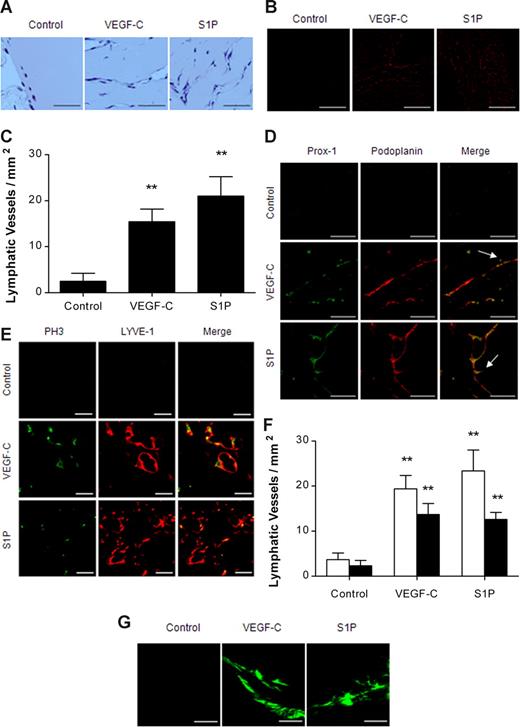
Since S1P stimulated migration and differentiation of HLECs and HMVECs-dLy in vitro, we performed a Matrigel plug assay to investigate whether S1P had in vivo lymphangiogenic activity. Matrigels containing S1P (0.4 μg) or VEGF-C (1 μg) were subcutaneously injected into C57BL/6 mice; after 10 days, the mice were killed and Matrigels were extracted. H&E staining and immunostaining of podoplanin revealed that both S1P and VEGF-C induced pronounced lymphatic vessel formation (Figure 2A-C), whereas lower numbers of lymphatic vessels were observed in the control. The podoplanin-positive lymphatic vessels were also positive for Prox-1, another lymphatic endothelial cell–specific marker, and sprouting lymphatic vessels were observed in S1P- and VEGF-C–treated Matrigel (Figure 2D). In the VEGF-C– and S1P-treated mice, respectively, 70.6% plus or minus 12.5% and 53.8% plus or minus 6.8% of LYVE-1–positive lymphatic vessels were stained by PH3, a marker of cell proliferation (Figure 2E,F). Furthermore, FITC-dextran microlymphography revealed that both S1P and VEGF-C were able to induce the growth of functional lymphatic vessels (Figure 2G). These observations suggest that S1P is a potent lymphangiogenic lipid molecule in vivo and that the lymphangiogenic activity of S1P is comparable to that of VEGF-C, a potent lymphangiogenic growth factor.
S1P promoted in vivo lymphangiogenesis. Ten days after subcutaneous injection of Matrigel containing none, VEGF-C (1 μg), or S1P (0.4 μg), in C57BL/6 mice (5 mice per group), the Matrigel was removed, fixed, embedded in paraffin, sectioned at 4 μm, and immunostained. (A) Cross-sections of Matrigel were stained by H&E. Scale bars represent 50 μm. (B,C) Lymphatic vessels in Matrigel were immunostained for podoplanin (red). Representative photographs and the number of podoplanin-positive lymphatic vessels per field are shown in panels B and C, respectively. Scale bars represent 200 μm. (D) Lymphatic vessels in Matrigel were immunostained for Prox-1 (green) and podoplanin (red). Arrows show sprouting lymphatic vessels. Scale bars represent 50 μm. (E,F) Proliferating lymphatic endothelial cells in Matrigel were double immunostained for PH3 (green) and LYVE-1 (red). Representative photographs are shown in panel E. Scale bars represent 20 μm. The number of LYVE-1+ (□) or LYVE-1+/PH3+ (■) vessels per field were counted (F). (G) Uptake of injected FITC-dextran (2000 kDa) into newly formed lymphatic vessels was visualized using confocal microscope. Scale bars represent 100 μm. All values are expressed as means (± SEM). ** indicates statistically significant difference (P < .01).
