Abstract
Von Willebrand factor (VWF) and factor VIII (FVIII) circulate in a tight noncovalent complex. At present, the cells that contribute to the removal of FVIII and VWF are of unknown identity. Here, we analyzed spleen and liver tissue sections of VWF-deficient mice infused with recombinant VWF or recombinant FVIII. This analysis revealed that both proteins were targeted to cells of macrophage origin. When applied as a complex, both proteins were codirected to the same macrophages. Chemical inactivation of macrophages using gadolinium chloride resulted in doubling of endogenous FVIII levels in VWF-null mice, and of VWF levels in wild-type mice. Moreover, the survival of infused VWF was prolonged almost 2-fold in VWF-deficient mice after gadolinium chloride treatment. VWF and FVIII also bound to primary human macrophages in in vitro tests. In addition, radiolabeled VWF bound to human THP1 macrophages in a dose-dependent, specific, and saturable manner (half-maximal binding at 0.014 mg/mL). Binding to macrophages was followed by a rapid uptake and subsequent degradation of the internalized protein. This process was also visualized using a VWF–green fluorescent protein fusion protein. In conclusion, our data strongly indicate that macrophages play a prominent role in the clearance of the VWF/FVIII complex.
Introduction
Von Willebrand factor (VWF) is produced in endothelial cells and megakaryocytes, where it undergoes several posttranslational modifications, including glycosylation, multimerization, and proteolytic cleavage of its propeptide.1 The mature protein exists as variably sized multimers that are either secreted in plasma or maintained in storage organelles. Functions of VWF include recruitment of platelets to sites of vascular injury via interactions with the platelet receptors glycoprotein Ibα and αIIbβ3.2 Furthermore, VWF is a carrier protein for factor VIII (FVIII). Factor VIII is critical to the coagulation cascade, and its deficiency is associated with a severe bleeding tendency known as hemophilia A.3
Complex formation with VWF is needed to maintain FVIII in the circulation, illustrated by strongly reduced FVIII levels in the absence of VWF, and also by reduced survival of FVIII in VWF-deficient patients.4,5 The importance of VWF for FVIII survival is further underscored by the strong correlation between FVIII half-life and preinfusion VWF levels.6 Several mechanisms may contribute to the protective effect of VWF: (1) stabilization of FVIII heterodimeric structure7 ; (2) protection of FVIII from proteolytic degradation by phospholipid-binding proteins such as activated protein C8 ; (3) interference with FVIII binding to negatively charged phospholipid surfaces, like on activated platelets9 ; (4) inhibition of FVIII binding to activated factor IX10 ; and (5) prevention of cellular uptake of FVIII.11
In the past decade, interest in clearance mechanisms of factor VIII and VWF has gained momentum. This was driven, for instance, by observations that increased clearance of VWF contributes to the pathogenesis of von Willebrand disease (VWD),12 whereas for FVIII, interest arose to improve hemophilia A treatment.13 Studies on FVIII clearance resulted in the identification of potential receptors that could mediate catabolism of FVIII. These include LDL receptor related protein-1 (LRP1) and other members of the LDL receptor family, asialoglycoprotein receptor and CD206 (for review see Lenting et al).14 In particular, contribution of LRP1 to regulation of FVIII levels has been evaluated in animal models and epidemiologic studies.15,16 Interestingly, interactions between FVIII and potential clearance receptors are blocked by VWF in experiments using purified components.3,17 This could imply that free FVIII (approximately 2%-5% of the total FVIII molecules)18,19 and the remaining VWF-bound FVIII (95%-98%) are cleared via different pathways. It seems conceivable that the majority of VWF-bound FVIII is cleared as part of the VWF-FVIII complex, although it cannot be excluded that this complex dissociates at the cellular surface under particular conditions.20
As for the molecular basis of VWF clearance, little is known. In contrast to FVIII, no dominant clearance receptors have been identified, apart from the asialoglycoprotein receptor that may recognize VWF in cases of hyposialylation or altered glycosylation.21,22 In addition, little information exists on which cells contribute to the removal of VWF, FVIII, or the VWF-FVIII complex. We have previously reported the use of a mouse model using VWF-null mice to study in vivo clearance of normal and mutated VWF.23,24 Here, we used this model to identify cells that contribute to the uptake of VWF and FVIII. Our data indicate that macrophages in both liver and spleen have the capacity to remove both proteins from the circulation.
Methods
Proteins and antibodies
Immunoaffinity-purified recombinant VWF was prepared as described.23,25 Green fluorescent protein–tagged VWF (VWF-GFP) was provided by Dr A. B. Meijer (Sanquin Research, Amsterdam, The Netherlands).26 Plasma-derived (pd)–VWF was purified from VWF/FVIII concentrate (Haemate P; Behring, Marburg, Germany).27 Antihemophilic factor (ReFacto; Wyeth, Madison, NJ) and recombinant antihemophilic factor (Kogenate; Bayer-Healthcare, Mijdrecht, The Netherlands) were used as sources for recombinant B-domainless and full-length FVIII, respectively. Polyclonal rabbit anti-VWF antibodies and polyclonal horseradish peroxidase (HRP)–conjugated antibodies were from DakoCytomation (Glostrup, Denmark). Sheep polyclonal anti-VWF antibodies were from Biozol (Eching, Germany). Mouse monoclonal anti-FVIII antibody CLB-CAg A was provided by Dr J. A. van Mourik (Sanquin Research). Monoclonal human anti–human FVIII antibody (LE2E9) was a gift from Dr J. M. Saint-Remy (University of Leuven, Leuven, Belgium). Monoclonal rat anti–mouse CD68 and rat anti–mouse F4/80 were from AbD Serotec (Oxford, United Kingdom), and monoclonal mouse anti–human CD16 antibodies were from BD-Pharmingen (San Diego, CA). Rhodamine-conjugated donkey anti–sheep antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Other rhodamine (TRITC)–conjugated and fluorescein (FITC)–conjugated secondary antibodies were from Jackson Immunoresearch Laboratories (West Grove, PA).
