Abstract
Recent observations have shown that Drm, a member the Dan family of bone morphogenic protein (BMP) antagonists, induces endothelial cell (EC) sprouting in vitro and angiogenesis in vivo by interacting with signaling EC receptors in a BMP-independent manner. Here, recombinant Drm (rDrm) up-regulates angiopoientin-1 (Ang-1) expression in EC without affecting Ang-2 and Tie-2 receptor expression. Ang-1 up-regulation is mediated by the activation of the transcription factor NF-κB. Specific inhibition of Ang-1 activity by anti–Ang-1 antibodies, soluble Tie-2 receptor, or Ang-1 siRNA transfection significantly reduced the rDrm-mediated sprouting of EC in three-dimensional fibrin and type I collagen gels. In addition, Ang-1 antagonists inhibited the angiogenic activity exerted by rDrm in the chick embryo chorioallantoic membrane. Taken together, the data indicate that the proangiogenic activity of Drm is mediated by the activation of an Ang-1–dependent autocrine loop of stimulation in EC.
Introduction
The angiopoietin/Tie receptor system, which consists of 4 angiopoietin (Ang) ligands and the Tie-1 and Tie-2 receptors, plays a nonredundant role in embryonic vascularization and tumor angiogenesis.1,2 An autocrine Ang-1/Tie-2 axis modulates blood vessel plasticity and contributes to vascular maintenance. Accordingly, Ang-1 enhances survival, migration, and network formation of endothelial cells (ECs) in vitro3 and induces neovascularization in vivo.4,5
Drm, a member the Dan family of bone morphogenic protein (BMP) antagonists,6,7 interacts with signaling EC receptors in a BMP-independent manner.8 The productive interaction stimulates EC sprouting and migration in vitro and induces angiogenesis in vivo.8 Drm is overexpressed in human cancers by tumor and stromal cells, thus suggesting that Drm may contribute to tumor neovascularization.8-10
Here, we investigated the role of the Ang/Tie-2 system in mediating the angiogenic activity of Drm. The results demonstrate that Drm up-regulates Ang-1 expression in EC via activation of the transcription factor NF-κB. This leads to an autocrine loop of stimulation that mediates Drm-induced EC sprouting in vitro and angiogenesis in vivo.
Methods
Cell cultures
Approval for these studies was obtained from the Ethical Committee of the School of Medicine of the University of Brescia. Subcutaneous murine microvascular EC (SIE cells) and vascular endothelial growth factor (VEGF) receptor-2–transduced murine aortic EC (VEGFR2-MAE cells) were cultured in DMEM supplemented with 10% calf serum (Invitrogen, Carlsbad, CA). Human umbilical vein ECs (HUVECs) were grown as described.11 ECs were transfected (70%-80% efficiency) with siRNAs using the SiRNA Transfection Reagent (Santa Cruz Biotechnology, Santa Cruz, CA) according to the manufacturer's instructions.
Western blotting
Western blotting was performed according to standard procedures on the conditioned media (0.5 mL), total cell lysates (20 μg), nuclear extracts (5 μg), and cytoplasmic fractions (10 μg) from serum-starved EC cultures stimulated with 50 ng/mL of recombinant Drm (rDrm, R&D Systems, Minneapolis, MN).
Electrophoretic mobility shift assay
Nuclear extracts (2 μg) were incubated with biotin-labeled NF-κB double-stranded DNA oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC) according to LightShift Chemiluminescent EMSA Kit instructions (Pierce, Rockford, IL) and analyzed on a native 6% polyacrylamide gel.
EC sprouting
Artery ring assay
One-millimeter human umbilical artery rings were embedded in fibrin gel14 and cultured in human EC medium SFM (Invitrogen) with different stimuli. After 3 days, EC sprouts were counted under an inverted microscope at ×10 magnification.
Gene expression analysis
cDNA was generated from total RNA using the MMLV-RT kit (Invitrogen). Reverse-transcribed polymerase chain reaction (RT-PCR) was performed using murine specific primers for Ang-1 (NM_009640: 5′-TCATGCTAACAGGAGGTTGGT-3′; 5′-ATGGTGGTGGAACGTAAGGA-3′); Ang-2 (NM_007426: 5′-TCGGGAGCCCTCTGGGAGAGTACT-3′; 5′-AGCGAATGCGCCTCGTTGC-3′); β-actin (NM_009073: 5′-CGTAAAGACCTCTATGCCAACA-3′; 5′-CCACCGATCCACACAGAGTA-3′).