S1P promoted in vivo lymphangiogenesis. Ten days after subcutaneous injection of Matrigel containing none, VEGF-C (1 μg), or S1P (0.4 μg), in C57BL/6 mice (5 mice per group), the Matrigel was removed, fixed, embedded in paraffin, sectioned at 4 μm, and immunostained. (A) Cross-sections of Matrigel were stained by H&E. Scale bars represent 50 μm. (B,C) Lymphatic vessels in Matrigel were immunostained for podoplanin (red). Representative photographs and the number of podoplanin-positive lymphatic vessels per field are shown in panels B and C, respectively. Scale bars represent 200 μm. (D) Lymphatic vessels in Matrigel were immunostained for Prox-1 (green) and podoplanin (red). Arrows show sprouting lymphatic vessels. Scale bars represent 50 μm. (E,F) Proliferating lymphatic endothelial cells in Matrigel were double immunostained for PH3 (green) and LYVE-1 (red). Representative photographs are shown in panel E. Scale bars represent 20 μm. The number of LYVE-1+ (□) or LYVE-1+/PH3+ (■) vessels per field were counted (F). (G) Uptake of injected FITC-dextran (2000 kDa) into newly formed lymphatic vessels was visualized using confocal microscope. Scale bars represent 100 μm. All values are expressed as means (± SEM). ** indicates statistically significant difference (P < .01).
S1P stimulated lymphangiogenesis via the S1P1/Gi pathway
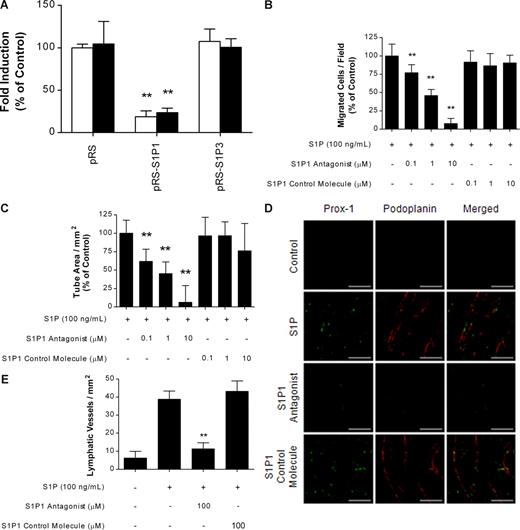
S1P1, S1P2, and S1P3 are widely expressed subtypes in vascular endothelial cells,25-27 and S1P stimulates proliferation and migration of vascular endothelial cells via S1P1 and S1P3.20,28 To investigate whether S1P-induced lymphangiogenesis was mediated by the activation of S1P receptors, we performed real-time PCR analysis to identify the expression pattern of S1P receptors in HLECs. Real-time PCR analysis revealed that both HLECs and HUVECs expressed S1P1 and S1P3, but not S1P2 (Figure 3A). Although it has been reported that VEGF induces S1P1 receptors in endothelial cells,29 real-time PCR analysis revealed that VEGF-C did not affect the expression of S1P1 and S1P3 in HLECs (data not shown). Because 2 distinctive S1P receptors were expressed on HLECs, we next carried out migration and tube formation assays to determine which receptor was mainly involved in S1P-induced lymphangiogenesis. S1P-induced migration and tube formation of HLECs were almost completely blocked by treatment with 100 ng/mL PTX, a Gi protein–specific inhibitor, whereas treatment with PTX in the control did not affect HLECs (Figure 3B,C). Both S1P1 and S1P3 convert external signals into intracellular signals via heterotrimeric G proteins coupled to them; S1P1 is coupled with the Gi protein, but S1P3 is coupled with the Gq and G12/13 proteins as well as the Gi protein.4 Therefore, our results suggest that the activation of Gi proteins coupled to S1P1 may be involved in S1P-induced migration and differentiation of HLECs.
S1P-induced in vitro lymphangiogenesis was mediated through the S1P1/Gi protein. (A) Total RNA (100 ng) from HUVECs (□) or HLECs (■) was amplified using primers for S1P receptors and β-actin. For quantification, the targets were normalized to β-actin as an internal standard. (B) After a 2-hour preincubation with 100 ng/mL PTX, HLECs were treated with 100 ng/mL S1P and 100 ng/mL PTX for an additional 4 hours. The migrated HLECs were stained and counted in 3 random fields. (C) HLECs were laid on a GFR Matrigel-coated 24-well plate and incubated with 100 ng/mL S1P and 100 ng/mL PTX for 6 hours. Two randomly chosen fields per well were photographed and the total tube area was analyzed using Scion Image. All values are expressed as means (± SD). Data are representative of 3 independent experiments with similar results. NS and ** indicate no significant difference and a statistically significant difference (P < .01), respectively.