VWF antigen assay
VWF antigen was quantified as described.28 FVIII activity was assayed using a one-stage clotting assay. Normal pooled mouse plasma was used as a reference, using concentrations of 0.1 μg/mL and 10 μg/mL for FVIII and VWF, respectively.
Mice
VWF-deficient mice and wild-type mice used were on a C57BL/6J background and were between 8 and 12 weeks of age.29 Housing and experiments were done as recommended by French regulations and the experimental guidelines of the European Community.
Tissue collection
VWF-deficient mice were injected intravenously in the tail-vein with human recombinant VWF (10 μg/mouse), human recombinant FVIII (100 IU/kg corresponding to 0.25 μg/mouse), or both recombinant FVIII and recombinant VWF (0.25 μg and 10 μg per mouse, respectively), which were preincubated for 30 minutes prior to injection to allow complex formation. After injection (5 minutes for FVIII and 30 minutes for VWF or VWF/FVIII), mice were bled and killed, and tissue was collected, perfused with PBS, embedded in Tissue-Tek OCT-compound (Miles Laboratories, Elkhart, IN), and immediately frozen in liquid nitrogen. Tissue was cut into 8-μm sections on a freezing microtome and mounted on slides.
Immunohistochemical analysis of mouse spleen and liver
Cryostat sections were immunostained by the indirect immunoperoxidase method using the following antibodies: monoclonal rat anti–mouse CD68, monoclonal rat anti–mouse F4/80, or peroxidase-conjugated polyclonal rabbit anti–human VWF. Subsequently, sections were incubated with peroxidase-conjugated goat anti–rat antibodies or goat anti–rabbit antibodies. Antibodies were visualized using brown-staining DAB substrate (Vector Laboratories, Burlingame, CA). Slides were counterstained with hematoxylin and examined by light microscopy. For immunofluorescence analysis, cryostat sections were immunostained using monoclonal rat anti–mouse CD68 or monoclonal rat anti–mouse F4/80 antibody to detect macrophages. TRITC-conjugated goat anti–rat immunoglobulins were used as secondary antibodies. VWF was detected either using polyclonal rabbit anti–human VWF IgG (with FITC-conjugated goat anti–rabbit antibodies as secondary antibodies) or polyclonal sheep anti–human VWF IgG (with TRITC-conjugated donkey anti–sheep antibodies as secondary antibodies). To detect FVIII, sections were incubated with human monoclonal anti–human FVIII antibody LE2E9, followed by incubation with FITC-conjugated anti–human antibodies. FITC signal was amplified using alexa-fluor-488 signal amplification kit (Invitrogen, Paisley, United Kingdom). The binding site for LE2E9 may overlap with that of VWF or LRP. Therefore, control experiments have been performed to confirm that LE2E9 is able to detect FVIII in the presence of VWF or LRP (not shown). All antibodies were incubated for 30 minutes at room temperature. After staining, sections were embedded in Mowiol mounting medium (Merck, Schiphol, The Netherlands) containing 2.5% DABCO (Fluka-Chemie AG, Buchs, Switzerland) and analyzed using a Leitz-DMIB fluorescence microscope, with 40× Planapo objective (Leica, Voorburg, The Netherlands), interfaced with a Leica-TCS4D confocal laser microscope (Leica Lasertechnik, Heidelberg, Germany).
Gadolinium chloride treatment of mice
Gadolinium chloride (GdCl3, 50 mg/kg) was given to mice via intravenous tail injection 24 to 48 hours prior to collection of blood samples (for analysis of endogenous VWF or FVIII levels) or intravenous tail-vein injection of VWF (for clearance studies). Control mice received equivalent injections of saline. Liver was collected as described in “Tissue collection” to perform immunohistochemical analysis. Depletion of macrophages in the liver due to GdCl3 treatment was quantified as the average number of Kupffer cells per field from at least 10 randomly selected fields per section. Kupffer cells were identified as CD68+ cells with an appropriate nuclear morphology and sinusoidal location.
Clearance of purified recombinant VWF in mice
Radiolabeling of pd-VWF
Pd-VWF was labeled with Na125I (GE Healthcare, Little Chalfont, United Kingdom) using the IODO-GEN method (Pierce Chemical, Rockford, IL) as described.23 Radiolabeled protein was separated from free iodine on a PD10-disposable column and equilibrated in Hepes-buffered saline/0.005% Tween-20. The content of free iodine was routinely less than 6% as determined by precipitation in 10% trichloroacetic acid.
Preparation of human macrophages
Monocytes were isolated from peripheral blood drawn from healthy individuals by centrifugation on a Ficoll-Paque gradient (GE Healthcare Biosciences, Piscataway, NJ) and magnetically sorted using CD14+ magnetic beads (MACS; Miltenyi Biotec, Auburn, CA). Cells were seeded on glass coverslips coated with coating buffer (phosphate-buffered saline [PBS] containing 4% bovine albumin [BSA]) in 24-well culture plates in growth medium (RPMI1640 medium supplemented with 5% heat-inactivated FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μM β-mercaptoethanol). Cells were allowed to differentiate into macrophages for 5 days. Routinely, these cells reach the following phenotype: CD14+CD68+CD71+CD1a−. Alternatively, monocytic human THP-1 cells (ATCC TIB-202; ATCC, Manassas, VA) were maintained in growth medium and differentiated into macrophages by growing cells on glass coverslips, coated with coating buffer, in 24-well culture plates in growth medium supplemented with 20 ng/mL phorbol-12-myristate-13-acetate (Biomedicals, Aurora, OH) for 72 hours.30 Phenotypic characterization of these cells indicates low expression of surface markers CD4 and CD64 and high expression of CD36 and CD11b.
