Abstract
Inhibition of multiple myeloma (MM) plasma cells in their permissive bone marrow microenvironment represents an attractive strategy for blocking the tumor/vessel growth associated with the disease progression. However, target specificity is an essential aim of this approach. Here, we identified platelet-derived growth factor (PDGF)–receptor beta (PDGFRβ) and pp60c-Src as shared constitutively activated tyrosine-kinases (TKs) in plasma cells and endothelial cells (ECs) isolated from MM patients (MMECs). Our cellular and molecular dissection showed that the PDGF-BB/PDGFRβ kinase axis promoted MM tumor growth and vessel sprouting by activating ERK1/2, AKT, and the transcription of MMEC-released proangiogenic factors, such as vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8). Interestingly, pp60c-Src TK-activity was selectively induced by VEGF in MM tumor and ECs, and the use of small-interfering (si)RNAs validated pp60c-Src as a key signaling effector of VEGF loop required for MMEC survival, migration, and angiogenesis. We also assessed the antitumor/vessel activity of dasatinib, a novel orally bioactive PDGFRβ/Src TK-inhibitor that significantly delayed MM tumor growth and angiogenesis in vivo, showing a synergistic cytotoxicity with conventional and novel antimyeloma drugs (ie, melphalan, prednisone, bor-tezomib, and thalidomide). Overall data highlight the biologic and therapeutic relevance of the combined targeting of PDGFRβ/c-Src TKs in MM, providing a framework for future clinical trials.
Introduction
Multiple myeloma (MM) is characterized by a clonal proliferation of immunoglobulin-secreting plasma cells in the bone marrow (BM), with clinical manifestations including lytic bone lesions, anemia, renal failure, immunodeficiency, and hypercalcemia.1 For patients treated with conventional and high-dose chemotherapies, the median survival time from diagnosis is 3 to 4 years.2 Approximately one-half of patients with newly diagnosed MM achieve remission from these therapies, but options are more limited for primary resistant or relapsing disease. Partial remission occurs in only 20% of resistant patients and in 45% of relapsing patients. Survival time improves in approximately 50% of those younger than 60 who are able to undergo high-dose chemotherapy followed by autologous stem cell transplantation, but approximately 30% of them are late in the graft intake or even develop myelodysplasia that prevents additional chemotherapy while relapse or resistant disease occurs.
Induction of MM plasma cells is thought to involve genetic lesions (ie, translocations between immunoglobulin enhancers and oncogenes) and secondary events underlying the activation of bidirectional MM tumor-microenvironment interactions.3-5 Indeed, homing and survival of MM plasma cells are sustained by overangiogenic sprouting of microvascular endothelial cells (ECs),6,7 as well as by osteoclasts, fibroblasts, monocytes, macrophages, and mast cells,4,8,9 thus resulting in a multiplicity of autocrine/paracrine growth loops in the BM milieu. A number of cytokines, including vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8), are released by MM tumor and ECs (MMECs), thereby contributing to the marked BM microvessel density, which is the constant hallmark of active MM but not of monoclonal gammopathies of undetermined significance (MGUS),10-13 and to the acquired refractoriness of MM tumor cells to conventional (ie, melphalan and prednisone) and novel (ie, bortezomib and thalidomide) therapies.2,3
Validation of new tumor/vessel-targeted approaches is therefore needed to provide more selective treatment options for MM patients.2,14 Small-molecule tyrosine kinase (TK) inhibitors (TKIs) of symmetric VEGF-receptors, such as VEGFR1 (Flt-1) on MM plasma cells and VEGFR2 (Flk-1/KDR) on MMECs, were reported to target both MM tumor and EC proliferation and impair their VEGF-triggered autocrine and/or paracrine loops.15-17 These pan VEGFRs antagonists (PTK/ZK222584 and GW654652) showed significant anti-MM efficacy in vitro, thus sparking major interest on the inhibition of multiple signaling cascades by TKIs with different growth-factor receptor specificities (Pazopanib or GW786034B).18
In the present paper, we show that several points of intervention at the level of a distinctive receptor itself, as well as along intracellular transducing effectors, could be identified and therapeutically effective. Specifically, we validated the biologic relevance of platelet-derived growth factor (PDGF)-receptor beta (PDGFRβ) and VEGF-induced c-Src kinase activity in MM pathogenesis, by testing primary patient-derived plasma cells and ECs. We observed that the responsiveness of MM tumor and ECs to dasatinib (BMS-354825/Sprycel), a novel orally bioactive TKI currently used for treating patients with hematologic and solid malignancies,19-22 correlated with a combined targeting of PDGFRβ and VEGFR/c-Src signaling pathways, with ensuing decreased MM tumor/vessel growth in vitro and in vivo. Dasatinib could therefore represent a valuable treatment option for MM patients.
Methods
Patients
Patients fulfilling the International Myeloma Working Group diagnostic criteria23 for active MM (n = 30) and MGUS (n = 14) were studied. According to clinical features and M-component level,24 MM patients were studied at diagnosis (n = 23), relapse (n = 5), or leukemic phase (n = 2). They were 18 males and 12 females 44 to 75 years of age (median, 61.5 years), staged as IIA (n = 6), IIB (n = 4), IIIA (n = 15), and IIIB (n = 5), and their BM plasmacytosis was 35% plus or minus 16%. MGUS patients (8 males and 6 females), 43 to 78 years of age (median, 65.7 years), had 4% plus or minus 2% plasmacytosis. Individuals with benign anemia (iron or vitamin B12 deficiency) were included as controls. The study was approved by the Ethics Committee at the University of Bari, and all patients gave their informed consent in accordance with the Declaration of Helsinki.
Isolation of plasma cells and ECs from MM patients
Bone marrow mononuclear cells (BMMCs) were separated on Ficoll gradient (GE Healthcare, Little Chalfont, United Kingdom), and analyzed by flow cytometry (FACS Canto II; BD Biosciences, San Jose, CA) with the plasma cell marker anti-CD138 or the endothelial marker anti-Factor VIII-Related Antigen (FVIII-RA; both from Immunotech, Marseille, France) phycoerythrin (PE)–labeled monoclonal antibodies (mAbs; Sigma-Aldrich, St Louis, MO), in combination with an anti–PDGF-Rβ fluorescein isothiocyanate (FITC)–labeled mAb (Santa Cruz Biotechnology, Santa Cruz, CA). BMMCs were immunodepleted of plasma cells and microvascular ECs, as previously described.7,25
Cell cultures
Human umbilical vein ECs (HUVECs) were purchased from Lonza Walkersville (Walkersville, MD) and cultured in EGM-2MV media (Lonza Walkersville). MM cell lines (MM.1S, MM.1R, RPMI-8226, Dox-40, and OPM-1) were from ATCC (Manassas, VA), and maintained in RPMI 1640 with 10% fetal bovine serum (FBS), and 100 μg/mL streptomycin/penicillin (Invitrogen, Paisley, United Kingdom) at 37°C.
Reagents
Dasatinib (Bristol-Myers Squibb, Stamford, CT) was dissolved at 1 mM in dimethyl sulfoxide (DMSO, Sigma-Aldrich), filtered and stored at −20°C. VEGF165 was from R&D Systems (Minneapolis, MN) and PDGF-BB from Invitrogen.
Conditioned media and enzyme-linked immunosorbent assay test
Preparation of conditioned media (CM) was previously described.7 VEGF and PDGF-BB were quantified by enzyme-linked immunosorbent assay (ELISA; Human Angiogenesis Array 2, SearchLight; Pierce Chemical, Rockford, IL).
