Abstract
Dendritic cells (DCs) play a crucial role in naive T-cell priming. Recent data suggested that natural killer (NK) cells can influence the capability of DCs to promote Th1 polarization. This regulatory function is primarily mediated by cytokines released in the microenvironment during inflammatory responses involving NK cells. In this study, we show that human NK cells exposed for short time to interleukin (IL)–12, IL-2, or IL-18, promote distinct pathways of Th1 priming. IL-12– or IL-2–conditioned NK cells induce maturation of DCs capable of priming IFN-γ–producing Th1 cells. On the other hand, IL-18–conditioned NK cells induce Th1 polarization only when cocultured with both DCs and T cells. In this case, IL-2 released by T cells and IL-12 derived from DCs during the priming process promote interferon (IFN)–γ production. In contrast, when NK cells are exposed to IL-4, nonpolarized T cells releasing only low levels of IL-2 are generated. Thus, the prevalence of IL-12, IL-2, IL-18, or IL-4 at inflammatory sites may differentially modulate the NK-cell interaction with DCs, leading to different outcomes in naive T-cell polarization.
Introduction
Natural killer (NK) cells and dendritic cells (DCs) are 2 specialized cell types of the innate immunity that act as liaisons between innate and Ag-specific adaptive immune responses.1-4 DCs function as sentinels of the immune system and display the unique capability of inducing primary immune responses by providing all the signals necessary to prime naive T cells.5 NK cells play an active role in innate responses against viruses, bacteria, and tumors thanks to their potent cytotoxic activity and rapid production of cytokines, particularly interferon (IFN)-γ and tumor necrosis factor (TNF)–α.6,7 Because of its important role in shaping the immune response, the crosstalk between NK cells and DCs has been a field of extensive study in recent years.8-15 It has been shown that the cytolytic activity of fresh NK cells is strongly up-regulated by the interaction with monocyte-derived DCs (MoDCs) that had been exposed to maturation stimuli such as lipopolysaccharide (LPS), interferon (IFN)–α, or Mycobacterium tuberculosis.9-12 Reciprocally, upon interaction with immature DCs (iDCs) in the presence of maturation stimuli, fresh NK cells can strongly enhance DC maturation and cytokine production.8,10,11,14 In addition, IL-2–activated NK cells could directly induce DC maturation and enhance their ability to stimulate alloproliferative responses of naive CD4+ T cells.10 In this case, DC maturation occurs at low NK/DC ratios and is dependent on cell-to-cell interactions mediated via the NKp30 and DNAM-1 NK receptors and the release of TNF-α and IFN-γ by NK cells.8,13-16 Regarding the effect exerted by DCs on NK cells, most NK cells become partially activated upon interaction with pathogen-activated DCs, as revealed by the expression of CD69 and by the increase of their cytotoxic activity against different targets.
In general, major histocompatibility complex (MHC) class I–deficient aberrant cells are susceptible to NK cell–mediated lysis, whereas normal cells are protected from NK cells because they express MHC class I molecules. iDCs represent a remarkable exception. In an autologous setting, they are highly susceptible to lysis by activated polyclonal NK-cell populations but become resistant when they undergo maturation.17 Killing of iDCs is confined to those NK cells that lack killer immunoglobulin-like receptor (KIR) specific for self–human leukocyte antigen (HLA) class I alleles and express the HLA-E–specific CD94/NKG2A inhibitory receptor.18 Based on these data, an additional function of NK cells would be to keep in check the quality of DCs undergoing maturation in peripheral tissues and to control the amplitude of DC responses and subsequent T-cell priming. This function is based mostly on the ability of NK cells to selectively kill DCs that do not express sufficient amounts of HLA-E at the cell surface.18,19 Because iDCs express very low amounts of HLA-E molecules that are acquired progressively during DC maturation, NK cells would operate a positive selection of DCs undergoing efficient maturation (“editing” process).