Cell-binding experiments
Macrophages were washed once with growth medium and incubated for 30 minutes at 37°C, once with ice-cold RPMI1640 medium and then cooled for 15 minutes on ice. Subsequently FVIII, pd-VWF, radiolabeled pd-VWF, or VWF-GFP was added at indicated concentrations and incubated for 1 hour, maintaining temperature at 4°C to avoid internalization. After removing excess of unbound protein by washing once with ice-cold RPMI1640 medium, coverslips were transferred to wells containing ice-cold RPMI1640 medium and washed once more. Cells were then either fixed using 4% paraformaldehyde for 15 minutes at 4°C (for immunofluorescence studies) or lysed with PBS/1% Triton-X100 for 1 hour at room temperature (for radiolabeled VWF). In cases of cells that were lysed, supernatant was collected and analyzed for the presence of radioactivity. In cases of cells that were fixed for immunofluorescence studies, cells were washed with PBS and the presence of FVIII, VWF, or CD16 was detected using the following antibodies: FITC-labeled monoclonal antibody CLB-CAg A for FVIII, polyclonal rabbit anti–human VWF antibodies in combination with FITC-conjugated goat anti–rabbit antibodies for VWF, and monoclonal mouse anti–human CD16 antibody in combination with TRITC-conjugated goat anti–mouse antibodies for CD16. Finally, coverslips were embedded in Mowiol mounting medium containing 2.5% DABCO and analyzed using a Leitz-DMIB fluorescence microscope, with 63× Planapo objective interfaced with a Leica-TCS4D confocal laser microscope. Nonspecific binding in experiments was determined by incubating VWF (either radiolabeled or VWF-GFP fusion protein) with coverslips in the absence of cells. No fluorescence was observed when assessed for VWF-GFP, while nonspecific radioactivity was routinely less than 15% compared with the radioactivity found in the presence of cells. In other control experiments, binding of radiolabeled VWF was examined in the presence of various concentrations of nonlabeled VWF, which resulted in a dose-dependent decrease in the amount of bound radioactive protein.
Cell-mediated degradation experiments
Differentiated THP-1 macrophages were grown as described in “Cell-binding experiments” in 24-well culture plates, and incubated with radiolabeled pd-VWF (13.5 μg/mL) or VWF-GFP (65 μg/mL) for 1 hour at 4°C to allow VWF to bind to these cells. Cells were washed with ice-cold RPMI1640 medium to remove unbound protein and once with RPMI1640 medium at 37°C. After placing cells at 37°C to initiate degradation, incubation was continued at 37°C in a volume of 500 μL. When radiolabeled VWF was used, samples were taken at indicated time points to determine the amount of degraded material. Degraded material is defined as the radioactivity that is soluble in 10% trichloroacetic acid. In all experiments, controls were included to determine the amount of nonspecific degradation in the absence of cells. When VWF-GFP was used, coverslips were transferred at indicated time points to wells containing ice-cold RPMI1640 medium, to avoid further internalization and degradation and washed with ice-cold RPMI1640 medium. Subsequently, cells were fixed using 4% paraformaldehyde for 15 minutes at room temperature. Coverslips were embedded and analyzed as described in “Cell-binding experiments.”
Data analysis and statistics
Analyses of data were performed using GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA). P values were determined using Student unpaired t test, and values less than .05 were considered significant.
Results
Spleen and liver are efficient in the uptake of VWF
Previously, we reported on the tissue distribution of radiolabeled VWF when applied intravenously to VWF-deficient mice.23 A summary of this distribution during the first 30 minutes after injection is provided in Table 1. These data indicate that liver takes up the majority of VWF from the circulation (40%-60% of the amount of radioactivity found back in organs), whereas the other organs such as spleen and kidney take up relatively small amounts. Since liver is the largest in size of the examined organs, it was of interest to compare the relative efficiency by which organs take up VWF. By calculating uptake of VWF per weight of tissue, again the liver proved to be among the organs that were most efficient in taking up VWF (Figure 1). Apart from liver, spleen also efficiently takes up VWF (17.0% ± 4.9% and 14.6% ± 3.4% VWF/mg tissue for spleen and liver, respectively; Figure 1), although its absolute contribution to the overall clearance remains limited.
Biodistribution of radiolabeled pd-VWF. Radiolabeled pd-VWF (5 μg/mouse) was injected intravenously into VWF-deficient mice. After 30 minutes, mice were killed and organs were collected. Indicated organs were weighed and residual radioactivity was analyzed. This allowed us to calculate the percentage of radioactivity present in the individual organs relative to the total amount of radioactivity injected (percentage of injected). The relative uptake was then determined as the ratio of the percentage of injected over organ weight (percentage of injected/mg tissue). Data represent mean values plus or minus SEM of 3 mice.
Biodistribution of radiolabeled pd-VWF. Radiolabeled pd-VWF (5 μg/mouse) was injected intravenously into VWF-deficient mice. After 30 minutes, mice were killed and organs were collected. Indicated organs were weighed and residual radioactivity was analyzed. This allowed us to calculate the percentage of radioactivity present in the individual organs relative to the total amount of radioactivity injected (percentage of injected). The relative uptake was then determined as the ratio of the percentage of injected over organ weight (percentage of injected/mg tissue). Data represent mean values plus or minus SEM of 3 mice.