S1P-induced in vitro lymphangiogenesis was mediated through the S1P1/Gi protein. (A) Total RNA (100 ng) from HUVECs (□) or HLECs (■) was amplified using primers for S1P receptors and β-actin. For quantification, the targets were normalized to β-actin as an internal standard. (B) After a 2-hour preincubation with 100 ng/mL PTX, HLECs were treated with 100 ng/mL S1P and 100 ng/mL PTX for an additional 4 hours. The migrated HLECs were stained and counted in 3 random fields. (C) HLECs were laid on a GFR Matrigel-coated 24-well plate and incubated with 100 ng/mL S1P and 100 ng/mL PTX for 6 hours. Two randomly chosen fields per well were photographed and the total tube area was analyzed using Scion Image. All values are expressed as means (± SD). Data are representative of 3 independent experiments with similar results. NS and ** indicate no significant difference and a statistically significant difference (P < .01), respectively.
We next examined the effect of specific knockdown of S1P1 and S1P3 by RNA interference on S1P-induced migration and differentiation of HLECs. Down-regulation of S1P1 significantly inhibited S1P-induced migration and tube formation of HLECs, whereas down-regulation of S1P3 did not (Figure 4A). To further confirm that S1P promotes lymphangiogenesis by stimulating S1P1, we investigated the effect of an S1P1 antagonist30 on S1P-induced in vitro and in vivo lymphangiogenesis. The S1P1 antagonist blocked S1P-induced migration and tube formation of HLECs in a dose-dependent manner, whereas an inactive S1P1 control molecule did not (Figure 4B,C). Furthermore, Matrigel plug assays showed that the S1P1 antagonist almost completely blocked S1P-induced lymphatic vessel formation, whereas the inactive S1P1 control molecule did not (Figure 4D,E). These results strongly suggest that the activation of Gi proteins coupled to S1P1 may be involved in S1P-induced migration and differentiation of HLECs in vitro and lymphangiogenesis in vivo.
S1P-induced lymphangiogenesis was mediated through the S1P1. (A) HLECs were infected with retroviruses carrying S1P1 and S1P3 shRNA expression vectors in pRS plasmid, after which stably transfected cells were obtained by selection with puromycin. pRS plasmid was used as control. After a 4-hour incubation with 100 ng/mL S1P, migrated HLECs were stained and counted in 3 random fields (□). HLECs were laid on a GFR Matrigel-coated 24-well plate and incubated with 100 ng/mL S1P for 6 hours. Two randomly chosen fields per well were photographed and the total tube area was analyzed using Scion Image (■). (B,C) Effect of S1P1 antagonist or S1P1 control molecule on the S1P-induced migration (B) and capillary-like tube formation (C) of HLECs was examined as described in panel A. (D,E) Ten days after subcutaneous injection of Matrigel in C57BL/6 mice (5 mice per group), the Matrigel was removed, fixed, embedded in paraffin, sectioned at 4 μm, and immunostained using antibodies specific for Prox-1 (green) and podoplanin (red). (D) Representative photographs of untreated control mice, and mice treated with S1P (0.4 μg) in the absence or presence of S1P1 antagonist (100 μM) or S1P1 control molecule (100 μM). Scale bars represent 50 μm. (E) The number of Prox-1+/podoplanin+ lymphatic vessels per field was counted. All values are expressed as means plus or minus SD (A-C) and means plus or minus SEM (E). In panels A-C, data are representative of 3 independent experiments with similar results. ** indicates a statistically significant difference (P < .01).
S1P-induced lymphangiogenesis was mediated through the S1P1. (A) HLECs were infected with retroviruses carrying S1P1 and S1P3 shRNA expression vectors in pRS plasmid, after which stably transfected cells were obtained by selection with puromycin. pRS plasmid was used as control. After a 4-hour incubation with 100 ng/mL S1P, migrated HLECs were stained and counted in 3 random fields (□). HLECs were laid on a GFR Matrigel-coated 24-well plate and incubated with 100 ng/mL S1P for 6 hours. Two randomly chosen fields per well were photographed and the total tube area was analyzed using Scion Image (■). (B,C) Effect of S1P1 antagonist or S1P1 control molecule on the S1P-induced migration (B) and capillary-like tube formation (C) of HLECs was examined as described in panel A. (D,E) Ten days after subcutaneous injection of Matrigel in C57BL/6 mice (5 mice per group), the Matrigel was removed, fixed, embedded in paraffin, sectioned at 4 μm, and immunostained using antibodies specific for Prox-1 (green) and podoplanin (red). (D) Representative photographs of untreated control mice, and mice treated with S1P (0.4 μg) in the absence or presence of S1P1 antagonist (100 μM) or S1P1 control molecule (100 μM). Scale bars represent 50 μm. (E) The number of Prox-1+/podoplanin+ lymphatic vessels per field was counted. All values are expressed as means plus or minus SD (A-C) and means plus or minus SEM (E). In panels A-C, data are representative of 3 independent experiments with similar results. ** indicates a statistically significant difference (P < .01).