Mailliard et al proposed the concept of a “helper” function exerted by certain NK cells.20 Thus, NK cells treated with IL-18 acquire the unusual CD56+CD83+CCR7+CD25+ phenotype, distinct from that induced by IL-2. These NK cells display high migratory capacity in response to the lymph node–associated chemokines CCL19 and CCL21. Although they do not secrete IFN-γ spontaneously, these cells acquire the capability of producing high amounts of IFN-γ upon exposure to either DC-related signals (IFN-α or IL-12) or T cell–related signals (IL-2). These NK cells also display an increased ability to promote IL-12p70 secretion by DC upon CD40L-mediated stimulation. Thus, their presence during priming of naive CD4+ Th cells facilitates their polarization toward IFN-γ-producing Th1 cells.
Recently, we showed that short-time exposure of freshly isolated NK cells to exogenous IL-12 (but not to IL-4) induced strong cytolytic activity against tumor cells and iDCs.21 Moreover, MoDCs cocultured with IL-12–conditioned NK cells expressed higher levels of CD83 and CD86 compared with control iDCs or DCs cocultured with IL-4–conditioned NK cells. According to the editing process model, these results suggested that, after the selection operated by IL-12–conditioned NK cells, the resulting DCs would migrate to lymph nodes, where they could promote efficient priming of Th1 responses. On the other hand, IL-4–conditioned NK cells, being unable to perform a DC editing program, would generate DCs favoring T-cell tolerization or Th2 priming.
In the present study, we assessed directly whether and how different cytokines may modulate the NK-cell capability of regulating the process of naive CD4+ T-cell polarization.
Methods
Monoclonal antibodies
The following mAbs produced in our laboratory were used in this study: anti-CD16 (KD1; IgG2a), anti-CD56 (c218; IgG1), anti-CD69 (FST3; IgG3), and anti-CD8 (B9.4; IgG2b). The anti-CD45RO mAb (UCHL1; IgG2a) was a kind gift of A. Poggi (Laboratory of Experimental Oncology, National Institute for Cancer Research, Genoa, Italy). FITC-conjugated anti-CD19 (IgG1), PE-conjugated anti-CD14 (IgG2a) and anti-CD86 (IgG2b), a mixture of PC5-conjugated anti-CD56 mAb and FITC-conjugated anti-CD3 mAb, and purified anti-CD19 (IgG1) and anti-CD14 (IgG2a) were purchased from Beckman Coulter (Marseille, France).
NK-cell purification and culture in the presence of cytokines
NK cells were separated from heparinized whole blood by an NK-cell separation cocktail (Rosette Sep; StemCell Technologies, Vancouver, BC) and Ficoll gradient centrifugation (Sigma-Aldrich, St Louis, MO).21 The purity of NK cells was greater than 96% as assessed by staining with a mixture of anti-CD56-PC5 and anti-CD3-FITC, anti-CD14-PE and anti-ILT3-PC5 mAb. Presence of T cells, monocytes, and contaminating DCs in purified NK cells was less than 1%.
These cells were resuspended in medium supplemented with 2 mM glutamine, 50 μg/mL penicillin/streptomycin, and 10% heat-inactivated fetal calf serum (FCS) in the presence of 0.1 ng/mL IL-12 (PeProTech, London, United Kingdom), or 50 ng/mL IL-4 (PeProTech) plus 0.1 μg/mL anti-IL-12 mAb, or 0.1 μg/mL IL-18, with or without 100 U/mL IL-2 (proleukin; Chiron, Emeryville, CA). Cells were plated in round-bottomed 96-well tissue culture plates. After a 40-hour culture, the activation state of NK cells was checked by flow cytometry before coculture with DCs.
Generation of DCs and coculture with cytokine-treated NK cells
MoDCs were obtained by plastic adherence of peripheral blood mononuclear cells (PBMCs) and culture 5 days in RPMI 1640 medium in the presence of 20 ng/mL IL-4 and 50 ng/mL GM-CSF (PeProTech).18 After 5 days of culture, cells were characterized by the CD14−CD1a+CD83− phenotype corresponding to immature MoDCs.