Chick embryo chorioallantoic membrane assay
Alginate beads (5 μL) containing vehicle, rDrm (100 ng/embryo), parental or Drm-overexpressing COS cells8 (10 000 cells/embryo) were placed on the chorioallantoic membrane (CAM) of fertilized White Leghorn chicken eggs at day 11 of incubation.15 After 72 hours, new blood vessels converging toward the implant were counted under a stereomicroscope at ×5 magnification.16
Results and discussion
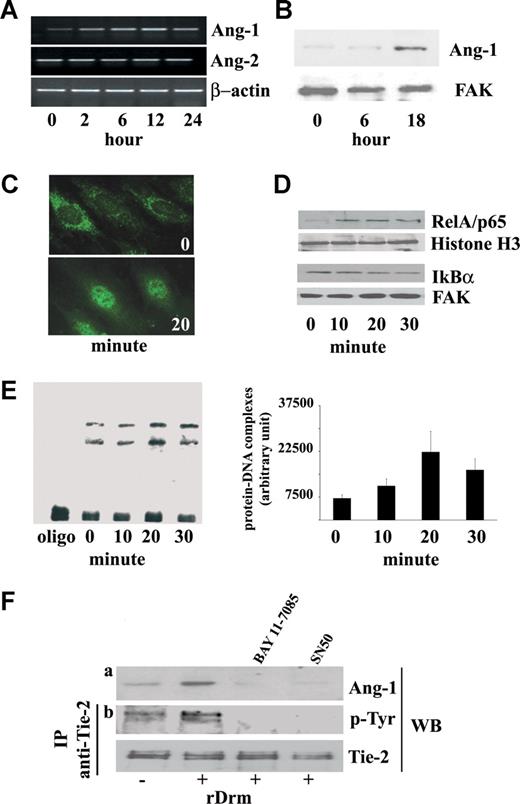
Steady-state levels of Ang-1, Ang-2, Tie-2, and VEGF-A transcripts were evaluated in rDrm-treated SIE cells by RT-PCR analysis. Within 2 hours, rDrm causes the up-regulation of Ang-1 expression without affecting the expression of Ang-2 (Figure 1A) or of Tie-2 and VEGF-A (data not shown). Accordingly, Western blot analysis of rDrm-treated EC extracts demonstrates an increase in the levels of cell-associated Ang-1 protein at 18 hours after stimulation (Figure 1B). The effect was dose-dependent, maximal stimulation occurring at 50 ng/mL of rDrm (data not shown). Secreted Ang-1 induces Tie-2 phosphorylation in SIEC cells (Figure 1F) that was prevented by neutralizing anti–Ang-1 antibodies (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), pointing to an Ang-1/Tie-2 autocrine loop of stimulation in Drm-activated ECs.
rDrm induces Ang-1 expression in EC in a NF-κB–dependent manner. (A) Serum-starved SIE cells were stimulated for 2, 6, 12, and 24 hours with 50 ng/mL of rDrm. At the end of incubation, total RNA was extracted and Ang-1 and Ang-2 transcripts were analyzed by semiquantitative RT-PCR. β-Actin was used as a control. (B) Cell lysates were prepared from SIE cells stimulated for 0, 6, and 18 hours with 50 ng/mL of rDrm. Then, 20-μg aliquots were probed by Western blotting with anti–Ang-1 antibodies (Santa Cruz Biotechnology). Uniform loading of the gels was confirmed by incubation of the membranes with anti–focal adhesion kinase (FAK) antibodies. (C) Serum-starved ECs were incubated for 20 minutes with 50 ng/mL of rDrm and immunostained with an anti-RelA/p65 antibody (Santa Cruz Biotechnology) followed by antirabbit FITC (Invitrogen). Control cells show a diffuse cytoplasmic RelA/p65 immunoreactivity (top panel) that localizes into the nucleus after rDrm stimulation (bottom panel). Analysis was performed using a Zeiss Axiovert 200M epifluorescence microscope equipped with a Plan-Apochromat 63×/1.4 NA oil objective. (D) SIE cells were stimulated for 0 to 30 minutes with 50 ng/mL of rDrm. Then, nuclear (5 μg) and cytoplasmic (10 μg) extracts were probed by Western blotting with anti-RelA/p65 and anti-IkBα (Santa Cruz Biotechnology) antibodies, respectively. Uniform loading of the gels was confirmed by incubation of the membranes with antihistone H3 (Santa Cruz Biotechnology) and anti-FAK antibodies, respectively. (E) Nuclear extracts (2 μg) of SIE cells treated for 0 to 30 minutes with 50 ng/mL of rDrm were incubated with a biotin-labeled NF-κB double-stranded DNA oligonucleotide probe. (Left panel) The protein-DNA complexes were analyzed by EMSA onto a native 6% polyacrylamide gel; the first lane shows the migration of the labeled probe in the absence of nuclear extract. (Right panel) Densitometric analysis of the protein-DNA complexes shown in the left panel; data are the mean plus or minus SEM of 3 independent experiments. (F) SIE cells were treated for 1 hour with NF-κB inhibitors SN50 (45 pg/mL, US Biological, Swampscott, MA) or