VWF is targeted to macrophages in spleen and liver
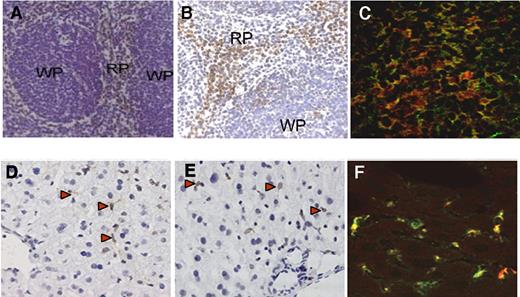
To evaluate the cellular destination of VWF in liver and spleen, tissues were isolated from VWF-deficient mice that were injected with recombinant VWF (10 μg/mouse) and prepared for immunohistochemical analysis. As controls, tissue sections of noninjected VWF-null mice were used, which all stained negative for VWF (not shown). As for tissue sections from VWF-injected mice, we observed that VWF was present in distinct regions within the spleen (Figure 2A). These regions seem to be located within the red pulp area bordering the white pulp. In view of its close location toward the white pulp, it seems that part of the protein is also found in the marginal zone.31 Staining of noncontinuous spleen sections of the same mouse using the antimouse macrophage marker F4/80 revealed that the regions where VWF is found is enriched in macrophages (Figure 2B; see also Oldenborg et al32 ). When another series of spleen tissue sections were analyzed via costaining for VWF and macrophages, a considerable overlap of both markers was observed (Figure 2C). This indicates that VWF is colocalized with these macrophages. As for the liver sections, a pattern was observed upon VWF staining that seemed similar to the pattern found upon staining of other noncontinuous liver sections for macrophages, that is, Kupffer cells using antimurine CD68 antibodies (Figure 2D and 2E, respectively).33 Indeed, costaining experiments for VWF and Kupffer cells using another series of liver tissue sections demonstrated that the majority of VWF colocalizes with these macrophages (Figure 2F). These data indicate that macrophages contribute to the cellular uptake of VWF in both liver and spleen.
Identification of macrophages as main target for VWF in spleen and liver. (A,B) Spleen sections of VWF-deficient mice treated with recombinant VWF (10 μg/mouse). Spleen was collected 30 minutes after injection. Sections are stained using polyclonal anti-VWF antibodies (A) or the anti–mouse macrophage marker F4/80 (B), and counterstained using hematoxylin (magnification 200×). RP indicates red pulp; WP, white pulp. (C) Merged image of a spleen tissue section stained with anti–mouse F4/80 antibody (red) and polyclonal antihuman antibodies VWF (green). Original magnification 400×. (D,E) Liver sections of VWF-deficient mice treated with recombinant VWF (10 μg/mouse). Liver was collected 30 minutes after injection. Sections are stained using polyclonal anti-VWF antibodies (D) or a monoclonal anti–mouse CD68 antibody (E), and counterstained using hematoxylin (magnification 200×). (F) Merged image of liver tissue section stained with anti–mouse CD68 antibody (red) and polyclonal anti–human VWF antibodies (green). Original magnification 400×. Red arrowheads in panels D and E point to examples of positively stained cells (brown color).
Identification of macrophages as main target for VWF in spleen and liver. (A,B) Spleen sections of VWF-deficient mice treated with recombinant VWF (10 μg/mouse). Spleen was collected 30 minutes after injection. Sections are stained using polyclonal anti-VWF antibodies (A) or the anti–mouse macrophage marker F4/80 (B), and counterstained using hematoxylin (magnification 200×). RP indicates red pulp; WP, white pulp. (C) Merged image of a spleen tissue section stained with anti–mouse F4/80 antibody (red) and polyclonal antihuman antibodies VWF (green). Original magnification 400×. (D,E) Liver sections of VWF-deficient mice treated with recombinant VWF (10 μg/mouse). Liver was collected 30 minutes after injection. Sections are stained using polyclonal anti-VWF antibodies (D) or a monoclonal anti–mouse CD68 antibody (E), and counterstained using hematoxylin (magnification 200×). (F) Merged image of liver tissue section stained with anti–mouse CD68 antibody (red) and polyclonal anti–human VWF antibodies (green). Original magnification 400×. Red arrowheads in panels D and E point to examples of positively stained cells (brown color).
FVIII is targeted to macrophages in spleen and liver
To investigate cellular targeting of FVIII, we again used VWF-null mice, to avoid VWF-mediated targeting of FVIII. VWF-deficient mice are characterized by residual FVIII plasma levels of 20% to 25%, due to the complete absence of VWF.29 To confirm specificity of the human anti–human FVIII monoclonal antibody LE2E9, liver and spleen tissue sections of VWF- and FVIII-deficient mice were analyzed, and for both mouse strains staining was found to be absent (not shown). Thus, the anti-FVIII antibody LE2E9 does not cross-react with murine proteins under the conditions used. VWF-deficient mice were subsequently infused with FVIII (0.25 μg/mouse), and tissues were collected already 5 minutes after injection because of the short half-life of FVIII in the absence of VWF. Analysis of sections stained using the monoclonal human antihuman FVIII antibody LE2E9 and antimouse macrophage-specific antibodies (anti-F4/80 and anti-CD68 for spleen and liver, respectively), revealed that the majority of FVIII is targeted to cells positive for these macrophage markers (Figure 3). Of interest, full-length FVIII displayed similar distribution compared with B-domainless FVIII when injected in VWF-deficient mice (not shown).
Localization of FVIII in macrophages in spleen and liver. (A) Liver section of a VWF-deficient mouse treated with recombinant FVIII (0.25 μg) stained with anti–mouse CD68 antibody. (B) Same liver section as depicted in panel A, but stained with monoclonal anti–human FVIII antibody LE2E9. (C) Merged image of panels A and B. (D) Spleen section of a VWF-deficient mouse treated with recombinant FVIII (0.25 μg) stained with anti–mouse F4/80 antibody. (E) Same spleen section as depicted in panel A, but stained with monoclonal anti–human FVIII antibody LE2E9. (F) Merged image of panels D and E. Both organs were collected 5 minutes after injection.
Localization of FVIII in macrophages in spleen and liver. (A) Liver section of a VWF-deficient mouse treated with recombinant FVIII (0.25 μg) stained with anti–mouse CD68 antibody. (B) Same liver section as depicted in panel A, but stained with monoclonal anti–human FVIII antibody LE2E9. (C) Merged image of panels A and B. (D) Spleen section of a VWF-deficient mouse treated with recombinant FVIII (0.25 μg) stained with anti–mouse F4/80 antibody. (E) Same spleen section as depicted in panel A, but stained with monoclonal anti–human FVIII antibody LE2E9. (F) Merged image of panels D and E. Both organs were collected 5 minutes after injection.