Before coculture, NK cells and iDCs were washed extensively to remove the cytokines used for their treatment. NK cells were then cocultured with allogeneic iDCs at a ratio of 1:5 (104 NK cells for 5 × 104 iDCs) in round-bottomed 96-well tissue culture plates for 24 hours. Control mature DCs were obtained by addition of 1 μg/mL LPS for 24 hours, and iDCs were obtained by culture for 24 hours in medium alone.
Mixed lymphocyte reaction and Th polarization
CD4+ naive T cells were purified by immunomagnetic depletion (Dynal; Invitrogen, Frederick, MD) using a mixture of mAb anti-CD19, anti-CD56 (c218), anti-CD8 (B9.4), anti-CD16 (KD1), and anti-CD45RO (UCHL1). Naive T lymphocytes were more than 97% pure as assessed by CD4 and CD45RA labeling. Primary mixed lymphocyte reaction (MLR) was conducted in 96-well flat-bottomed culture plates. DCs recovered at day 6 were washed extensively and NK cells were removed or not by magnetic depletion. DC were then resuspended in RPMI 1640/10% FCS and cocultured in triplicate with 105 naive T cells in 200 μL at DC/T-cell ratios ranging from 1:10 to 1:40.22 When indicated, neutralizing Abs were added at the beginning of the MLR: against IL-2 (0.5 μg/mL; PeProTech) and/or IL-12 (1 μg/mL; PeProTech); against NCR (F252, anti-NKp30, IgM, 5 μg/mL; KS38, anti-NKp44, IgM, 5 μg/mL; KL247, anti-NKp46, IgM, 5 μg/mL); against NKG2D (BAT221, IgG1, 5 μg/mL); against 2B4 (MA344, IgM, 5 μg/mL); against MHC class I molecules (A6/136, IgM, 5 μg/mL). Supernatants were recovered after 2 days for IL-2 quantification and after 5 days of coculture for IFN-γ, IL-4, IL-10, IL-5, and IL-13 measurement.
Cytokine measurement and statistical analysis
IL-8, IL-10, IL-12p40 or p70, IL-17, IL-23, IFN-γ, and TNF-α levels were determined using cytokine-specific enzyme-linked immunosorbent assay (ELISA) kits (Biosource International, Camarillo, CA). IL-4, IL-5, and IL-13 were assayed using ELISA kits from Euroclone. IL-2 was measured by a chemiluminescent ELISA (Pierce Biotechnology, Endogen, Rockford, IL).
Viable IFN-γ–secreting T cells were also detected by fluorescence-activated cell sorting (FACS) using a cytokine secretion assay (Miltenyi Biotec, Bergisch Gladbach, Germany) after 5 days of MLR according to the manufacturer's recommendations. Statistical analysis was performed with Student t test; analysis of variance (ANOVA) test was used to assess significance.