BAY 11-7085 (5 μM; Sigma-Aldrich, St Louis, MO) before rDrm administration. After 18 hours, cell extracts were probed by Western blotting with anti-Ang-1 antibodies (a). In addition, conditioned media from the corresponding cell cultures were added for 10 minutes to naive serum-starved SIE cells. Then, immunoprecipitation with anti–Tie-2 antibodies was performed on the cell extracts followed by Western blotting with antiphosphotyrosine antibodies (Santa Cruz Biotechnology) (b). Uniform loading of the gel was confirmed by incubation of the membrane with anti–Tie-2 antibodies. Similar results were obtained in 2 independent experiments.
rDrm induces Ang-1 expression in EC in a NF-κB–dependent manner. (A) Serum-starved SIE cells were stimulated for 2, 6, 12, and 24 hours with 50 ng/mL of rDrm. At the end of incubation, total RNA was extracted and Ang-1 and Ang-2 transcripts were analyzed by semiquantitative RT-PCR. β-Actin was used as a control. (B) Cell lysates were prepared from SIE cells stimulated for 0, 6, and 18 hours with 50 ng/mL of rDrm. Then, 20-μg aliquots were probed by Western blotting with anti–Ang-1 antibodies (Santa Cruz Biotechnology). Uniform loading of the gels was confirmed by incubation of the membranes with anti–focal adhesion kinase (FAK) antibodies. (C) Serum-starved ECs were incubated for 20 minutes with 50 ng/mL of rDrm and immunostained with an anti-RelA/p65 antibody (Santa Cruz Biotechnology) followed by antirabbit FITC (Invitrogen). Control cells show a diffuse cytoplasmic RelA/p65 immunoreactivity (top panel) that localizes into the nucleus after rDrm stimulation (bottom panel). Analysis was performed using a Zeiss Axiovert 200M epifluorescence microscope equipped with a Plan-Apochromat 63×/1.4 NA oil objective. (D) SIE cells were stimulated for 0 to 30 minutes with 50 ng/mL of rDrm. Then, nuclear (5 μg) and cytoplasmic (10 μg) extracts were probed by Western blotting with anti-RelA/p65 and anti-IkBα (Santa Cruz Biotechnology) antibodies, respectively. Uniform loading of the gels was confirmed by incubation of the membranes with antihistone H3 (Santa Cruz Biotechnology) and anti-FAK antibodies, respectively. (E) Nuclear extracts (2 μg) of SIE cells treated for 0 to 30 minutes with 50 ng/mL of rDrm were incubated with a biotin-labeled NF-κB double-stranded DNA oligonucleotide probe. (Left panel) The protein-DNA complexes were analyzed by EMSA onto a native 6% polyacrylamide gel; the first lane shows the migration of the labeled probe in the absence of nuclear extract. (Right panel) Densitometric analysis of the protein-DNA complexes shown in the left panel; data are the mean plus or minus SEM of 3 independent experiments. (F) SIE cells were treated for 1 hour with NF-κB inhibitors SN50 (45 pg/mL, US Biological, Swampscott, MA) or BAY 11-7085 (5 μM; Sigma-Aldrich, St Louis, MO) before rDrm administration. After 18 hours, cell extracts were probed by Western blotting with anti-Ang-1 antibodies (a). In addition, conditioned media from the corresponding cell cultures were added for 10 minutes to naive serum-starved SIE cells. Then, immunoprecipitation with anti–Tie-2 antibodies was performed on the cell extracts followed by Western blotting with antiphosphotyrosine antibodies (Santa Cruz Biotechnology) (b). Uniform loading of the gel was confirmed by incubation of the membrane with anti–Tie-2 antibodies. Similar results were obtained in 2 independent experiments.