GdCl3-induced macrophage reduction alters VWF and FVIII catabolism
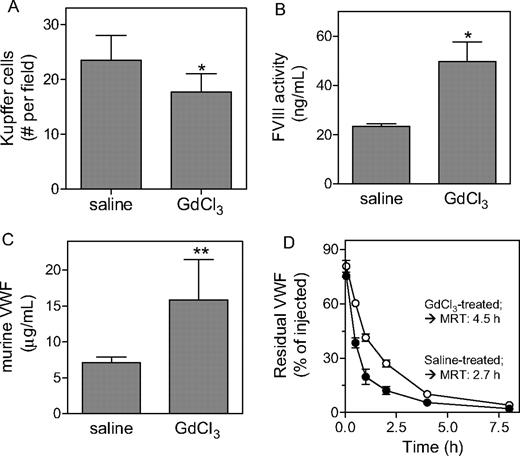
In an attempt to further explore the contribution of macrophages to the catabolism of FVIII and VWF in vivo, we first treated VWF-null mice (in case of FVIII) and wild-type mice (in case of VWF) with GdCl3 (50 mg/kg). GdCl3 is an agent known to efficiently reduce the number and function of macrophages.34-36 Indeed, analysis of livers taken from mice 24 hours after treatment with GdCl3 revealed a significant 25% reduction of the number of CD68+ Kupffer cells (Figure 4A). This reduction was associated with an increase in FVIII activity levels in VWF-deficient mice (23 ± 1 ng/mL and 50 ± 8 ng/mL for saline- and GdCl3-treated mice, respectively; P = .028; Figure 4B) and in VWF antigen levels in wild-type mice (7.1 ± 0.8 μg/mL and 15.9 ± .5.6 μg/mL for saline- and GdCl3-treated mice, respectively; P < .001; Figure 4C). In a second approach, we studied the effect of GdCl3 treatment on the survival of VWF in VWF-deficient mice. Recombinant VWF (5 μg/mouse) was applied to saline- or GdCl3-treated VWF-deficient mice, and plasma was collected at indicated time points during a 24-hour period and analyzed for the presence of residual VWF antigen. The survival of VWF in saline-treated mice was similar as previously reported for untreated VWF-deficient mice (mean residence time [MRT] = 2.70 ± 0.35 hours).23 In contrast, the survival of VWF in GdCl3-treated mice was significantly prolonged (MRT = 4.53 ± 0.82 hours; P < .05 compared with control mice, Figure 4C). Thus, our data point to macrophages as physiological relevant elements in the clearance of VWF and FVIII.
Increased VWF and FVIII levels upon gadolinium chloride treatment. (A) Liver sections of wild-type mice treated with saline or GdCl3 were stained with antimouse CD68. Kupffer cells were identified as CD68+ cells with an appropriate nuclear morphology and sinusoidal location. Cells were counted from at least 10 randomly selected fields per section. One field corresponds to a surface of 0.1 mm2. (B,C) VWF-deficient mice (B) or wild-type mice (C) were treated with saline or GdCl3, and 24 to 48 hours after treatment plasma samples were taken. Samples were analyzed for FVIII activity (B) or VWF antigen (C). (D) VWF-deficient mice were treated with saline (•) or GdCl3 (○) 24 hours prior to intravenous injection with VWF (5 μg/mouse). At indicated time points, samples were drawn and analyzed for residual VWF antigen. For clarity, data for the first 8 hours are shown. Calculation of MRT values was based on data obtained over a 24-hour period. (A-D) Data represent mean plus or minus SD of 3 to 9 experiments. *P < .05; **P < .001.
Increased VWF and FVIII levels upon gadolinium chloride treatment. (A) Liver sections of wild-type mice treated with saline or GdCl3 were stained with antimouse CD68. Kupffer cells were identified as CD68+ cells with an appropriate nuclear morphology and sinusoidal location. Cells were counted from at least 10 randomly selected fields per section. One field corresponds to a surface of 0.1 mm2. (B,C) VWF-deficient mice (B) or wild-type mice (C) were treated with saline or GdCl3, and 24 to 48 hours after treatment plasma samples were taken. Samples were analyzed for FVIII activity (B) or VWF antigen (C). (D) VWF-deficient mice were treated with saline (•) or GdCl3 (○) 24 hours prior to intravenous injection with VWF (5 μg/mouse). At indicated time points, samples were drawn and analyzed for residual VWF antigen. For clarity, data for the first 8 hours are shown. Calculation of MRT values was based on data obtained over a 24-hour period. (A-D) Data represent mean plus or minus SD of 3 to 9 experiments. *P < .05; **P < .001.
Human macrophages bind FVIII and VWF in vitro
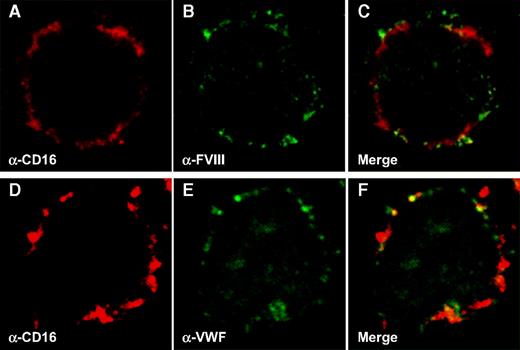
In view of the observations that both FVIII and VWF are targeted to macrophages in our mouse model, the next step was to test whether human macrophages are able to bind both proteins in vitro. Primary monocytes were isolated from human blood and allowed to differentiate into macrophages. Macrophages were incubated with human recombinant FVIII (50 μg/mL) or recombinant VWF (10 μg/mL) for 1 hour at 4°C to avoid endocytosis. This approach revealed that human macrophages are indeed capable of binding either FVIII (Figure 5B) or VWF (Figure 5E). As is apparent from costaining with the macrophage surface marker CD16 (Figure 5A,C and 5D,F [merge]), binding was limited to the cellular surface as expected. The interaction between VWF and macrophages was further investigated in more detail using 125I-radiolabeled VWF and human THP1 cell–derived macrophages. Binding of 125I-VWF (0-250 μg/mL) to these cells was dose dependent and saturable (half-maximal binding at 19.7 ± 11.1 μg/mL; Figure 6A). Moreover, binding of radiolabeled VWF (13.5 μg/mL) was markedly inhibited when applied in the presence of increasing amounts of unlabeled VWF (Figure 6B), supporting the specificity of the VWF-macrophage interaction.