S1P-induced lymphangiogenesis was mediated via the PLC/Ca2+ pathway
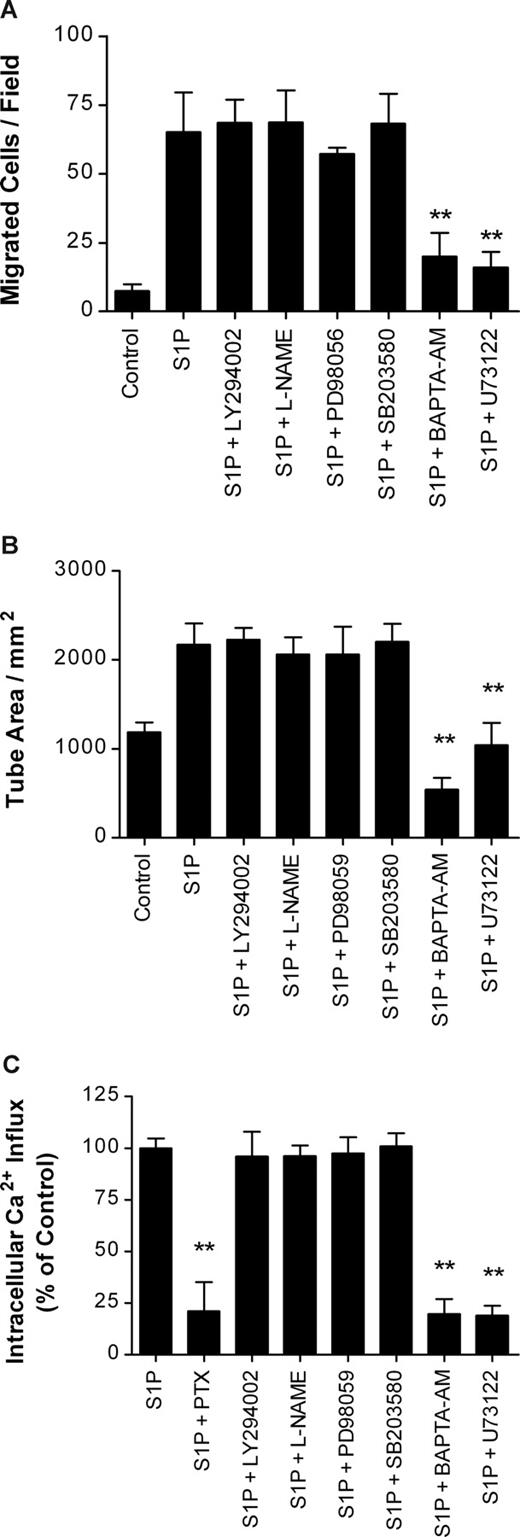
After activation of the Gi protein complex by an upstream receptor such as S1P1, the Gi protein complex is dissociated into Giα and Giβγ subunits to activate downstream signaling molecules. To investigate downstream signaling events involved in the S1P-induced lymphangiogenesis of HLECs, we performed migration and tube formation assays with LY294002, L-NAME, PD98059, SB203580, BAPTA-AM, and U73122, which are specific inhibitors for phosphatidylinositol 3-kinase (PI3K), nitric oxide synthase (NOS), p44/42 mitogen-activated protein kinase (MAPK), p38 MAPK, intracellular Ca2+ chelator, and PLC, respectively. Approximately 78.3% and 85.2% of S1P-induced migration of HLECs was efficiently blocked when 100 ng/mL S1P was applied to HLECs with 30 μM BAPTA-AM and 5 μM U73122, respectively (Figure 5A). Furthermore, S1P-induced capillary-like tube formation of HLECs was completely inhibited by treatment with 30 μM BAPTA-AM and 5 μM U73122 (Figure 5B). However, treatment with the inhibitors for PI3K, NOS, p44/42 MAPK, and p38 MAPK did not show any inhibitory effect on S1P-induced migration and tube formation of HLECs. All these results suggest that PLC and intracellular Ca2+ mobilization, but not NOS, PI3K, p44/42 MAPK, and p38 MAPK, are major signaling pathways involved in S1P-induced lymphangiogenesis.