Results
Effect of polarizing cytokines on NK cell–induced iDC maturation
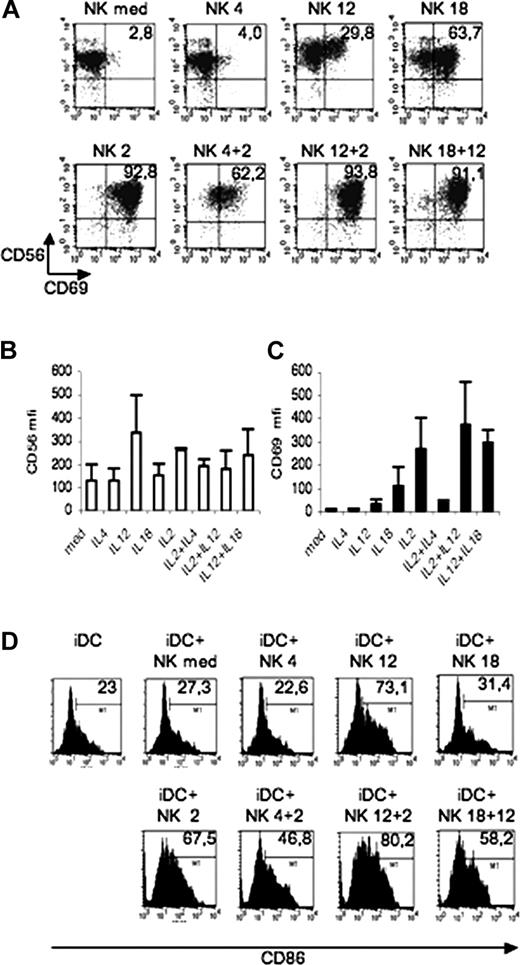
We first analyzed the state of activation of fresh NK cells according to the surface expression of CD69 after they had been cultured in the absence or in the presence of polarizing Th1 or Th2 cytokines and/or IL-2. The fraction of CD56+CD69+ NK cells was very low in NK cells cultured in the absence of cytokines. In NK cells cultured in IL-4, there was no significant modification in the proportion of this cell subset. On the other hand, CD56+CD69+ cells were substantially increased after cell exposure to IL-12, IL-18, or IL-2. The effect of IL-2 was partially inhibited by the simultaneous presence of IL-4. Remarkably, very large proportions of NK cells cultured in IL-18 plus IL-12 acquired the CD56+CD69+ phenotype, whereas cell culture in IL-12 plus IL-2 had only a minor additive effect (Figure 1A). Up-regulation of CD56 expression occurred in NK cells cultured in IL-12 or IL-2, but not in those cultured in IL-4 or IL-18. The effect of IL-2 on CD56 up-regulation was partially inhibited by IL-4, while no synergy was observed between IL-12 and IL-2 or IL-18 (Figure 1B). CD69 was barely detectable on control NK cells or NK cells cultured with IL-4, but was clearly up-regulated on NK cells that had been exposed to either IL-2 or IL-18 (Figure 1C). In line with previous data,20 a variable fraction of IL-18–conditioned NK cells expressed CCR7, whereas NK cells conditioned by the other cytokines did not (data not shown).
Expression of activation markers on NK cells and MoDCs. (A-C) Freshly isolated NK cells were cultured 40 hours in medium alone or in the presence of IL-4, IL-12, IL-2, IL-18, or a combination of these cytokines. Expression of CD56 and CD69 were analyzed by 2-color immunofluorescence (A). Values in the upper right square indicate the percentage of cells stained by both Abs. (B,C) Mean fluorescence intensity for CD56 (B) and CD69 (C). Means plus or minus SD of 3 independent experiments. (D) Induction of iDC maturation by NK cells. NK cells that had been exposed to IL-4, IL-12, IL-2, IL-18, or a combination of these cytokines for 40 hours were washed and cocultured with iDCs for 24 hours. These iDCs were then analyzed for the expression of CD86. In control cultures, iDCs were plated in medium (iDC). The mean fluorescence intensity is indicated in the right corner. Data are representative of 3 independent experiments.
Expression of activation markers on NK cells and MoDCs. (A-C) Freshly isolated NK cells were cultured 40 hours in medium alone or in the presence of IL-4, IL-12, IL-2, IL-18, or a combination of these cytokines. Expression of CD56 and CD69 were analyzed by 2-color immunofluorescence (A). Values in the upper right square indicate the percentage of cells stained by both Abs. (B,C) Mean fluorescence intensity for CD56 (B) and CD69 (C). Means plus or minus SD of 3 independent experiments. (D) Induction of iDC maturation by NK cells. NK cells that had been exposed to IL-4, IL-12, IL-2, IL-18, or a combination of these cytokines for 40 hours were washed and cocultured with iDCs for 24 hours. These iDCs were then analyzed for the expression of CD86. In control cultures, iDCs were plated in medium (iDC). The mean fluorescence intensity is indicated in the right corner. Data are representative of 3 independent experiments.