Proangiogenic cytokines and growth factors cause the activation of the transcription factor NF-κB.17 Immunofluorescence and Western blot analysis demonstrate the rapid nuclear translocation of the NF-κB RelA/p65 subunit in rDrm-treated ECs paralleled by the degradation of the NF-κB inhibitor IKBα (Figure 1C,D). Electrophoretic mobility shift assay (EMSA) of the nuclear extracts with a NF-κB double-stranded DNA oligonucleotide probe revealed an increase in the formation of protein-DNA complexes 20 minutes after rDrm stimulation (Figure 1E). Complex formation was prevented by an excess of unlabeled NF-κB probe but not of a mutant NF-κB probe and the presence of RelA/p65 in the complex(es) was confirmed by EMSA supershifting (Figure S2).
NF-κB activation may cause Ang-1 induction.18 Accordingly, inhibition of NF-κB activity by the NF-κB nuclear translocation inhibitor SN50 or by the IkBα phosphorylation inhibitor BAY 11-7085 abrogates rDrm-mediated Ang-1 up-regulation and release in SIE cells, thus preventing Tie-2 phosphorylation (Figure 1F). Accordingly, RelA/p65 down-modulation by siRNA transfection inhibits Ang-1 up-regulation (Figure S3). These data demonstrate that Ang-1 induction by rDrm in EC is mediated, at least in part, by NF-κB activation.
Drm stimulates EC sprouting and invasive activity.8 Ang-1 siRNA transfection, which suppresses Ang-1 up-regulation in rDrm-treated cells, prevents rDrm-mediated sprouting of murine EC aggregates in fibrin gel (Figure 2A) without affecting cell survival and serum-dependent proliferation (data not shown). No inhibition was instead exerted by Ang-1 siRNA on human Ang-1–transfected ECs either in the absence or in the presence of rDrm treatment (Figure S4). Similar to Ang-1 siRNA transfection, neutralizing anti–Ang-1 antibodies affected rDrm-mediated EC sprouting (Figure 2A). In addition, a soluble Tie-2 receptor (sTie-2) inhibited rDrm-mediated invasion of type I collagen matrix by HUVEC spheroids without affecting Ang-1 up-regulation, as shown by Ang-1 immunostaining (Figure 2B). Moreover, anti–Ang-1 antibodies and sTie-2 abrogate the capacity of rDrm to induce EC sprouting from fibrin-embedded human umbilical artery rings (Figures 2C, S5A). Accordingly, anti–Ang-1 antibodies prevent EC sprouting in a murine aorta ring assay (data not shown). Finally, the 2 Ang-1 antagonists significantly inhibit the angiogenic response triggered in vivo by rDrm or by Drm-overexpressing COS cells8 on the chick embryo CAM (Figures 2D, S5B).