S1P-induced in vitro lymphangiogenesis was mediated through the PLC/Ca2+ pathway. (A) After a 4-hour isncubation with 100 ng/mL S1P containing 10 μM LY294002, 1 mM L-NAME, 10 μM PD98059, 25 μM SB203580, 30 μM BAPTA-AM, or 5 μM U73122, the migrated HLECs were stained and counted in 3 random fields. (B) HLECs were laid on a GFR Matrigel-coated 24-well plate and incubated with 100 ng/mL S1P containing 10 μM LY294002, 1 mM L-NAME, 10 μM PD98059, 25 μM SB203580, 30 μM BAPTA-AM, or 5 μM U73122 for 6 hours. Two randomly chosen fields per well were photographed and the total tube area was analyzed using Scion Image. (C) HLECs were loaded with fura-2/AM for 30 minutes. The cells were resuspended in Ca2+-free Locke solution, transferred to a quartz cuvette, and exposed to 100 ng/mL S1P with 100 ng/mL PTX, 10 μM LY294002, 1 mM L-NAME, 10 μM PD98059, 25 μM SB203580, 30 μM BAPTA-AM, or 5 μM U73122. Relative intracellular Ca2+ influx was calculated from the tracing. All values are expressed as means (± SD). Data are representative of 3 independent experiments with similar results. ** indicates a statistically significant difference (P < .01).
S1P-induced in vitro lymphangiogenesis was mediated through the PLC/Ca2+ pathway. (A) After a 4-hour isncubation with 100 ng/mL S1P containing 10 μM LY294002, 1 mM L-NAME, 10 μM PD98059, 25 μM SB203580, 30 μM BAPTA-AM, or 5 μM U73122, the migrated HLECs were stained and counted in 3 random fields. (B) HLECs were laid on a GFR Matrigel-coated 24-well plate and incubated with 100 ng/mL S1P containing 10 μM LY294002, 1 mM L-NAME, 10 μM PD98059, 25 μM SB203580, 30 μM BAPTA-AM, or 5 μM U73122 for 6 hours. Two randomly chosen fields per well were photographed and the total tube area was analyzed using Scion Image. (C) HLECs were loaded with fura-2/AM for 30 minutes. The cells were resuspended in Ca2+-free Locke solution, transferred to a quartz cuvette, and exposed to 100 ng/mL S1P with 100 ng/mL PTX, 10 μM LY294002, 1 mM L-NAME, 10 μM PD98059, 25 μM SB203580, 30 μM BAPTA-AM, or 5 μM U73122. Relative intracellular Ca2+ influx was calculated from the tracing. All values are expressed as means (± SD). Data are representative of 3 independent experiments with similar results. ** indicates a statistically significant difference (P < .01).
Increased mobilization of intracellular Ca2+ by S1P was mediated through the Gi/PLC/Ca2+ pathway
The activation of PLC by extracellular S1P evokes the induction of robust Ca2+ mobilization in HUVECs, which is important to the S1P-induced migration of vascular endothelial cells.31,32 Since we demonstrated the involvement of S1P-mediated PLC activation and intracellular Ca2+ release in the migration and capillary-like tube formation of HLECs, we investigated whether the S1P-mediated increase in intracellular Ca2+ release was dependent on the Gi/PLC pathway in HLECs. The application of S1P to fura-2/AM-loaded HLECs caused a dramatic increase in Ca2+ release into the cytosol in a dose-dependent manner (data not shown). The increase in Ca2+ influx by 100 ng/mL S1P was significantly inhibited by treatment with 100 ng/mL PTX (78.9% ± 14.0%), 30 μM BAPTA-AM (80.3% ± 7.3%), and 5 μM U73122 (81.3% ± 4.7%), but treatment with the signaling inhibitors for PI3K, NOS, p44/42 MAPK, and p38 MAPK did not show any inhibitory effect on Ca2+ mobilization (Figure 5C). These results suggest that S1P-induced migration and differentiation of HLECs may be mediated by intracellular Ca2+ release following Gi/PLC activation.
Discussion
The lymphatic system has important roles in regulating tissue fluid balance for homeostasis and facilitating interstitial protein transport and immunologic function through lymph nodes. Clinical evidence suggests that the dissemination of malignant tumors to regional lymph nodes via the lymphatic vessels is important in tumor metastasis and that chronic inflammation causes lymphangiogenesis and lymphedema, although the molecular mechanisms regulating lymphangiogenesis are largely unclear. In addition to VEGF-C, a potent lymphangiogenic growth factor, VEGF-A, bFGF, HGF, angiopoietin-1, IGF-1/2, and PDGF-BB, previously known as proangiogenic factors, have lymphangiogenic activity. However, no previous studies have examined the effects of bioactive lipid molecules, including S1P, on lymphangiogenesis. In this report, we provide evidence that S1P induces in vitro and in vivo lymphangiogenesis by stimulating the migration and differentiation of lymphatic endothelial cells via a S1P1/Gi/PLC/Ca2+ signaling pathway. To the best of our knowledge, this study is the first to establish that S1P is a lymphangiogenic lipid mediator.