Small interfering RNA
MMECs (5 × 105/well) at 80% confluence were washed with Opti-MEM I Reduced Serum Medium (Invitrogen) and transiently transfected with 50, 100, or 250 nM of c-Src siRNAs or 250 nM of control siRNAs (SMARTpool; Dharmacon RNA Technologies, Lafayette, CO) using Oligofectine (Qiagen, Dorking, United Kingdom) according to the manufacturer's instructions.
Proliferation and apoptosis assay
Cells (104/well in 200 μL) were incubated with 1 μCi 3[H]-thymidine 8 hours before measuring DNA synthesis using a filter scintillation counter (1430 MicroBeta; PerkinElmer Wallac, Turku, Finland). The IC50 inhibitory drug concentration indicates a 50% decrease in proliferation compared with control.
For survival analysis, cells were washed with ice-cold phosphate-buffered saline (PBS) and fixed in 70% cold ethanol, treated with DNase-free RNase (Roche Diagnostics, Indianapolis, IN), and stained with 10 μg/mL propidium iodide (Sigma-Aldrich). Cell-cycle analysis was assessed using FACScalibur flow cytometry and Cell-quest software (BD Biosciences). The ranges for G0/G1, S, G2/M, and sub-G1 phase cells were established on the basis of the corresponding DNA content of histograms. At least 10 000 cells/sample were considered in the gate regions used for calculations.
Functional studies
Chemotaxis, angiogenesis on Matrigel, EC adhesion to fibronectin, and chick embryo chorioallantoic membrane assay were previously described.25,26 MM cell adhesion was evaluated with CellTrace CFSE Cell Proliferation Kit (Invitrogen). Briefly, MM tumor cells labeled with CSFE (5 μM) for 5 minutes were added, after intensive washing in complete medium, to confluent monolayers of 105 unstained MMECs. Adhesion cocultures were incubated with or without dasatinib for 24 hours. Subsequently, nonadherent cells were washed off 3 times, and the remaining adherent MM tumor and ECs were trypsinized. Ratios of the adherent CSFE-stained MM plasma cells and unstained MMECs were quantified by fluorescence-activated cell sorter (FACS) analysis. To perform a wound-healing assay, cells were grown to confluence on 6-well tissue culture plates. A “wound” was made by scraping with a P200 pipette tip the middle of the cell monolayer. Floating cells were removed by extensive washing with cold PBS, and fresh complete media containing DMSO or dasatinib was added for 4 days. The rate of motility was quantified by counting the cells migrating into the total wound area of each 10× field using a Leitz Laborlux D microscope (Leitz Wetzlar Leica, Hicksville, NY). At least 3 different fields were randomly chosen across the wound length.
Immunoprecipitation and Western blotting analysis
Cells were washed once in PBS at 4°C and resuspended in lysis buffer (50 mM of Tris HCl, pH 7.4, 1% Triton X-100, 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 1 mM of Na3VO4, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), and protease inhibitor cocktail (10 μM benzamidine-HCl and 10 μg each aprotinin, leupeptin, and pepstatin A per milliliter) followed by incubation on ice for 30 minutes. Lysates were clarified by centrifugation at 13 000g for 15 minutes at 4°C, and protein concentration was determined using the BCA protein assay (Pierce Chemical). Total proteins (100 μg) were incubated for 1 hour with 1 μg of the indicated antibody at 4°C. Immunocomplexes were precipitated with 30 μg of protein A-Sepharose (GE Healthcare) for 4 hours before separation on sodium dodecyl sulfate (SDS)-polyacrylamide gels. Immunoblotting was performed using Immobilon-P nitrocellulose membrane (Millipore, Billerica, MA). Primary incubations were 1 to 3 hours. Secondary incubations were for 1 hour with horseradish peroxidase-conjugated antimouse or antirabbit antibodies (GE Healthcare). Proteins were visualized by chemiluminescence (Super Signal; Pierce Chemical). Abs against VEGF (A-20), VEGFR1 (C-17), VEGFR2 (C-1158), and actin were from Santa Cruz Biotechnology. Abs against PDGFRβ, p-Y716 PDGFRβ, antiphosphotyrosine (pY, clone 4G10), c-Src (clone GD11), p-Ser473 AKT, and p-Thr202/Tyr204 ERK-1/2 were from Upstate Biotechnology (Charlottesville, VA). Phospho-Y418 c-Src Ab was from BioSource International (Camarillo, CA), and p-Y1054 VEGFR2 was from Abcam (Cambridge, United Kingdom).
RT-PCR
Reverse transcription–polymerase chain reaction (RT-PCR), primers (Invitrogen), and PCR profiles for VEGF, bFGF, Ang1, Ang2, HGF, IL-8, and GAPDH were described elsewhere.27,28 Primers used were as follows: 5′-GATCCGCTCCTTTGATGATC-3′ and 5′-GTCTCACACTTGCATGCCAG-3′ for PDGF-BB; 5′-AATGTCTCCAGCACCTTCGT-3′ and 5′-AGCGGATGTGGTAAGGCATA-3′ for PDGF-Rβ, 5′-GGACCATGGGTAGCAACAAG-3′ and 5′-AGGGAATTCGCCTGGATGGAGTCG-3′ for c-Src. PCR profiles were PDGF-BB: denaturation at 94°C for 3 minutes, 30 cycles at 94°C, 60°C, and 72°C for 1 minute; PDGF-Rβ: denaturation at 94°C for 3 minutes, 20 cycles at 94°C, 58°C, and 72°C for 1 minute; c-Src: denaturation at 94°C for 90 seconds, 30 cycles at 94°C, 50°C, and 72°C for 45 seconds.
In vivo experiments
CB-17 severe combined immunodeficient mice (Charles River Laboratories, Les Oncins, France) inoculated with OPM-1 cells and bearing measurable tumors (8 mice/group) were treated once a day with 75 mg/kg dasatinib, 150 mg/kg dasatinib, or with vehicle alone, for 15 days. Dasatinib was dissolved in propylene glycol:water (50:50; Sigma-Aldrich) and given in a volume of 0.01 mL/mouse. Animals were killed when their tumors reached 2 cm in diameter or when paralysis or major compromise in their life occurred. Tumor volume was calculated using an electronic caliper and the following formula: V = 0.5a × b2, where a and b are the long and short diameter of the tumor, respectively. Survival was evaluated from the first day of treatment until death.
Immunohistochemistry
Formalin-fixed, 4-μm-thick tumor sections were stained with anti-CD31 and hematoxylin and eosin (BD Biosciences). Sections were observed under an Olympus photomicroscope (Olympus, Tokyo, Japan) equipped with a CCD camera (Princeton Scientific Instruments, Monmouth Junction, NJ). The images were analyzed using Image Analysis software (Olympus Italia, Rozzano, Italy), and the results expressed as percentage of CD31+ vessels area referred to the total area of the section.
Isobologram analysis
The interaction between dasatinib and the indicated agents was analyzed using the CalcuSyn software program (Biosoft, Ferguson, MO), based on the Chou-Talalay method.29 Data from cell viability assay (MTT; Sigma-Aldrich) were expressed as the fraction of cells (Fa) killed by the individual drugs or the combination in drug-treated versus untreated cells. The software analysis is based on the following equation: combination index (CI) = (D)1/(Dx)1 + (D)2/(Dx)2 + (D)1(D)2/(Dx)1(Dx)2, where (D)1 and (D)2 are the doses of drug1 and drug2 that have x effect when used in combination, and (Dx)1 and (Dx)2 are the doses of drug1 and drug2 that have x effect when used alone.29 A CI less than 1 indicates synergism, whereas 1 indicates additive effects.