Ang-1 modulates the proangiogenic activity of rDrm. (A) Cell aggregates from naive VEGFR2-MAE cells (−) or from VEGFR2-MAE cells transfected with fluorescein-conjugated control siRNA (c) or 4 different Ang-1 siRNA (1-3, 3 distinct single oligonucleotides, [Qiagen, Valencia, CA]; 4, one pool of 3 different oligonucleotides, Santa Cruz Biotechnology) were embedded in fibrin gel. Then, 50 ng/mL of rDrm was added on the top of the gel in medium containing 10 μg/mL of aprotinin. In addition, some rDrm-treated aggregates were incubated in the presence of 1.0 μg/mL of neutralizing anti–Ang-1 antibodies or irrelevant IgGs. Formation of radially growing cell sprouts was observed during the next 48 hours.8 Sprouts were photographed at ×40 magnification with an IX51 inverted microscope equipped with a 4×/0.10 NA objective and a Camedia C-4040 digital camera (Olympus, Melville, NY). Sprouting was quantified by computerized analysis of the digitalized images.8 Data are expressed as means plus or minus SEM (n = 10). * Statistically different from rDrm-treated naive cells (P < .01, Student t test). Conditioned media from the corresponding transfectants were analyzed for Ang-1 protein content by Western blotting (inset). (B) HUVEC spheroids prepared in medium supplemented with carboxymethylcellulose were embedded in type I collagen gel and treated with vehicle or 30 ng/mL of rDrm in the absence or in the presence of 100 ng/mL sTie-2 (Relia-Tech, Woburn, MA). After 24 hours, cell sprouts were photographed at ×200 magnification using an Axiovert 200M microscope equipped with a 20× objective (LD A PLAN 20×/0.30PH1, Zeiss, Jena, Germany). Aggregates were fixed in 4% paraformaldehyde, permeabilized in TBS-0.2% Triton X100, and costained with anti–Ang-1 antibodies/antirabbit Alexa594 (Invitrogen) (in red) and counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) (in blue). Images were acquired using a LSM510Meta confocal microscope equipped with objective LD A PLAN 32×/0.40PH1 and processed using the LSM software (Zeiss). (C) One-millimeter human umbilical artery rings were embedded in fibrin gel and incubated with the indicated concentrations of rDrm in the absence or in the presence of 1.0 μg/mL of neutralizing anti-Ang-1 antibodies or 100 ng/mL of sTie-2. After 3 days, EC sprouts were counted under an IX51 inverted microscope at ×10 magnification. * Statistically different from rDrm-treated rings (P < .01, Student t test). (D) Alginate beads containing vehicle, 100 ng of rDrm, parental ([GRAPHIC]) or Drm-overexpressing-COS cells (10 000 cells/embryo) were implanted on the top of chick embryo CAMs at day 11 of development. When indicated, pellets contained also 1.0 μg of neutralizing anti–Ang-1 antibodies or 100 ng of sTie-2. After 72 hours, newly formed blood vessels converging toward the implant were counted in ovo at ×5 magnification using a STEMI SR stereomicroscope equipped with an objective f equal to 100 mm with adapter ring 475070 (Zeiss). Data are expressed as mean plus or minus SEM (n = 6-8); * Statistically different from the corresponding stimulus in the absence of any inhibitor (P < .01, Student t test).
Ang-1 modulates the proangiogenic activity of rDrm. (A) Cell aggregates from naive VEGFR2-MAE cells (−) or from VEGFR2-MAE cells transfected with fluorescein-conjugated control siRNA (c) or 4 different Ang-1 siRNA (1-3, 3 distinct single oligonucleotides, [Qiagen, Valencia, CA]; 4, one pool of 3 different oligonucleotides, Santa Cruz Biotechnology) were embedded in fibrin gel. Then, 50 ng/mL of rDrm was added on the top of the gel in medium containing 10 μg/mL of aprotinin. In addition, some rDrm-treated aggregates were incubated in the presence of 1.0 μg/mL of neutralizing anti–Ang-1 antibodies or irrelevant IgGs. Formation of radially growing cell sprouts was observed during the next 48 hours.8 Sprouts were photographed at ×40 magnification with an IX51 inverted microscope equipped with a 4×/0.10 NA objective and a Camedia C-4040 digital camera (Olympus, Melville, NY). Sprouting was quantified by computerized analysis of the digitalized images.8 Data are expressed as means plus or minus SEM (n = 10). * Statistically different from rDrm-treated naive cells (P < .01, Student t test). Conditioned media from the corresponding transfectants were analyzed for Ang-1 protein content by Western blotting (inset). (B) HUVEC spheroids prepared in medium supplemented with carboxymethylcellulose were embedded in type I collagen gel and treated with vehicle or 