Next we analyzed the capability of NK cells exposed to IL-2, IL-12, IL-4, or IL-18 or to a combination of these cytokines to promote DC maturation, as assessed by the up-regulation of CD86 surface expression. In line with previous data,10,21 substantial increments in the surface expression of CD86 were detected when iDCs were cocultured for 24 hours with NK cells that had been exposed to IL-2 or IL-12. This effect was dependent on cell-to-cell interaction and was strongly inhibited by anti-NKp30 antibodies (data not shown).15 On the contrary, NK cells exposed to IL-4 or IL-18 did not induce CD86 up-regulation (Figure 1D).
The numbers of DC recovered after coculture with NK cells did not significantly vary between the different culture conditions (data not shown). This is in line with previous data showing that at low effector target (E:T) ratios (ie, 1:5), NK cells can drive DC maturation without displaying strong cytolytic activity against iDC.14,20
NK cells exposed to IL-12 or IL-2 induce an inflammatory cytokine environment
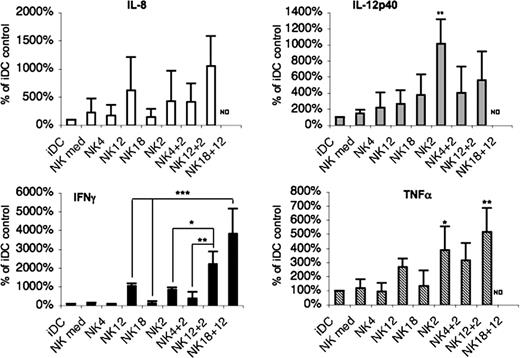
To analyze the cytokine environment resulting from the NK/DC interaction, we assessed the cytokines released in the coculture supernatants. The cocultures analyzed contained iDCs and NK cells that had been pre-exposed to different cytokines. In no instances were Th2 cytokines such as IL-4, IL-5, or IL-13 detected in supernatant of DC/NK cocultures. This finding is consistent with the previous observation that fresh peripheral blood NK cells cultured overnight with IL-4 do not release Th2 cytokines when stimulated by either anti-NKp46 mAb or PMA (phorbol-12-myristate-13-acetate) and ionomycin.21 Remarkably, IL-10 also could not be detected, whereas supernatants contained substantial amounts of IL-8, IL-12p40, TNF-α, and IFN-γ. These cytokines were abundant in supernatants from cocultures containing DCs and IL-2– (most cytokines) or IL-12 (IFN-γ only) conditioned NK cells. The other differences were not statistically different. The effect of IL-2 was partially inhibited by IL-4, whereas it was increased by IL-12 (with an exception for IL-12p40). The level of cytokines released in supernatants derived from cocultures of DCs and IL-4– or IL-18–conditioned NK cells was comparable with that of controls (Figure 2). Because high amounts of IL-12p40 were detected in the supernatants of certain culture conditions, these supernatants were further evaluated for the levels of IL-12p70 and IL-23. However, these cytokines were not detected, even after 48 hours of coculture (not shown).
DC cocultured with IL-2– or IL-12–conditioned NK cells induce an environment of inflammatory type. NK cells that had been exposed to IL-4, IL-12, IL-2, IL-18, or a combination of these cytokines during 40 hours were washed and further cultured together with iDCs for 24 hours. Cytokine levels were measured in supernatants of these cocultures. Cytokine secretions were normalized to 100% for control iDCs. Data represent means plus or minus SD of 3 independent experiments. ND indicates not determined. *P < .05; **P < .01; ***P < .001.
DC cocultured with IL-2– or IL-12–conditioned NK cells induce an environment of inflammatory type. NK cells that had been exposed to IL-4, IL-12, IL-2, IL-18, or a combination of these cytokines during 40 hours were washed and further cultured together with iDCs for 24 hours. Cytokine levels were measured in supernatants of these cocultures. Cytokine secretions were normalized to 100% for control iDCs. Data represent means plus or minus SD of 3 independent experiments. ND indicates not determined. *P < .05; **P < .01; ***P < .001.