During angiogenesis and lymphangiogenesis, the migration and proliferation of endothelial cells are important procedures to maintain net vascular structure by replenishing new endothelial cells in the empty space caused by the migration of endothelial cells toward stimuli. Several reports have supported the conclusion that S1P has potent migratory effects on vascular endothelial cells.21,33 In our study, the S1P (100 ng/mL)–induced migration of HLECs was approximately 5.3-fold higher than that induced by VEGF-C (500 ng/mL). This result indicates that the activity of S1P to induce migration of lymphatic endothelial cells was similar to that for vascular endothelial cells, in which S1P has 3- to 10-fold greater effects than induction by VEGF-A or bFGF.34 Furthermore, we also demonstrated that S1P (0.4 μg/0.5 mL in Matrigel) can promote pronounced lymphangiogenesis in vivo, similar to VEGF-C (1 μg/0.5 mL in Matrigel), suggesting that S1P might be an important regulator of lymphangiogenesis. Although the physiological concentration of S1P has been reported to be 0.2 μg/mL in serum, levels of S1P are elevated in pathological conditions: 0.4 μg/mL in synovial fluids from rheumatoid arthritis patients and 0.9 μg/mL in ascites of ovarian cancer patients.35,36 However, we do not know the local concentration of S1P in tissues.
Although S1P directly induces the proliferation of human aortic endothelial cells (HAECs) and bovine aortic endothelial cells,21,28,33 S1P (40 ng/mL) showed less potent mitogenic activities on these cells (approximately 1.3-fold increase of proliferation in HAECs and bovine aortic endothelial cells), suggesting that the mitogenic activity of S1P is less potent than that of VEGF-A (10 ng/mL). In this study, we found that S1P, up to 200 ng/mL, did not induce the proliferation of HLECs and HMVECs-dLy, but lymphatic vessels formed by S1P were proliferative in in vivo Matrigel plug assay. The discrepancies in in vitro and in vivo results might be explained as follows. During angiogenesis and lymphangiogenesis, migration is very earlier cellular response than proliferation by angiogenic or lymphangiogenic stimuli, and is induced by lower concentrations than those required for cell proliferation.37 Lymphatic endothelial cells can be dedifferentiated and stimulated to proliferate when contact inhibition is broken by migration. It is well known that soluble forms of E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 show angiogenic responses in vascular endothelial cells by induction of migration and differentiation without any in vitro mitogenic activity of vascular endothelial cells.22,38 Another possible explanation is that lymphatic endothelial cells can be proliferative by lymphangiogenic factors secreted from leukocytes recruited by S1P-induced migration. It has been known that S1P promotes trafficking of various kinds of leukocytes that secret lymphangiogenic factors such as VEGF-C/-D. However, the precise mechanisms by which S1P induces in vivo proliferation of lymphatic endothelial cells remain to be elucidated.
Although S1P receptors are coupled with various G proteins, such as Gi, Gq, and G12/13, S1P1 is coupled with only the Gi protein; other S1P receptors can bind to various G proteins. We identified 2 kinds of S1P receptors, S1P1 and S1P3, in HLECs. S1P-mediated migration and tube formation were completely blocked by the application of PTX. Since PTX specifically binds to the Gi protein and inhibits the dissociation of the Gi heterotrimer protein complex into 2 subunits, our results suggest that the Gi protein is an important signaling mediator of lymphangiogenesis induced by extracellular S1P. Although S1P3 can also bind to the Gi protein, PTX shows partial inhibition of the responsiveness mediated by S1P3/Gi, but complete inhibition of that mediated by S1P1/Gi. We further showed that shRNA-mediated down-regulation of S1P1 significantly blocked S1P-induced migration and differentiation of HLECs, whereas down-regulation of S1P3 did not. Moreover, an S1P1 selective antagonist significantly blocked S1P-induced in vitro and in vivo lymphangiogenesis, whereas an inactive S1P1 control molecule did not. These results strongly suggest that S1P-induced lymphangiogenesis is mediated mainly by activation of the Gi protein coupled to S1P1.