30 ng/mL of rDrm in the absence or in the presence of 100 ng/mL sTie-2 (Relia-Tech, Woburn, MA). After 24 hours, cell sprouts were photographed at ×200 magnification using an Axiovert 200M microscope equipped with a 20× objective (LD A PLAN 20×/0.30PH1, Zeiss, Jena, Germany). Aggregates were fixed in 4% paraformaldehyde, permeabilized in TBS-0.2% Triton X100, and costained with anti–Ang-1 antibodies/antirabbit Alexa594 (Invitrogen) (in red) and counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) (in blue). Images were acquired using a LSM510Meta confocal microscope equipped with objective LD A PLAN 32×/0.40PH1 and processed using the LSM software (Zeiss). (C) One-millimeter human umbilical artery rings were embedded in fibrin gel and incubated with the indicated concentrations of rDrm in the absence or in the presence of 1.0 μg/mL of neutralizing anti-Ang-1 antibodies or 100 ng/mL of sTie-2. After 3 days, EC sprouts were counted under an IX51 inverted microscope at ×10 magnification. * Statistically different from rDrm-treated rings (P < .01, Student t test). (D) Alginate beads containing vehicle, 100 ng of rDrm, parental ([GRAPHIC]) or Drm-overexpressing-COS cells (10 000 cells/embryo) were implanted on the top of chick embryo CAMs at day 11 of development. When indicated, pellets contained also 1.0 μg of neutralizing anti–Ang-1 antibodies or 100 ng of sTie-2. After 72 hours, newly formed blood vessels converging toward the implant were counted in ovo at ×5 magnification using a STEMI SR stereomicroscope equipped with an objective f equal to 100 mm with adapter ring 475070 (Zeiss). Data are expressed as mean plus or minus SEM (n = 6-8); * Statistically different from the corresponding stimulus in the absence of any inhibitor (P < .01, Student t test).
Vascular homeostasis is controlled by a balanced Tie-2 signaling.2 Ang-1 is constitutively expressed at low levels in adult vasculature.2 However, proangiogenic stimuli, including nitric oxide, hypoxia, TNFα, and VEGF, cause Ang-1 up-regulation.18-20 In addition, Ang-1 triggers neovascularization in vivo4,5 and modulates EC sprouting and vascular network formation in vitro.3 Here, we demonstrate the capacity of the proangiogenic factor Drm to increase Ang-1 production in murine and human ECs. Ang-1 up-regulation plays a nonredundant role in the angiogenic process triggered by Drm. Indeed, inhibition of the Ang-1/Tie-2 autocrine loop of stimulation by Ang-1 antagonists or by siRNA transfection hampers Drm-mediated EC sprouting in vitro and angiogenesis in vivo. Interestingly, Ang-1 antagonists cause also an approximately 50% inhibition of the angiogenic response triggered by VEGF-A or fibroblast growth factor-2 in the CAM assay (S. M., unpublished observations, January 2008), indicating that activation of the Ang-1/Tie-2 system may represent a common step in the angiogenic process.
Drm-induced Ang-1 up-regulation in EC is mediated, at least in part, by activation of NF-κB. NF-κB triggers a proinflammatory/proangiogenic transcription program in endothelium and regulates the production of various angiogenic factors.17 Accordingly, various NF-κB-modulated chemokines (ie, CCL2, CCL7, CXCL1, and CXCL2) and cell adhesion molecules (ie, ICAM1 and VCAM1) are up-regulated in rDrm-treated SIE cells in addition to Ang-1 (G.A., unpublished observations, September 2007). Interestingly, Ang-1 exerts anti-inflammatory effects.2 NF-κB-mediated Ang-1 up-regulation by Drm may represent a negative feedback mechanism of control of the inflammatory response during angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank F. Bussolino and L. Napione (IRCC Institute, Candiolo, Turin, Italy) for having provided the pcDNA3-human Ang-1 expression plasmid.
This work was supported by grants from Istituto Superiore di Sanità (Oncotechnological Program, Rome, Italy), Ministero dell'Istruzione, Università e Ricerca (Centro di Eccellenza per l'Innovazione Diagnostica e Terapeutica, Cofin projects, Rome, Italy), Associazione Italiana per la Ricerca sul Cancro (Milan, Italy), Fondazione Berlucchi (Brescia, Italy), and NOBEL Project Cariplo (Milan, Italy; M.P.).
Authorship
Contribution: S.M., E.M., G.A., and M.P. designed research; S.M., E.M., C.R., G.A., and M.B. performed research; S.M., E.M., and M.P. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Presta, General Pathology, Department of Biomedical Sciences and Biotechnology, University of Brescia, Viale Europa 11, 25123 Brescia, Italy; e-mail: presta@med.unibs.it.