Statistical analysis
Statistical significance of differences observed in drug-treated versus control cultures was determined using the Wilcoxon signed ranks test. The minimal level of significance was P less than .05.
Results
Expression and phospho-activation of PDGFRβ and c-Src TKs in MM
Although PDGFRβ could promote pathologic processes, such as autocrine and paracrine tumor growth and angiogenesis,30-32 its biologic relevance in the pathogenesis and progression of MM remains elusive.
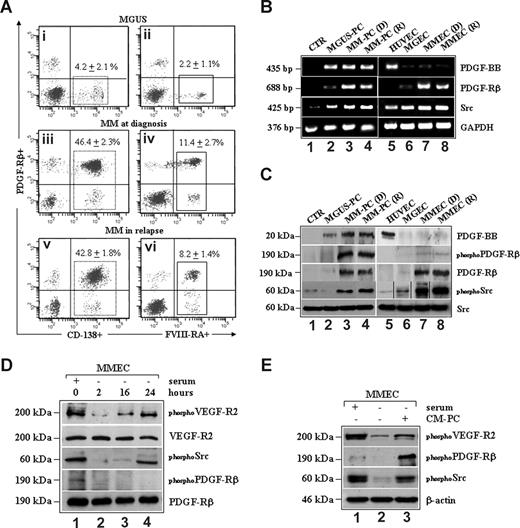
We analyzed freshly isolated BMMCs from patients with MGUS and MM by performing PDGFRβ+/CD138+ or PDGFRβ+/FVIII-RA+ double staining to immunophenotypically detect MM plasma cells and ECs expressing PDGFRβ in vivo, respectively. Figure 1A shows the results obtained in a representative patient with MGUS (Figure 1A,B) and symptomatic MM at diagnosis (Figure 1C,D) or in relapse (Figure 1E,F) with the highest extent of plasma cell BM infiltration. As expected,23,24 the proportion of CD138+ plasma cells was higher in MM than in MGUS (46.4% ± 2.3% [mean ± SD] and 42.8% ± 1.8% vs 4.2% ± 2%; P < .001), and significantly expressed PDGFRβ. Similarly, a higher number of FVIII-RA+ ECs in MM versus MGUS (11.4% ± 2.7% and 8.2% ± 1.4% vs 2.2% ± 1.1%; P < .005), a hallmark of the MM vascular phase,4,5 correlated with the expression of PDGFRβ.
Expression and phosphorylation of PDGFRβ and c-Src TKs in MM patient-derived plasma cells and ECs. (A) FACS analysis of BMMCs from a representative patient with MGUS or active MM at diagnosis and relapse. Numbers on plots are percentages (± SD) of total cells gated. RT-PCR (B) and Western blotting analysis (C) of PDGF-BB, PDGFRβ, and pp60c-Src expression in control BMMCs (CTR), plasma cells isolated from MGUS (MGUS-PC) MM at diagnosis (MM-PC, D), and relapse (MM-PC, R). HUVEC (D) and ECs isolated from a representative patient with MGUS (MGEC), MM at diagnosis (MMEC, D), and MM in relapse (MMEC, R) were also included. All cells were serum-starved for 16 hours before immunoblotting analysis of constitutive phosphorylation of PDGFRβ (phosphoPDGFRβ) and pp60c-Src (phosphoSrc). Vertical lines have been inserted to indicate repositioned gel lanes. (D) Total cell lysates from control (1) and serum-starved MMECs at different time points (1-4) were probed with the indicated antibodies. (E) MMECs cultured in the presence of serum (1) were starved for 2 hours (2) and incubated with the conditioned medium (CM) harvested from MM plasma cells (CM-PC; 3) for 15 minutes. Whole cell lysates were then prepared and analyzed by SDS-PAGE analysis as shown.
Expression and phosphorylation of PDGFRβ and c-Src TKs in MM patient-derived plasma cells and ECs. (A) FACS analysis of BMMCs from a representative patient with MGUS or active MM at diagnosis and relapse. Numbers on plots are percentages (± SD) of total cells gated. RT-PCR (B) and Western blotting analysis (C) of PDGF-BB, PDGFRβ, and pp60c-Src expression in control BMMCs (CTR), plasma cells isolated from MGUS (MGUS-PC) MM at diagnosis (MM-PC, D), and relapse (MM-PC, R). HUVEC (D) and ECs isolated from a representative patient with MGUS (MGEC), MM at diagnosis (MMEC, D), and MM in relapse (MMEC, R) were also included. All cells were serum-starved for 16 hours before immunoblotting analysis of constitutive phosphorylation of PDGFRβ (phosphoPDGFRβ) and pp60c-Src (phosphoSrc). Vertical lines have been inserted to indicate repositioned gel lanes. (D) Total cell lysates from control (1) and serum-starved MMECs at different time points (1-4) were probed with the indicated antibodies. (E) MMECs cultured in the presence of serum (1) were starved for 2 hours (2) and incubated with the conditioned medium (CM) harvested from MM plasma cells (CM-PC; 3) for 15 minutes. Whole cell lysates were then prepared and analyzed by SDS-PAGE analysis as shown.
These findings were further confirmed by RT-PCR and Western blotting analysis of purified cells in vitro: PDGFRβ mRNA transcripts (Figure 1B) and protein levels (Figure 1C) were increased in both tumor and ECs isolated from patients with active MM at diagnosis (lanes 3 and 7) or in relapse (lanes 4 and 8) compared with MGUS (lanes 2 and 6), control BMMCs (lane 1), or quiescent HUVECs (lane 5). As shown in Figure 1C, whereas a constitutive PDGFRβ and c-Src TK phosphorylation was detected in serum-starved MM plasma cells (lanes 3 and 4), only a growth factor–independent c-Src TK-activation still occurred in MMECs (lanes 7 and 8). Indeed, the lower expression of PDGF-BB mRNA or protein in MMECs compared with HUVECs and MM plasma cells (Figure 1B,C, lanes 7 and 8 vs lanes 5, 3, and 4) could be not sufficient to sustain an autocrine PDGFRβ phosphorylation in vitro. Accordingly, Figure 1D shows that c-Src, but not PDGFR, activation was gradually restored in serum-starved MMECs correlating with an autocrine VEGF-induced phosphorylation of VEGFR2 (lanes 2 to 4), as previously reported.33,34 To this, the phosphorylation of PDGFRβ in MMECs detected at time 0 (lane 1) could be explained by a contamination of PDGF in the culture media because exposure of MMECs to the CM harvested from MM plasma cells (CM-PC; Figure 1E) triggered VEGFR2 and c-Src activation, also increasing PDGFRβ TK phosphorylation (lane 3).
These results link the PDGF/PDGFRβ kinase axis to MM disease activity, being up-regulated in tumor/ECs from patients at diagnosis or in relapse, compared with MGUS or normal BMMCs. PDGFRβ is constitutively activated in MM plasma cells, which, in turn, secrete PDGF-BB by triggering the phosphorylation of PDGFRβ in MMECs, in a paracrine fashion. In addition, these data indicate that MM plasma cells and MMECs have increased phospho-levels of c-Src TK, which appears to be activated independently of PDGFRβ in MMECs.