Human-derived macrophages bind VWF and FVIII. Monocytes were derived from blood and differentiated into macrophages as described in “Preparation of human macrophages.” Binding of pd-VWF (10 μg/mL) and recombinant FVIII (50 μg/mL) was allowed for 1 hour at 4°C. Cells were stained using TRITC-conjugated anti-CD16 antibodies (A,D) in combination with either FITC-conjugated anti-FVIII antibodies (B) or FITC-conjugated anti-VWF antibodies (E). Panels C and F represent merged images of panels A and B and panels D and E, respectively. Original magnification 630×.
Human-derived macrophages bind VWF and FVIII. Monocytes were derived from blood and differentiated into macrophages as described in “Preparation of human macrophages.” Binding of pd-VWF (10 μg/mL) and recombinant FVIII (50 μg/mL) was allowed for 1 hour at 4°C. Cells were stained using TRITC-conjugated anti-CD16 antibodies (A,D) in combination with either FITC-conjugated anti-FVIII antibodies (B) or FITC-conjugated anti-VWF antibodies (E). Panels C and F represent merged images of panels A and B and panels D and E, respectively. Original magnification 630×.
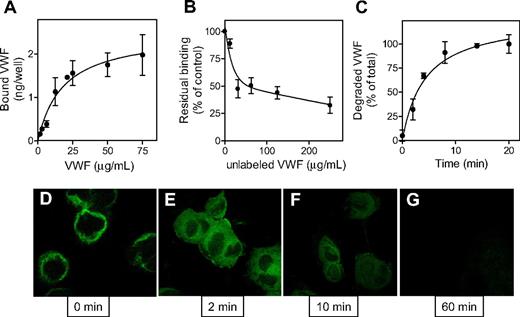
Binding and degradation of VWF by macrophages. THP-1 cells were differentiated into macrophages and incubated for 1 hour at 4°C with either radiolabeled pd-VWF (A-C) or VWF-GFP (D). (A) Binding of various concentrations of 125I-labeled pd-VWF (0-135 μg/mL) was determined as described in “Cell-binding experiments.” (B) Radiolabeled VWF (13.5 μg/mL) was added to THP-1 cells in the presence of unlabeled VWF (0-250 μg/mL). (C) Radiolabeled pd-VWF was added to THP-1 cells for a 1-hour period at 4°C. Cells were then washed, and incubation was continued at 37°C to initiate endocytosis. At indicated time points, samples were taken to determine the amount of degraded material. Degraded material is defined as the radioactivity that is soluble in 10% trichloroacetic acid. In all experiments, controls were included to determine the amount of nonspecific degradation in the absence of cells, which routinely was less than 10% of degradation in the presence of cells. Data represent mean plus or minus SEM of 6 to 9 experiments. (D) THP-1 cells were incubated with VWF-GFP (65 μg/mL) for 1 hour at 4°C. After washing, cells were fixed and analyzed for the presence of bound VWF-GFP. (E-G) After incubation at 4°C and washing, cells were placed at 37°C to initiate endocytosis. After 2, 10, and 60 minutes, cells were fixed and processed for analysis (original magnification 630×).
Binding and degradation of VWF by macrophages. THP-1 cells were differentiated into macrophages and incubated for 1 hour at 4°C with either radiolabeled pd-VWF (A-C) or VWF-GFP (D). (A) Binding of various concentrations of 125I-labeled pd-VWF (0-135 μg/mL) was determined as described in “Cell-binding experiments.” (B) Radiolabeled VWF (13.5 μg/mL) was added to THP-1 cells in the presence of unlabeled VWF (0-250 μg/mL). (C) Radiolabeled pd-VWF was added to THP-1 cells for a 1-hour period at 4°C. Cells were then washed, and incubation was continued at 37°C to initiate endocytosis. At indicated time points, samples were taken to determine the amount of degraded material. Degraded material is defined as the radioactivity that is soluble in 10% trichloroacetic acid. In all experiments, controls were included to determine the amount of nonspecific degradation in the absence of cells, which routinely was less than 10% of degradation in the presence of cells. Data represent mean plus or minus SEM of 6 to 9 experiments. (D) THP-1 cells were incubated with VWF-GFP (65 μg/mL) for 1 hour at 4°C. After washing, cells were fixed and analyzed for the presence of bound VWF-GFP. (E-G) After incubation at 4°C and washing, cells were placed at 37°C to initiate endocytosis. After 2, 10, and 60 minutes, cells were fixed and processed for analysis (original magnification 630×).
Human macrophages rapidly degrade VWF
To test whether human macrophages are also capable of degrading VWF, THP1 cells were incubated with 125I-labeled VWF (13.5 μg/mL) for 1 hour at 4°C to allow surface binding. Cells were then incubated at 37°C to initiate endocytosis. Samples were taken at indicated time points and analyzed for the presence of TCA-soluble radioactive fragments, which represent degraded VWF protein. Within minutes after inducing endocytosis, VWF degradation products appeared and degradation was virtually complete after 20 minutes (Figure 6C). Binding and uptake of VWF by macrophages were also visualized using a recombinant VWF-GFP fusion protein. As expected, incubation of THP1 macrophages with VWF-GFP fusion protein (65 μg/mL) at 4°C resulted in binding of this protein at the cellular surface (0 minutes; Figure 6D). Increasing the temperature to 37°C resulted in rapid internalization, as illustrated by the fluorescent signal homogenously present within the cells (2 minutes; Figure 6D). In time, the intracellular fluorescent signal disappeared, which could be compatible with VWF-GFP protein being degraded within the cells. In conclusion, our data demonstrate that macrophages are able to bind and deliver VWF to their intracellular degradation pathway.