![Figure 2. Ang-1 modulates the proangiogenic activity of rDrm. (A) Cell aggregates from naive VEGFR2-MAE cells (−) or from VEGFR2-MAE cells transfected with fluorescein-conjugated control siRNA (c) or 4 different Ang-1 siRNA (1-3, 3 distinct single oligonucleotides, [Qiagen, Valencia, CA]; 4, one pool of 3 different oligonucleotides, Santa Cruz Biotechnology) were embedded in fibrin gel. Then, 50 ng/mL of rDrm was added on the top of the gel in medium containing 10 μg/mL of aprotinin. In addition, some rDrm-treated aggregates were incubated in the presence of 1.0 μg/mL of neutralizing anti–Ang-1 antibodies or irrelevant IgGs. Formation of radially growing cell sprouts was observed during the next 48 hours.8 Sprouts were photographed at ×40 magnification with an IX51 inverted microscope equipped with a 4×/0.10 NA objective and a Camedia C-4040 digital camera (Olympus, Melville, NY). Sprouting was quantified by computerized analysis of the digitalized images.8 Data are expressed as means plus or minus SEM (n = 10). * Statistically different from rDrm-treated naive cells (P < .01, Student t test). Conditioned media from the corresponding transfectants were analyzed for Ang-1 protein content by Western blotting (inset). (B) HUVEC spheroids prepared in medium supplemented with carboxymethylcellulose were embedded in type I collagen gel and treated with vehicle or 30 ng/mL of rDrm in the absence or in the presence of 100 ng/mL sTie-2 (Relia-Tech, Woburn, MA). After 24 hours, cell sprouts were photographed at ×200 magnification using an Axiovert 200M microscope equipped with a 20× objective (LD A PLAN 20×/0.30PH1, Zeiss, Jena, Germany). Aggregates were fixed in 4% paraformaldehyde, permeabilized in TBS-0.2% Triton X100, and costained with anti–Ang-1 antibodies/antirabbit Alexa594 (Invitrogen) (in red) and counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) (in blue). Images were acquired using a LSM510Meta confocal microscope equipped with objective LD A PLAN 32×/0.40PH1 and processed using the LSM software (Zeiss). (C) One-millimeter human umbilical artery rings were embedded in fibrin gel and incubated with the indicated concentrations of rDrm in the absence or in the presence of 1.0 μg/mL of neutralizing anti-Ang-1 antibodies or 100 ng/mL of sTie-2. After 3 days, EC sprouts were counted under an IX51 inverted microscope at ×10 magnification. * Statistically different from rDrm-treated rings (P < .01, Student t test). (D) Alginate beads containing vehicle, 100 ng of rDrm, parental ([GRAPHIC]) or Drm-overexpressing-COS cells (10 000 cells/embryo) were implanted on the top of chick embryo CAMs at day 11 of development. When indicated, pellets contained also 1.0 μg of neutralizing anti–Ang-1 antibodies or 100 ng of sTie-2. After 72 hours, newly formed blood vessels converging toward the implant were counted in ovo at ×5 magnification using a STEMI SR stereomicroscope equipped with an objective f equal to 100 mm with adapter ring 475070 (Zeiss). Data are expressed as mean plus or minus SEM (n = 6-8); * Statistically different from the corresponding stimulus in the absence of any inhibitor (P < .01, Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/4/10.1182_blood-2007-09-111450/6/m_zh80160822000002.jpeg?Expires=1768036794&Signature=oV2Pz~mUY6RvVqevAhBdNKQr5Aj52faHkZiinxd3vZ7iuhED-apo5sc3bF8VGdIbbP2denK1Mh8v~iNYsYQ5p0Mh1XBB0aMiVtXXXRZcgQALKzb2wUUZw2w8QMfe92JdvO~9A9aLsoRv8MkACZXrx0FgaKq8rcV~CT-fu0SwwLcRQ6ELzuBWqXNlWACcN-Mw6Qj-3p12BWekr8faCaWwWQ5HgXhdphIlxh8KToTAEySYbwBEkUkff69C8IgLAdwWG9lhdinKzPP2imtZ1yEykmjT3HyY9n4gcWmhWByzDENmNc8JF--t2nUCdt6sKx6kGI-vWJP-H-MsYIQGvZOLMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