Dasatinib inhibits the proliferative and survival effects of VEGF165 and PDGF-BB in MM plasma cells
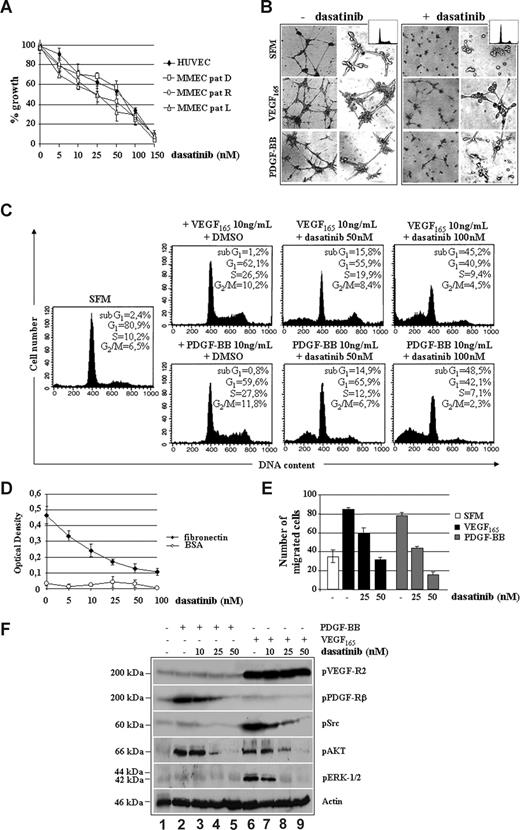
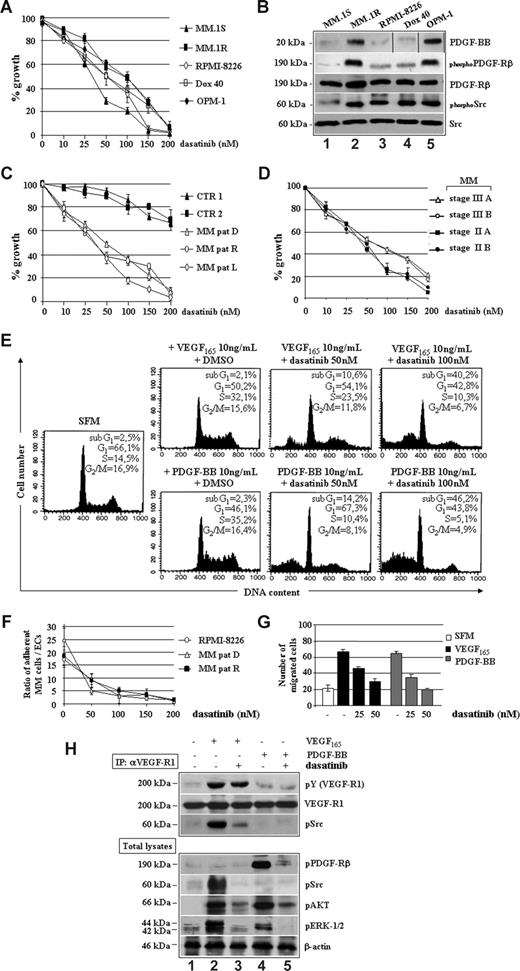
Dasatinib inhibited several MM cell lines, including dexamethasone-sensitive MM.1S, dexamethasone-resistant MM.1R, doxorubicin (Dox)–sensitive RPMI, Dox-resistant RPMI (Dox-40), and OPM-1, with an IC50 value ranging between 25 and 100 nM (Figure 2A). This effect was seemingly specific because increased responsiveness of MM cell lines to dasatinib correlated with increased baseline expression of PDGF-BB and PDGFRβ as well as of PDGFRβ and c-Src TK phosphorylation (Figure 2B).
Dasatinib reduces MM cell growth and survival by targeting VEGF165 and PDGF-BB downstream signaling. (A) Proliferation of human MM cell lines treated with dasatinib for 48 hours. Data indicate the percentage of growth inhibition (± SD) of triplicate experiments. (B) Western blotting analysis of PDGF-BB, PDGFRβ, and pp60c-Src expression/activation status in the indicated MM cell lines. Vertical lines have been inserted to indicate a repositioned gel lane. (C) Antiproliferative effect of dasatinib in control BMMCs (CTR1 and CTR2) and plasma cells isolated from one representative patient with MM at diagnosis (MM-PC, D), relapse (MM-PC, R), or leukemic progression (L). (D) Antiproliferative effect of dasatinib in representative MM cases staged as indicated. (E) MM plasma cells were serum-starved (SFM) for 16 hours and then incubated with VEGF165 and PDGF-BB in the presence of DMSO or dasatinib for 48 hours. Survival and cell- cycle distribution were assessed by propidium iodide staining and FACS analysis. (F) Adhesion cocultures of confluent MM patient-derived ECs (MMECs, 106) and CSFE-prelabeled RPMI 8226 or MM plasma cells from patients (2 × 106) incubated with or without dasatinib for 24 hours. Values represent the mean ratios of adherent MM plasma cells over MMECs from 3 independent experiments (P = .001). (G) Growth factor-deprived MM plasma cells were pretreated with or without dasatinib for 30 minutes, plated on a fibronectin-coated polycarbonate membrane in a Boyden chamber, and exposed to 10 ng/mL VEGF165 or PDGF-BB for 6 hours. Data are means (± SD) of migrated cells in 5 fields (original magnification ×400). (H) Serum-starved MM plasma cells (−) pretreated with 50 nM of dasatinib (30 minutes) were stimulated with 10 ng/mL VEGF165 or PDGF-BB for 15 minutes. VEGFR1 (IP: αVEGFR1) immunoprecipitates and total cell lysates were immunoblotted as indicated.
Dasatinib reduces MM cell growth and survival by targeting VEGF165 and PDGF-BB downstream signaling. (A) Proliferation of human MM cell lines treated with dasatinib for 48 hours. Data indicate the percentage of growth inhibition (± SD) of triplicate experiments. (B) Western blotting analysis of PDGF-BB, PDGFRβ, and pp60c-Src expression/activation status in the indicated MM cell lines. Vertical lines have been inserted to indicate a repositioned gel lane. (C) Antiproliferative effect of dasatinib in control BMMCs (CTR1 and CTR2) and plasma cells isolated from one representative patient with MM at diagnosis (MM-PC, D), relapse (MM-PC, R), or leukemic progression (L). (D) Antiproliferative effect of dasatinib in representative MM cases staged as indicated. (E) MM plasma cells were serum-starved (SFM) for 16 hours and then incubated with VEGF165 and PDGF-BB in the presence of DMSO or dasatinib for 48 hours. Survival and cell- cycle distribution were assessed by propidium iodide staining and FACS analysis. (F) Adhesion cocultures of confluent MM patient-derived ECs (MMECs, 106) and CSFE-prelabeled RPMI 8226 or MM plasma cells from patients (2 × 106) incubated with or without dasatinib for 24 hours. Values represent the mean ratios of adherent MM plasma cells over MMECs from 3 independent experiments (P = .001). (G) Growth factor-deprived MM plasma cells were pretreated with or without dasatinib for 30 minutes, plated on a fibronectin-coated polycarbonate membrane in a Boyden chamber, and exposed to 10 ng/mL VEGF165 or PDGF-BB for 6 hours. Data are means (± SD) of migrated cells in 5 fields (original magnification ×400). (H) Serum-starved MM plasma cells (−) pretreated with 50 nM of dasatinib (30 minutes) were stimulated with 10 ng/mL VEGF165 or PDGF-BB for 15 minutes. VEGFR1 (IP: αVEGFR1) immunoprecipitates and total cell lysates were immunoblotted as indicated.