S1P effectively activates PI3K, p44/42 MAPK, p38 MAPK, and PLC in vascular endothelial cells, and these activations are dramatically inhibited by treatment with PTX.21,39 However, PI3K and p44/42 MAPK signaling pathway are less important to S1P-induced vascular endothelial cell migration.21,28,40-42 Furthermore, whether the involvement of the p38 MAPK pathway is important to S1P-induced vascular endothelial cell migration remains unclear.21,28 In this study, we found that inhibition of PLC activation and chelation of intracellular Ca2+ ions by treatment with U73122 and BAPTA-AM resulted in a significant reduction in S1P-induced migration and tube formation, as well as Ca2+ influx to lymphatic endothelial cells. This suggests that Ca2+ ions generated by active PLC are an important second messenger in S1P-induced migration and differentiation of lymphatic endothelial cells. Furthermore, application of inhibitors for PI3K, p44/42 MAPK, and p38 MAPK did not affect S1P-induced migration and differentiation of HLECs, suggesting that PI3K, p44/42 MAPK, and p38 MAPK are not involved in S1P-induced lymphangiogenesis.
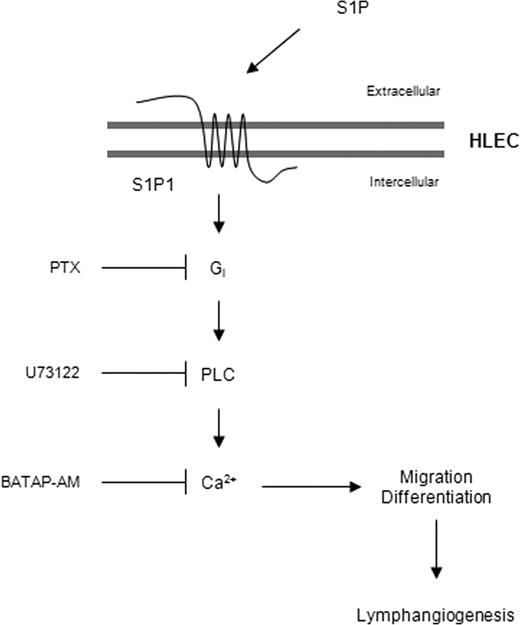
Our present data strongly suggest that extracellular S1P promotes new lymphatic vessel formation with the following mechanisms (Figure 6). Extracellular S1P binds to and stimulates its receptor S1P1, which is expressed on HLECs, resulting in the activation of coupled Gi proteins. Dissociation of active heterotrimeric Gi proteins from activated S1P1 stimulates PLC. Activated PLC stimulates the release of intracellular Ca2+ that causes the migration and differentiation of HLECs, resulting in lymphangiogenesis. S1P is generated by the phosphorylation of sphingosine, which is mediated by SphK1 and SphK2. In many primary tumor cells or inflammatory cells, the expression or activity of SphK1, also known as oncogenic kinase,43 is up-regulated by many angiogenic and inflammatory cytokines such as PDGF, epidermal growth factor, tumor necrosis factor-α, and interleukin-1β.44 Although it remains unclear how increased intracellular S1P is secreted to the extracellular milieu in tumor-associated cells or inflammatory cells, extracellular S1P can be generated by exported SphK1 in HUVECs.45,46 Hence, the possibility exists that extracellular S1P secreted from tumor-surrounded stromal cells, platelets, or inflammatory cells,47 as well as tumor cells, can activate vascular and lymphatic endothelial cells; this activation induces angiogenesis and lymphangiogenesis for tumor cell survival and tumor metastasis to regional lymph nodes.
Plausible mechanism for S1P-mediated lymphangiogenesis. Extracellular S1P, which is derived from tumor or inflammatory cells, binds to its receptor S1P1 and stimulates the activation of coupled Gi proteins. Dissociation of active heterotrimeric Gi proteins from activated S1P1 stimulates PLC, which causes the release of intracellular Ca2+, resulting in the stimulation of HLEC migration and differentiation. Extracellular S1P activates lymphatic endothelial cells to induce lymphangiogenesis, as well as vascular endothelial cells to induce angiogenesis, for tumor metastasis or an immune response.
Plausible mechanism for S1P-mediated lymphangiogenesis. Extracellular S1P, which is derived from tumor or inflammatory cells, binds to its receptor S1P1 and stimulates the activation of coupled Gi proteins. Dissociation of active heterotrimeric Gi proteins from activated S1P1 stimulates PLC, which causes the release of intracellular Ca2+, resulting in the stimulation of HLEC migration and differentiation. Extracellular S1P activates lymphatic endothelial cells to induce lymphangiogenesis, as well as vascular endothelial cells to induce angiogenesis, for tumor metastasis or an immune response.