Remarkably, although no IFN-γ could be detected in supernatants of DCs cocultured with IL-18–conditioned NK cells, it was produced in large amounts when NK cells were exposed to both IL-12 and IL-18, thus supporting the notion that IL-18–conditioned NK cells strictly require a second signal to produce IFN-γ20,23 (Figure 2).
NK cells treated with IL-2 or IL-12 can induce DCs to prime a Th1 response
The question to what extent DCs interacting with cytokine-treated NK cells can actually induce Th-cell polarization was addressed by investigating the profile of cytokines released by CD4+ naive T cells upon stimulation in allogeneic MLR. In a first set of experiments, DCs were incubated as described above together with NK cells that had been conditioned with cytokines for 40 hours. They were subsequently purified and cocultured with naive CD4+ T cells isolated from the same donor from whom NK cells were derived. Thus, in these experiments, DCs and T cells were isolated from different donors (allogeneic background). It should be stressed that the cytokines used for pretreating NK cells were no longer present during either the coculture of NK cells with DCs or the subsequent MLR. As shown in Figure 3A, iDCs induced a basal secretion of IL-2 by T cells. This IL-2 secretion was not affected when DCs were previously cocultured with either untreated or IL-4– or IL-12–conditioned NK cells. It was slightly decreased when DCs had been exposed to IL-18–conditioned NK cells. However, iDCs cocultured with NK cells conditioned by IL-2 or IL-2 plus IL-12 were able to induce T cells to secrete a greater amount of IL-2 (Figure 3A and data not shown). Regarding the production of IFN-γ, iDCs cultured in MLR with naive CD4+ T cells were poor inducers of IFN-γ secretion (Figure 3B). On the other hand, DCs cocultured with NK cells conditioned by IL-12 or IL-2 (but not with IL-4 or IL-18) induced production of IFN-γ by T cells (Figure 3B). The effect of IL-2 could be partially inhibited by IL-4, whereas only a minor synergy between IL-2 and IL-12 occurred (data not shown). Typical Th2 cytokines such as IL-4, IL-5, and IL-13, the immunosuppressive cytokine IL-10, or the cytokine IL-17 were not detected in the same coculture supernatants (data not shown).
NK cells conditioned with IL-2 or IL-12 instruct DCs to prime a Th1 response. (A,B) MLRs were performed with purified DCs and naive allogeneic CD4+ T cells. IL-2 (A) and IFN-γ (B) were measured in MLR supernatants by ELISA after 2 (A) or 5 days of coculture (B). Means plus or minus SD of triplicate of 1 representative experiment of 3 performed. (C) MLRs were performed with purified DCs and autologous CD4+ naive T cells. Cytokines were measured in MLR supernatants by ELISA after 5 days of coculture. Data represent means plus or minus SD of triplicates of 1 representative experiment of 3.
NK cells conditioned with IL-2 or IL-12 instruct DCs to prime a Th1 response. (A,B) MLRs were performed with purified DCs and naive allogeneic CD4+ T cells. IL-2 (A) and IFN-γ (B) were measured in MLR supernatants by ELISA after 2 (A) or 5 days of coculture (B). Means plus or minus SD of triplicate of 1 representative experiment of 3 performed. (C) MLRs were performed with purified DCs and autologous CD4+ naive T cells. Cytokines were measured in MLR supernatants by ELISA after 5 days of coculture. Data represent means plus or minus SD of triplicates of 1 representative experiment of 3.
Similar results were obtained when these experiments were perfomed in an autologous background (ie, DCs and naive CD4+ T cells derived from the same donor; Figure 3C).