Similar IC50 values were observed for patient-derived tumor cells, regardless of MM phases (Figure 2C) and stages (Figure 2D), whereas control BMMCs were slightly affected at high concentrations of dasatinib, thus suggesting a large therapeutic window. As shown in Figure 2E, incubation of starved MM plasma cells with PDGF-BB or VEGF165 enhanced proliferation, as judged by the increased number of cells in S-phase, which was further confirmed by measuring 3[H]-thymidine incorporation (data not shown). In contrast, dasatinib abrogated the proliferative stimuli elicited by VEGF165 or PDGF-BB by increasing, dose-dependently, the percentages of cells undergoing apoptosis (sub-G1 peaks). Adhesion of MM cell lines and patients' plasma cells to ECs (Figure 2F), as well as the VEGF165/PDGF-BB–triggered MM plasma cell migration (Figure 2G), was reduced by dasatinib in a dose-dependent manner. As shown in Figure 2H, dasatinib prevented tyrosine phosphorylation of c-Src, which was physically recruited and activated by VEGFR1 in response to VEGF165, without apparent changes in VEGFR1 kinase activity (IC50 > 2000 nM). Other VEGF165/VEGFR1 downstream signaling mediators, such as AKT and ERK1/2, were also inhibited. Furthermore, dasatinib directly blocked PDGF-BB–triggered activation of PDGFRβ, with ensuing reduced phosphorylation of AKT and ERK1/2.
These data indicate that (1) MM plasma cells are responsive to PDGF-BB/PDGFRβ signaling, which promotes their growth advantage independently of VEGF165 through the activation of surviving-kinases, such as AKT and ERK1/2; and (2) dasatinib may potentially act as therapeutic agent by blocking MM cell proliferation, survival, and adhesion elicited by VEGF165 and PDGF-BB.
Dasatinib suppresses VEGF165/PDGF-BB–triggered proliferation, survival, and angiogenic potential of MMECs
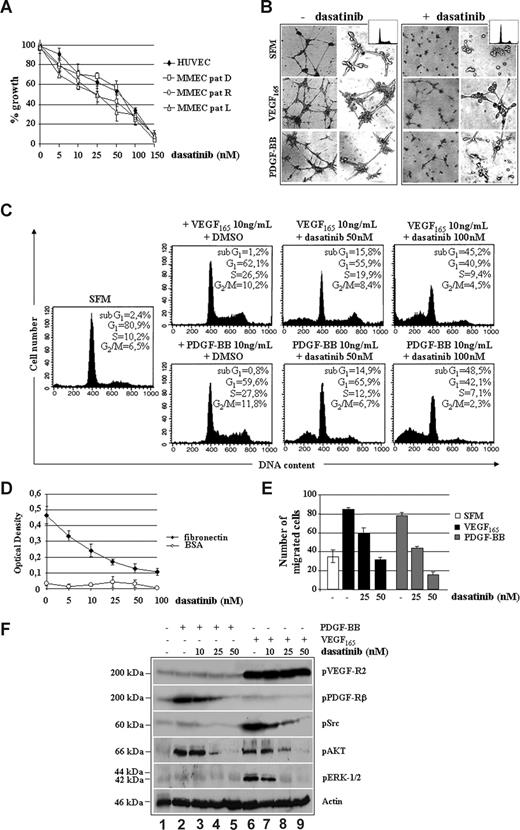
To further characterize the antitumor/vessel activity of dasatinib, we also studied its effects on MMECs. Figure 3A shows a dose-dependent inhibition of proliferation in HUVECs as well as in ECs purified from a representative patient with newly diagnosed MM (D), in relapse (R) or leukemic progression (L), with an IC50 value approximately 50 nM. The spontaneous ability of angiogenic MMECs to form branching and thick anastomosing capillaries in vitro (Figure 3B), when seeded on a Matrigel surface in absence of serum (SFM, top left panels), was completely abrogated by 50 nM dasatinib (SFM, top right panels). This antiangiogenic effect was observed in the absence of cytotoxicity, as assessed by cell cycle analysis (SFM, top little squares). After exposure to VEGF165 (middle left panels) or PDGF-BB (bottom left panels), MMECs increased the numbers of their tube multicentric junctions, which gave rise to a more closely knit network of capillary-like structures. Dasatinib blocked both VEGF165 and PDGF-BB promoted angiogenesis, as evidenced by isolated cell clumps with few sprouting capillaries (middle and bottom right panels). Although serum-starved MMECs increased their DNA synthesis in response to VEGF165 or PDGF-BB (Figure 3C), treatment with dasatinib abrogated these proliferative effects, decreasing EC survival in a dose-dependent manner, similarly to MM plasma cells (Figure 2C).
Dasatinib inhibits VEGF165 and PDGF-BB induced proliferation, angiogenesis, and fibronectin-mediated adhesion of MM patient ECs. (A) Proliferation of HUVECs and ECs from a representative patient with MM at diagnosis (MM-PC, D), relapse (MM-PC, R), or leukemic progression (L) treated with the indicated doses (nM) of dasatinib for 48 hours (104 cells/well). Data were expressed as percentage of growth inhibition. Error bars represent SD of triplicate experiments. (B) MMECs (with or without 10 ng/mL VEGF165 or PDGF-BB) were premixed with 50 nM of dasatinib and seeded on a Matrigel surface for 16 hours (5 × 103/well). Capillary formation was assessed by an inverted light microscope at ×4 and ×10. Photographs are representative of 3 independent experiments. (C) MMECs were serum-starved (SFM) for 16 hours and then incubated with VEGF165 and PDGF-BB in the presence of DMSO, as a solvent control, or the indicated concentrations of dasatinib for 48 hours. Cell- cycle distribution was assessed by propidium iodide staining and FACS analysis. (D) MM patient-derived ECs (MMECs) in serum-free medium or treated with the indicated doses of dasatinib were plated (5 × 103/well) in fibronectin- or BSA-coated 96-well plates in triplicate for 30 minutes. Cells were then fixed with 2.5% glutaraldehyde in PBS and quantified by a colorimetric assay (Cristal violet staining). Data are means (± SD) of 1 of 4 experiments. (E) Growth factor-deprived MMECs were pretreated with or without dasatinib for 30 minutes, plated on a fibronectin-coated polycarbonate membrane in a Boyden chamber, and exposed to 10 ng/mL VEGF165 or PDGF-BB for 6 hours. Data are mean (± SD) of migrated cells in 5 fields (original magnification ×400). (F) Serum-starved MMECs (−) pretreated with the indicated doses of dasatinib (10-50 nM) for 30 minutes were stimulated with 10 ng/mL VEGF165 or PDGF-BB for 15 minutes. Whole cell lysates were prepared and probed with the indicated antibodies.