Cotargeting of FVIII and VWF
One issue that remains relates to the cellular targeting of FVIII and VWF when applied as a complex. To get insight into this issue, recombinant FVIII (1.25 μg/mL, corresponding to 7.5 nM) and recombinant VWF (50 μg/mL, corresponding to 200 nM) were preincubated for 30 minutes prior to injection. These concentrations are well above the apparent dissociation constant of complex formation, allowing more than 95% of the FVIII molecules to be driven into complex formation with VWF. Thirty minutes after injection in VWF-deficient mice, spleen was collected and prepared for staining with anti-FVIII and anti-VWF antibodies. A clear staining for VWF could be observed in spleen (Figure 7). Interestingly, FVIII staining was found only in cells that were also positive for VWF. In addition, a surplus of VWF-positive cells was found compared with FVIII/VWF-positive cells. This may be explained by the excess of VWF over FVIII that was applied (27-fold in terms of subunits, or 2.7-fold in terms of VWF molecules, assuming an average of 10 subunits/multimer and 1 FVIII molecule bound per VWF multimer). Nevertheless, these data are in support of the possibility that when applied as a complex, FVIII and VWF are cotargeted to the same cells.
Colocalization of FVIII and VWF in macrophages. Merged image of a spleen tissue section of a VWF-deficient mouse infused with VWF/FVIII complex (10 and 0.25 μg/mouse, respectively) stained with anti–human VWF antibodies (red) and monoclonal anti–human FVIII antibody LE2E9 (green).
Colocalization of FVIII and VWF in macrophages. Merged image of a spleen tissue section of a VWF-deficient mouse infused with VWF/FVIII complex (10 and 0.25 μg/mouse, respectively) stained with anti–human VWF antibodies (red) and monoclonal anti–human FVIII antibody LE2E9 (green).
Discussion
Previously, we have developed a mouse model using VWF-deficient mice to study clearance mechanisms of VWF.23 This model has been used to investigate the effect of von Willebrand disease–related mutations on VWF survival, and to gain more insight into the molecular basis of VWF clearance.23,24 Our studies using radiolabeled VWF revealed main targeting of this protein to the liver. However, liver was the largest of the organs tested, and additional analysis revealed that spleen also efficiently takes up VWF (Figure 1). Thus, both organs contain cells that efficiently remove VWF from the circulation. It should be noted that in absolute terms the contribution of spleen to the clearance process is limited because of its relatively small size (Table 1). This is illustrated by patients who underwent splenectomy. Desmopressin treatment of these patients resulted in the expected rise in VWF levels. The subsequent decline of VWF levels in these asplenic patients occurred at an equal rate compared with healthy individuals.37
VWF uptake by both organs was confirmed in immunohistochemical analysis of tissue sections obtained after injection with nonlabeled recombinant VWF (Figure 2). We used polyclonal anti-VWF antibodies raised against pd-VWF. Since such preparations could potentially contain traces of residual FVIII, we considered the possibility that this polyclonal antibody cross-reacts with endogenous FVIII. However, neither in systems using purified recombinant human FVIII nor in tissue sections of noninjected VWF-deficient mice could any cross-reaction with FVIII (or other murine proteins) be observed. Although the human anti–human FVIII antibody was not expected to cross-react with murine proteins or human VWF because of its monoclonal nature, we did thoroughly examine specificity of this antibody. No cross-reaction with human VWF or significant staining in control tissue sections of FVIII- or VWF-deficient mice could be detected. Thus, the observed staining patterns originated exclusively from human VWF and human FVIII that were given to these mice. One of the concerns we addressed was whether binding of antibody LE2E9 to FVIII could be modulated by VWF, potentially affecting identification of surface-bound FVIII when in complex with VWF. However, control experiments confirmed that FVIII in complex with VWF was readily recognized by this antibody (not shown).
A similar pattern of staining was detected for FVIII and VWF (Figures 2,3), which coincided with staining of macrophage-like cells. These cells represented CD68+ cells in liver, most likely Kupffer cells.33 In spleen, FVIII- and VWF-positive cells correlated with cells stained positive for F4/80 antigen, a mouse macrophage marker.32 This marker stains macrophages present in the red pulp of spleen, while also macrophages in the marginal zone can be detected.31 Of note, the marginal zone of murine spleen is rather diffuse, which makes it difficult to assess where red pulp ends and the marginal zone starts.38 It is possible therefore that FVIII and VWF are targeted to macrophages present in red pulp and marginal zone areas of the spleen. Furthermore, it has been reported that F4/80 may also stain few nonmacrophage cells, most likely a subset of dendritic cells.31,39 Thus, our data do not exclude these cells as players in the uptake of FVIII or VWF.
Macrophage-like cells are well known for their capacity to rapidly bind and endocytose proteins. The functional contribution of macrophage-like cells to the uptake of proteins or particulate matter in vivo has previously been examined via functional depletion of these cells using GdCl3.34-36 Indeed, a 25% reduction of visible macrophages was observed under the conditions used (Figure 4). In a separate study, the residual functionality of the remaining macrophages has also been examined. Preliminary data indicate that our treatment procedure reduces functionality of macrophages by approximately 40% (C.V.D. and D. Geldwerth, unpublished observations, February 2006). Despite the incomplete reduction in the number of functional macrophages, we were able to detect a 2-fold increase in endogenous concentrations of VWF or FVIII (Figure 4). Whether this increase is the resultant of increased synthesis, decreased clearance, or both cannot be answered by measuring endogenous FVIII or VWF levels. However, our clearance experiments using intravenously administered VWF in GdCl3-treated VWF-deficient mice clearly demonstrated that MRT increased 2-fold, indicating that reduced clearance contributes to a significant extent to the increased VWF levels after macrophage depletion.