In this study, we demonstrated that S1P has in vitro and in vivo lymphangiogenic activity and identified S1P as the first lipid lymphangiogenic factor. This report may allow us to categorize S1P, a bioactive lipid molecule, as a new lymphangiogenic factor. Because several bioactive lipids, including lysophosphatidic acid, sphingosylphosphorylcholine, gangliosides, and sphingomyelin, have proangiogenic activity,48 investigations of other bioactive lymphangiogenic lipids may be warranted to clarify the mechanisms of lymphangiogenesis. In addition, because chronic inflammation and cancer are highly related to each other, coordinated regulation of lymphangiogenesis and inflammation by S1P suggests that they may be pharmacological targets for development of novel anti-inflammatory and antitumor metastatic therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mr Hee-Yeoul Park and Ms Kyung Young Ji (Pohang University of Science and Technology, Pohang, Republic of Korea) for immunohistologic and confocal analysis, respectively.
This work was supported by the Korea Science and Engineering Foundation (KOSEF, Daejeon, Republic of Korea) grant funded by the Korea government (MOST, no. R15-2004-033-05001-0), supported by a grant of the National R&D Program for Cancer Control (Goyang, Republic of Korea), Ministry of Health & Welfare, Republic of Korea (0320380-2), and supported by the Korea Basic Science Institute (Daejeon, Republic of Korea) K-MeP (T27021; Y.S.G.). B.S.H., H.G.M., and S.L. were recipients of Brain Korea 21 fellowships.
Authorship
Contribution: C.M.Y. designed research, performed research, analyzed data, and drafted the paper; B.S.H. performed research, analyzed data, and drafted the paper; H.G.M. and S.L. performed research and analyzed data; P.-G.S. and Y.-K.K. designed and advised research; C.-B.C. designed research, advised research, and reviewed the paper; Y.S.G. designed research, advised research, and drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yong Song Gho, Division of Molecular and Life Sciences, Pohang University of Science and Technology, Pohang 790-784, Republic of Korea; e-mail: ysgho@postech.ac.kr.

![Figure 1. S1P induced migration and the formation of capillary-like tube structure of human lymphatic endothelial cells. (A) After 4 hours of incubation with S1P or VEGF-C, migrated HLECs were stained and counted in 3 random fields. (B) S1P or VEGF-C was added to serum-starved HLECs for 24 hours, followed by additional incubation for 12 hours with 0.5 μCi (0.0185 MBq) [3H]-thymidine in HE-SFM. Results are expressed as the percentage [3H]-thymidine incorporation of the control versus S1P- or VEGF-C–treated HLECs. (C) HLECs were laid on a 24-well, GFR Matrigel-coated plate and incubated with S1P or VEGF-C for 6 hours. Two randomly chosen fields per well were photographed and the total tube area was analyzed using Scion Image. (D,E) Effect of S1P on the migration (D) and capillary-like tube formation (E) of HMVECs-dLy, lymphatic endothelial cells derived from dermal skins, was examined as described in panels A and C, respectively. (F) Cultured HLECs and HMVECs-dLy were fixed, and immunostained using antibodies against Prox-1 (green), LYVE-1 (red), and podoplanin (green). The nuclei were counterstained by Hoechst (blue). Scale bars represent 50 μm. Note that HLECs and HMVECs-dLy were not immunolabeled by isotype-matched control IgG antibodies (data not shown). All values are expressed as means (± SD). Data are representative of 3 independent experiments with similar results. * and ** indicate statistically significant differences (P < .05 and P < .01, respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2007-11-125203/6/m_zh80170822930001.jpeg?Expires=1769350828&Signature=BAf5rioSZ96F9ieGQAei97KEbKeATVXRfsOqLctjrXdt2TT9AQxi7sZ9N6JbvLR8iW6ZOk0wU11-~zqwshzsh67t-1eA3mZ9MzUJWRk51T4zm6ZeJfzkNJsMFxlnohbWniD3ziVeciILXT8PHrqYPqMEX5oZAHho3Gusj21-iap-OQuwjEFMGzQpjWBZChwx4b34SrOBgnUw5F0PNqrhAeBnh1ERLnA2-~Os5ysApWbjaby4ihKvAQp6nVO4zxxBSUPO0-HnPfri8d1hoaUkXniGHaR8-mL~8-ihhy7V7JryJG8THZBcgiAVSXSf3dFc9FNJ9wokmviV-NfFExGcXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)