Dasatinib inhibits VEGF165 and PDGF-BB induced proliferation, angiogenesis, and fibronectin-mediated adhesion of MM patient ECs. (A) Proliferation of HUVECs and ECs from a representative patient with MM at diagnosis (MM-PC, D), relapse (MM-PC, R), or leukemic progression (L) treated with the indicated doses (nM) of dasatinib for 48 hours (104 cells/well). Data were expressed as percentage of growth inhibition. Error bars represent SD of triplicate experiments. (B) MMECs (with or without 10 ng/mL VEGF165 or PDGF-BB) were premixed with 50 nM of dasatinib and seeded on a Matrigel surface for 16 hours (5 × 103/well). Capillary formation was assessed by an inverted light microscope at ×4 and ×10. Photographs are representative of 3 independent experiments. (C) MMECs were serum-starved (SFM) for 16 hours and then incubated with VEGF165 and PDGF-BB in the presence of DMSO, as a solvent control, or the indicated concentrations of dasatinib for 48 hours. Cell- cycle distribution was assessed by propidium iodide staining and FACS analysis. (D) MM patient-derived ECs (MMECs) in serum-free medium or treated with the indicated doses of dasatinib were plated (5 × 103/well) in fibronectin- or BSA-coated 96-well plates in triplicate for 30 minutes. Cells were then fixed with 2.5% glutaraldehyde in PBS and quantified by a colorimetric assay (Cristal violet staining). Data are means (± SD) of 1 of 4 experiments. (E) Growth factor-deprived MMECs were pretreated with or without dasatinib for 30 minutes, plated on a fibronectin-coated polycarbonate membrane in a Boyden chamber, and exposed to 10 ng/mL VEGF165 or PDGF-BB for 6 hours. Data are mean (± SD) of migrated cells in 5 fields (original magnification ×400). (F) Serum-starved MMECs (−) pretreated with the indicated doses of dasatinib (10-50 nM) for 30 minutes were stimulated with 10 ng/mL VEGF165 or PDGF-BB for 15 minutes. Whole cell lysates were prepared and probed with the indicated antibodies.
We also observed that the capacity of MMECs to attach to and spread on fibronectin-coated plates was inhibited by dasatinib (Figure 3D), in a dose-dependent manner and within 30 minutes, thus suggesting an involvement of αvβ3 integrins.35 Furthermore, VEGF165 and PDGF-BB–induced EC migration was blocked in a concentration-dependent manner by dasatinib (Figure 3E).
To assess at which level dasatinib could impact on MMEC signaling pathways (Figure 3F), serum-starved MMECs were stimulated with VEGF165 or PDGF-BB in absence (−) or presence (+) of dasatinib. Although VEGF165 treatment markedly promoted VEGFR2 activation also in the presence of dasatinib (blot: pVEGFR2), the drug blocked VEGF165-triggered tyrosine phosphorylation of c-Src (blot: pSrc), AKT (blot: pAKT), and ERK1/2 (blot: pERK1/2), in a dose-dependent manner. Dasatinib prevented PDGF-BB–mediated activation of PDGFRβ (blot: pPDGFRβ), also affecting the activation of phosphatidylinositol 3-kinase (PI3K)/AKT (blot: pAKT), which appeared the most important pathway for PDGF-BB induction in MMECs.
In conclusion, our data highlight the antiangiogenic potential of dasatinib for its ability to abolish the proliferative and antiapoptotic stimuli of PDGF-BB, which could therefore be an important paracrine mitogen for MMECs in vivo, and of c-Src as a VEGF165-downstream signaling mediator. The antiangiogenic effect of dasatinib was observed in all MM stages and phases.
Contribution of individual targets to the antiangiogenic activity of dasatinib in MMECs
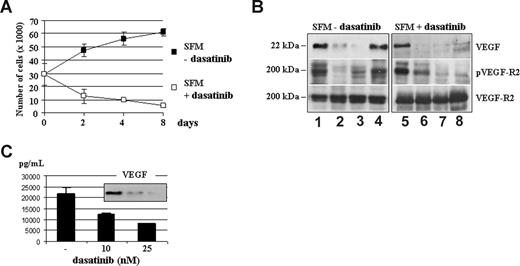
We previously demonstrated that MMEC proliferation and angiogenesis are promoted and sustained via an autocrine VEGF165/VEGFR2 loop.33 This growth signaling cascade was blocked by dasatinib in MMECs (Figure 4A), in accordance with its inhibitory effect on basal angiogenic activity of MMECs in vitro (Figure 3B SFM panels). As reported in Figure 4B, a prolonged exposure of MMECs to dasatinib annulled their ability to respond to VEGF, preventing the expression of endogenous VEGF in a time-dependent manner (lane 8 vs lane 4), and the levels of secreted VEGF in the CM of MMECs, in a dose-dependent manner (Figure 4C).
Dasatinib blocks the spontaneous proliferation of MM patient ECs (MMECs) by preventing constitutive VEGF/VEGFR2 loop. (A) Proliferation of MMECs cultured in serum-free medium (SFM) in the absence or in the presence of dasatinib 50 nM for 2, 4, and 8 days (104 cells/well). Data were expressed as percentage of growth inhibition. Error bars represent SD of triplicate experiments. (B) Whole lysates prepared from cells treated as described were examined by SDS-PAGE analysis, as indicated. (C) VEGF concentrations were measured in triplicate by ELISA in conditioned media (CM) of MMECs cultured with dasatinib (10-25 nM) for 48 hours. Protein levels of secreted VEGF in the CM were also assessed by Western blotting analysis, as indicated.
Dasatinib blocks the spontaneous proliferation of MM patient ECs (MMECs) by preventing constitutive VEGF/VEGFR2 loop. (A) Proliferation of MMECs cultured in serum-free medium (SFM) in the absence or in the presence of dasatinib 50 nM for 2, 4, and 8 days (104 cells/well). Data were expressed as percentage of growth inhibition. Error bars represent SD of triplicate experiments. (B) Whole lysates prepared from cells treated as described were examined by SDS-PAGE analysis, as indicated. (C) VEGF concentrations were measured in triplicate by ELISA in conditioned media (CM) of MMECs cultured with dasatinib (10-25 nM) for 48 hours. Protein levels of secreted VEGF in the CM were also assessed by Western blotting analysis, as indicated.
This notion that c-Src activation was preferentially triggered by VEGF165 in MMECs (Figure 3F) prompted us to test whether an siRNA-silenced c-Src expression (siSrc) could affect the expression of VEGF165 (Figure 5A). Although a scrambled siCTR oligo had no effect on c-Src, a substantial down-regulation was observed at 250 nM siSrc, correlating with lower VEGF165 levels. In Figure 5B, siSrc alone impaired MMEC proliferation compared with siCTR, thus validating c-Src as a crucial mediator of VEGF-signaling in MMECs.34 Interestingly, the antiproliferative effect elicited by reduced c-Src expression was partially rescued by exposure of siSrc-transfected MMECs to PDGF-BB, with ensuing restored adhesion to fibronectin, cell migration (wound-healing assay), and angiogenic activity on Matrigel (Figure 5C).
Silencing of c-Src abrogates autocrine VEGF-induced MM patient ECs (MMECs) proliferation in vitro, but not the stimuli elicited by PDGF-BB. (A) MMECs were transfected with 50 to 250 nM (lanes 3-5) of siRNA for c-Src (siSrc), 250 nM of a control siRNA (2), or carrier alone (1). After 48 hours, total lysates were analyzed for c-Src and VEGF expression. (B) Proliferation of MMECs transfected with either 250 nM siCTR or siSrc for 24 hours and cultured with or without 10 ng/mL PDGF-BB for an additional 24 hours. Data are means (± SD) of 3 independent experiments (P < .05). (C) Morphologic features of siRNA-transfected MMECs when tested for the effects of PDGF-BB on cell adhesion to fibronectin-coated plates (left panels), motility (middle panels), and angiogenic activity (right panels). (D) Molecular dissection of VEGF expression and activation of signaling molecules in siRNA-transfected MMECs. (E) RT-PCR analysis of proangiogenic genes in serum-starved MGECs and MMECs and after exposure to recombinant PDGF-BB 10 ng/mL alone or with dasatinib 50 nM for 8 hours.