We realize that care should be taken when clearance of human proteins is studied in animals, such as our mouse model. To make proper comparison, we are currently developing means to study the survival and biodistribution of murine VWF in VWF-deficient mice. We would like to mention that Sodetz et al have previously shown that inhibition of the reticuloendothelial system was associated with a prolonged half-life of VWF in a rabbit model,27 indicating that recognition of human VWF is not limited to the mouse model. Moreover, human FVIII and human VWF are recognized by macrophages of human origin: primary monocyte-derived macrophages (Figure 5) and macrophages derived from human THP1 cells (Figure 6). The finding that macrophages from different origin (liver and spleen macrophages in the mouse, and primary and cell line–derived human macrophages) internalize VWF indicates that these cells share a common mechanism. The identification of macrophages as scavenger cells for VWF may seem surprising, but appears to be logical at the same time. VWF is heterogeneously sized considerably (between 0.5 and > 10 million Da), and the physical dimensions of the larger molecules (up to several hundred nanometers)40 exceed the size of clathrin-coated pits or caveolae. Both these cell surface structures are the classical elements used by cells for the uptake of proteins. The capacity of macrophages to rapidly endocytose larger particles and microbes is compatible with the uptake of the large VWF molecule. Interestingly, the identification of macrophages as scavenger cells for VWF provides a strategy to study VWF uptake in vitro. This approach may be used to test the effect of glycosylation status of VWF on endocytosis. For example, differences in endocytosis rates of VWF purified from blood group O or non-O individuals or from patients known for glycosylation defects could be addressed using this in vitro system.
One intriguing observation regarding the uptake of VWF relates to its rapid degradation following its internalization. In experiments using either radiolabeled VWF or the GFP-VWF fusion protein, degradation of VWF was virtually completed after 20 minutes. For a number of other proteins, this process has been found to proceed much slower. For instance, we recently demonstrated that protein S is efficiently bound and intracellularly degraded by macrophages.41 However, the rate of degradation proceeds much slower compared with VWF: degraded material was observed not before 15 minutes after internalization, and degradation was completed after 4 hours.41 The reason for this marked difference in internalization and degradation rate is yet unclear, but may originate from distinct molecular pathways involved in the uptake of both proteins.
We were surprised by the finding that also free FVIII is targeted to macrophages (Figure 3). This targeting was fully independent of VWF, since FVIII was infused in VWF-deficient mice. Targeting was also independent of the FVIII B-domain, since similar distribution was observed for full-length and B-domainless FVIII. This may suggest that the B-domain plays a minor role in the clearance process of FVIII, despite the presence of a binding site for asialoglycoprotein receptor.42 Alternatively, low endogenous FVIII levels (20-25 ng/mL in VWF-deficient mice) may interfere with the uptake of FVIII by hepatocytes. If endogenous levels were to saturate the clearance system, one would expect that the FVIII half-life increases when increasing doses of FVIII are applied to VWF-deficient mice. However, no increase in FVIII half-life is observed even when administered at doses up to 2 to 5 times those used in this study.43
It has previously been shown that LRP plays an important role in the regulation of FVIII plasma levels, both in animals and humans.15,16 In addition, conditional deficiency of LRP in the liver is associated with increased endogenous FVIII levels and a prolonged half-life of exogenously administered FVIII.15 Considering the various cell types present in the liver, both hepatocytes and macrophages have been demonstrated to express LRP.44,45 Apparently, no FVIII is endocytosed by hepatocytes as assessed in our model. Does that mean that FVIII is not recognized by hepatocytes? That is yet unclear, but it cannot be excluded that FVIII is internalized by hepatocytes at a much slower rate than Kupffer cells. This seems unlikely, given the short FVIII half-life in the absence of VWF. Another explanation could be that FVIII is taken up by hepatocytes, but subsequently degraded so rapidly that it cannot be detected anymore by our anti-FVIII antibody. In view of the time span after which the organs were collected (5 minutes after infusion), this possibility seems unrealistic. However, considering the marked excess of hepatocytes over Kupffer cells, we cannot rule out that dilution of FVIII over this large number of hepatocytes prevents its detection by antibody LE2E9, as the amount of FVIII per cell would be too low.
The notion that both free FVIII and VWF-bound FVIII are targeted to macrophages is also striking given their strong difference in half-life (2 hours versus 14 hours in humans). Apparently, different mechanisms are involved in macrophage-mediated clearance of free and VWF-bound FVIII. VWF-bound FVIII is probably cleared as part of the complex rather than as a separate entity. Since the majority of FVIII is found in the same cells that have endocytosed VWF, it seems that the uptake of both proteins is mediated by a coordinated action. Whether cellular entry involves the complex as a whole, or that surface binding is associated with dissociation of the complex and separate cellular entry of its constituents is yet unclear.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully thank Dr A. B. Meijer for providing VWF-GFP protein and Dr J. M. Saint-Remy for providing antibody LE2E9. We thank Mrs V. Korver for excellent technical assistance.
This study was supported by a Bayer Hemophilia Award to P.J.L.
Authorship
Contribution: C.J.S. performed experiments, analyzed data, worked on the draft version of the paper, and approved the final version; S.S. and E.G. performed experiments and approved the final version of the paper; B.D.O. analyzed data, revised the draft, and approved the final version of the paper; H.M.B. designed the study and approved the final version of the paper; C.V.D. conceived and designed the study, performed experiments, revised the draft version of the paper, and approved the final version of the paper; and P.J.L. conceived and designed the study, performed experiments, analyzed data, and wrote the draft and the final version of the paper.
Conflict-of-interest disclosure: P.J.L. is an employee of Crucell Holland BV. The remaining authors declare no competing financial interests.
Correspondence: P. J. Lenting, Laboratory for Clinical Chemistry & Haematology, G03.550, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail: p.j.lenting@umcutrecht.nl.