Silencing of c-Src abrogates autocrine VEGF-induced MM patient ECs (MMECs) proliferation in vitro, but not the stimuli elicited by PDGF-BB. (A) MMECs were transfected with 50 to 250 nM (lanes 3-5) of siRNA for c-Src (siSrc), 250 nM of a control siRNA (2), or carrier alone (1). After 48 hours, total lysates were analyzed for c-Src and VEGF expression. (B) Proliferation of MMECs transfected with either 250 nM siCTR or siSrc for 24 hours and cultured with or without 10 ng/mL PDGF-BB for an additional 24 hours. Data are means (± SD) of 3 independent experiments (P < .05). (C) Morphologic features of siRNA-transfected MMECs when tested for the effects of PDGF-BB on cell adhesion to fibronectin-coated plates (left panels), motility (middle panels), and angiogenic activity (right panels). (D) Molecular dissection of VEGF expression and activation of signaling molecules in siRNA-transfected MMECs. (E) RT-PCR analysis of proangiogenic genes in serum-starved MGECs and MMECs and after exposure to recombinant PDGF-BB 10 ng/mL alone or with dasatinib 50 nM for 8 hours.
These findings indicated that PDGFRβ could signal independently of VEGFR2, thereby enhancing the angiogenic activation of MMECs in vivo. In this regard, a molecular dissection of PDGFRβ signaling effects in siSrc-transfected MMECs (Figure 5D) showed that PDGF-BB treatment recovered VEGF expression and AKT phosphorylation, without changes on ERK1/2 activation, which was seemingly restricted to a VEGF signal.33
We further corroborated these data by showing that MMECs cultured in the presence of PDGF-BB up-regulated the mRNA transcription of VEGF, IL-8, and bFGF genes that are primarily involved in the angiogenic cascade, with the exception of Ang2, which was instead down-regulated (Figure 5E).
Dasatinib inhibits angiogenesis and growth of MM cells in vivo
Previous pharmacokinetic studies established tolerability and safety of dasatinib in xenograft mouse models, without any significant side effects.19,36,37 Here, the in vivo antitumor effects of dasatinib were evaluated in a subcutaneous murine model of MM. A cohort of severe combined immunodeficient mice was engrafted with human OPM-1 cells (2 × 106 cells/mouse) and treated with vehicle alone or dasatinib 75 mg/kg or 150 mg/kg, orally every day for 15 days. As shown in Figure 6A, a significant dose-dependent inhibition of the tumor growth was observed in treated mice compared with control mice (P = .003 for 75 mg/kg, and P = .001 for 150 mg/kg). Treatment with dasatinib did not affect body weight of the mice, and histopathologic analysis of the major organs (heart, lung, kidneys, liver, and intestine) did not indicate drug toxicity. We also observed a significantly prolonged survival in animals treated with 75 mg/kg per day (P = .003) and 150 mg/kg per day (P = .001) dasatinib versus control (Figure 6B). In addition to the tumor growth delay, treatment with dasatinib affected tumor vascular density and leakage, as judged by CD31 immunostaining of tumor sections (Figure 6C). The macroscopic observation showed a pale appearance of resected MM xenografts in dasatinib-treated mice compared with the red appearance of tumors from nontreated mice.
Dasatinib inhibits MM tumor growth and angiogenesis in vivo. (A) Animals were killed when their tumors reached 2 cm in diameter or when paralysis or major compromise in their life occurred. Tumor volume is indicated as mean (± SD). (B) Survival was evaluated from the first day of treatment until death. (C) Representative microscopic images of tumor sections costained with hematoxylin and oesin and anti-CD31. (D) Angiogenic responses induced by gelatin sponges soaked with MM patient plasma cells (ii) or EC (iv) conditioned-medium compared with the sponge treated with medium alone (i). After treatment of the CAM with 50 nM dasatinib (iii and v, respectively), significantly fewer vessels surrounded the sponges (original magnifications ×50). Vessel counts are indicated.
Dasatinib inhibits MM tumor growth and angiogenesis in vivo. (A) Animals were killed when their tumors reached 2 cm in diameter or when paralysis or major compromise in their life occurred. Tumor volume is indicated as mean (± SD). (B) Survival was evaluated from the first day of treatment until death. (C) Representative microscopic images of tumor sections costained with hematoxylin and oesin and anti-CD31. (D) Angiogenic responses induced by gelatin sponges soaked with MM patient plasma cells (ii) or EC (iv) conditioned-medium compared with the sponge treated with medium alone (i). After treatment of the CAM with 50 nM dasatinib (iii and v, respectively), significantly fewer vessels surrounded the sponges (original magnifications ×50). Vessel counts are indicated.
To further corroborate these data, the antivessel effects of dasatinib were also examined using the chick chorioallantoic membrane (CAM) sponge assay.26 Figure 6D shows that gelatin sponges, soaked with CM from MM plasma cells (Figure 6B) or MMECs (Figure 6D), triggered the formation of neovessels radially converging in a “spoked-wheel” pattern toward the CAM implant compared with vehicle alone (Figure 6A). Whereas dasatinib did not affect basal (physiologic) angiogenesis in the CAM (data not shown), it significantly reduced both MM tumor and EC CM-induced angiogenesis, as shown by the indicated vessel counts.
Altogether, these data indicate that dasatinib impairs both MM tumor growth and neovascularization in vivo, without measurable cytotoxic side effects.
Synergistic effects of dasatinib with conventional therapies in reducing MM plasma cell/EC growth
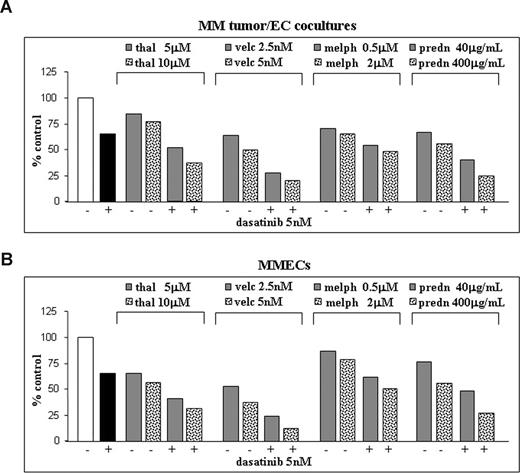
We have recently shown that the cytotoxic effects of small molecules, such as bortezomib (PS-1145/Velcade) and thalidomide, against MM tumor and ECs, are based on their ability to inhibit secretion of multiple growth factors, such as VEGF, bFGF (basic fibroblast growth factor), and HGF (hepatocyte growth factor).25,27,38 Here, we report that the addition of increasing doses of these 2 agents as well as of melphalan and prednisone to low-dose dasatinib resulted in increased cytotoxicity in either MM plasma cells/EC cocultures (Figure 7A) or purified MMECs (Figure 7B). Drug interactions were analyzed using the CalcuSyn software program based on the Chou-Talalay method,29 showing that each combination was synergistic (combination index, CI; Table 1).
Effects of dasatinib and conventional MM therapeutics on proliferation of plasma cell/EC growth. Dose-dependent inhibition of proliferation of MM plasma cells bound to ECs (A) or isolated MM patient ECs (MMECs) (B) cultured in absence (−) or in the presence of 5 nM dasatinib (+) and/or the indicated doses of bortezomib, thalidomide, melphalan, and prednisone for 48 hours. Data from a MMT-based cell viability assay were expressed as percentage of growth inhibition.
Effects of dasatinib and conventional MM therapeutics on proliferation of plasma cell/EC growth. Dose-dependent inhibition of proliferation of MM plasma cells bound to ECs (A) or isolated MM patient ECs (MMECs) (B) cultured in absence (−) or in the presence of 5 nM dasatinib (+) and/or the indicated doses of bortezomib, thalidomide, melphalan, and prednisone for 48 hours. Data from a MMT-based cell viability assay were expressed as percentage of growth inhibition.
The present data provide the rationale for combining these agents in future clinical practice to increase therapeutic efficacy in MM and overcoming drug resistance.
Discussion
Dasatinib is effective in patients with imatinib-resistant or -intolerant Philadelphia-positive (Ph+) leukemias and is under clinical evaluation in several human solid tumors.19-22 It is an orally ATP-competitive inhibitor of 5 critical oncogenic TKs: BCR-ABL, SRC family kinases (SFKs), c-KIT, PDGFRβ, FGFR3, and EPH receptors.20,39 Whereas BCR-ABL and c-KIT were not primarily implicated in the pathophysiology of MM, FGFR3, and multiple SFKs such as Fyn, Lyn, and Hck, are often strongly deregulated in MM tumor cells, thereby supporting the design of future clinical trials with dasatinib.40-43 In this regard, it would be easily anticipated that dasatinib may impact on proliferative/survival signals elicited by additional TK-receptors, such as insulin-like growth factor 1 (IGF-1) and interleukin-6 (IL-6), which recruit and activate Fyn and Lyn SFKs in MM plasma cells,44,45 and osteoclasts,46 also producing beneficial effects on bone density in patients with MM. Podar et al47 recently identified a “molecular-fingerprint” of MM cell sensitivity to dasatinib in vitro, associated with changes in the expression of diverse transcriptional genes (eg, MAF, MAFF, NFYC, PML, YY1, DAXX, CIAP1, IKKe), cell surface receptors (eg, EPH receptor B4 and CXCR4), proteasome subunits (PSMC3, PSMD12, PSME2), and regulators of apoptosis (eg, caspase-8/caspase-12).
We here extend these findings by dissecting the contribution of activity against individual targets, such as PDGFRβ and pp60c-Src TKs, to the antitumor/vessel efficacy of dasatinib (BMS-354825, Sprycel) in MM. The rationale of this study is based on the finding that increased responsiveness of several MM cell lines to dasatinib (IC50 value ranging between 25 and 100 nM), including those resistant to conventional anti-MM agents, such as dexamethasone and doxorubicin, correlated with increased protein levels of PDGF-BB and PDGFRβ as well as of PDGFRβ and pp60c-Src TK phosphorylation. To our knowledge, these are the first evidences of an up-regulated PDGF-BB/PDGFRβ kinase axis in freshly isolated tumor and ECs from patients with active newly diagnosed MM, but not with MGUS or vitamin B12/iron deficiency anemia. PDGFRβ was found to be constitutively phosphorylated in MM patient plasma cells which, in turn, expressed PDGF-BB, thus defining an autocrine growth loop that also controls EC chemotaxis and vessel sprouting, both mandatory for MM-associated neovascularization. Because PDGFRβ is usually undetectable in quiescent microvascular ECs,48,49 these findings emphasize the importance of epigenetic changes (ie, increased expression of PDGF-BB and PDGFRβ, at both mRNA and protein levels in MMECs) in promoting the “angiogenic switch” that occurs in the early stages of MM, and also potentially governs the MM cell resistance to drug-induced apoptosis in patients with progressive disease (ie, relapsed/refractory MM).6,10 Intriguingly, a recent study demonstrated that PDGFRβ is expressed on early hematopoietic/endothelial cell precursors (ie, hemangioblasts), and PDGF-BB is sufficient to promote their EC differentiation.50 Further studies are therefore warranted to elucidate how and whether the PDGF-BB released by MM plasma cells and other BM stromal inflammatory cells (ie, macrophages and platelets) may promote the differentiation of CD133+ hematopoietic stem cells in ECs,51 hence sustaining an active “vasculogenic/angiogenic phase,” as the disease evolves.7
Moreover, our data indicate that the constitutive autophosphorylation of pp60c-Src at tyrosine (Y)–418 in MM patient-derived tumor/ECs may further represent a robust read-out of SFKs activity during the clinical assessment of dasatinib in MM. Specifically, targeting of pp60c-Src by siRNA or dasatinib correlated with a reduced VEGF expression which, in turn, prevented VEGFR2 and ERK1/2 activation as well as spontaneous MMEC angiogenesis on a serum-free Matrigel. This implies that, despite quiescent HUVECs or MGUS ECs,7,33 only purified myeloma ECs per se have an autocrine VEGF-mediated survival/angiogenic mechanism that might involve pp60c-Src and/or multiple SFK activity, beyond their effects on integrin/cadherin-mediated adhesion,34,52 likely through the activation of different downstream transcriptional targets. Importantly, we observed that a PDGFRβ-mediated paracrine mechanism might still compensate for direct and/or indirect targeting of VEGF/Src signaling in MMECs, by promoting the transcription of IL-8, bFGF, and VEGF via alternative pathways involving AKT.
These data have clinical relevance indicating that a long-lasting antitumor/vessel effect in vivo could be achieved through a combined targeting of PDGF, as well as of VEGF, signaling pathways in MM. It seems therefore conceivable that dasatinib promises to be clinically more effective than imatinib,53-55 that is inactive against VEGF receptors and SRC family kinases, or pan VEGF receptor antagonists,15-18 that have a weaker activity versus PDGFRβ. In the present study, the in vivo anti-MM activity of dasatinib was evaluated in a subcutaneous murine model, thereby observing a significant effect on tumor growth and vascularization in treated mice versus control. Although a higher antitumor/vessel activity of dasatinib would be expected with repeated daily or twice daily dosing, our data show a synergistic effect of low-dose dasatinib with conventional and novel MM therapeutics, strongly supporting its future evaluation in combination clinical trials to increase efficacy and improve patient outcome. This strategy may result in induction and maintenance (ie, angioprevention) of an “avascular” phase of MM, with increased safety features compared with conventional cytotoxic therapy, thus allowing drug administration for prolonged uninterrupted periods at doses significantly below those maximum tolerated and preventing development of drug resistance or even overcoming drug resistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy; the Ministry of Education, University and Research (MUR, PRIN Projects 2005, Project CARSO no. 72/2), and the Ministry of Health, Progetto Oncologia 2006, Humanitas Mirasole S.P.A. (Rome, Italy). A.M.L.C. and T.C. are recipients of fellowships from Fondazione Italiana per la Ricerca sul Cancro (FIRC), Milan, Italy. The sponsors of this study are public or nonprofit organizations that support science in general. They had no role in gathering, analyzing, or interpreting the data.
Authorship
Contribution: A.M.L.C. designed and supervised all experiments and has all responsibility for this manuscript; N.D.R., T.C., and M.C.C. isolated MM tumor/ECs from the bone marrow of patients and performed proliferation, wound healing assays, and RT-PCR experiments; A.G. performed siRNA studies; D.M. performed the Matrigel assays; F.D. and D.R. performed CAM and immunohistochemistry analysis; P.N. and P.T. carried out in vivo experiments; and C.G.-P. and A.V. helped with the isobologram analysis and provided critical revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Addolorata M. L. Coluccia, Department of Internal Medicine and Clinical Oncology, Policlinico-Piazza Giulio Cesare, 11 I-70124 Bari, Italy; e-mail: malu.coluccia@libero.it.