These data suggest that DCs, when instructed by IL-2– or IL-12–conditioned NK cells, can promote an efficient differentiation of naive Th cells into IFN-γ–producing Th1 cells. On the other hand, DC cocultured with IL-4– or IL-18–conditioned NK cells did not induce detectable Th1 or Th2 (or Treg) responses, but rather induce nonpolarized T-cell responses resulting in the production of relatively low levels of IL-2. Similar results were obtained using 10-fold higher concentrations of IL-4 or IL-18, indicating that this effect was not strictly dependent on the concentration of these cytokines (data not shown).
NK cells treated with IL-2, IL-4, IL-12, or IL-18 differentially modulate Th1 polarization when cocultured with DC and naive T cells
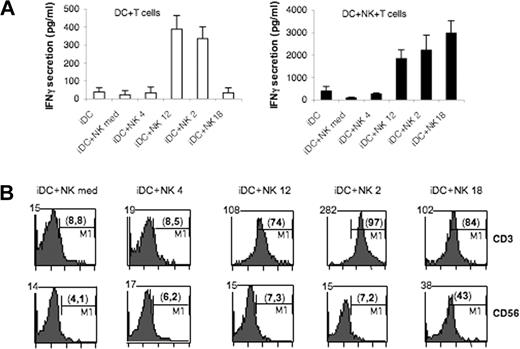
Previous studies indicated that the presence of IL-18–treated NK cells in cocultures of iDCs and CD4+ naive T cells can direct T-cell differentiation toward the Th1 pathway.20 Thus we comparatively analyzed the ability of NK cells conditioned by different cytokines to modulate T-cell polarization in MLR. In these experiments, DCs were incubated as above for 24 hours with cytokine-treated NK cells. Both NK cells and DCs were then harvested and extensively washed before coculture with naive CD4+ T cells. As shown in Figure 4A, the presence of IL-2– or IL-12–conditioned NK cells during MLR greatly enhanced IFN-γ secretion in the culture supernatants. In contrast, the presence of IL-4–conditioned or control untreated NK cells had only a minor impact on the basal IFN-γ secretion induced by iDCs. Despite their inability to induce DC maturation, IL-18–conditioned NK cells induced a strong IFN-γ secretion when present during MLR. This was consistently higher than in MLR performed in the presence of IL-2– or IL-12–conditioned NK cells (Figure 4A).
IL-18–conditioned NK cells exert a helper function during MLR. MLR were carried out with DC and allogeneic naive CD4+ T cells in the absence or in the presence of NK cells during the MLR. (A) IFN-γ was measured in MLR supernatants by ELISA. □: DC with T cells; ■: DC with T and NK cells. (B) Viable IFN-γ–secreting cells were detected by FACS after 5 days of coculture. The mean fluorescence intensity is stated on the upper left corner. The percentage of IFN-γ+ cells is shown in parentheses.
IL-18–conditioned NK cells exert a helper function during MLR. MLR were carried out with DC and allogeneic naive CD4+ T cells in the absence or in the presence of NK cells during the MLR. (A) IFN-γ was measured in MLR supernatants by ELISA. □: DC with T cells; ■: DC with T and NK cells. (B) Viable IFN-γ–secreting cells were detected by FACS after 5 days of coculture. The mean fluorescence intensity is stated on the upper left corner. The percentage of IFN-γ+ cells is shown in parentheses.
As shown above (Figure 2) and in agreement with Maillard et al,20 IL-18–conditioned NK cells do not secrete IFN-γ spontaneously, but release large amounts of this cytokine in the presence of a second signal such as IL-2 (derived from T cells) or IL-12 (derived from DCs). In view of these findings, we asked which cellular type(s) was responsible for IFN-γ secretion in our MLR settings. A FACS assay assessing the proportion of viable IFN-γ–secreting cells was performed by staining IFN-γ+ cells with a CD3/CD56 mixture and gating on each population. As shown in Figure 4B, in MLR containing IL-18–conditioned NK cells, both T cells and NK cells secreted IFN-γ. In contrast in MLR performed in the presence of IL-2– or IL-12–conditioned NK cells, only T cells produced IFN-γ.
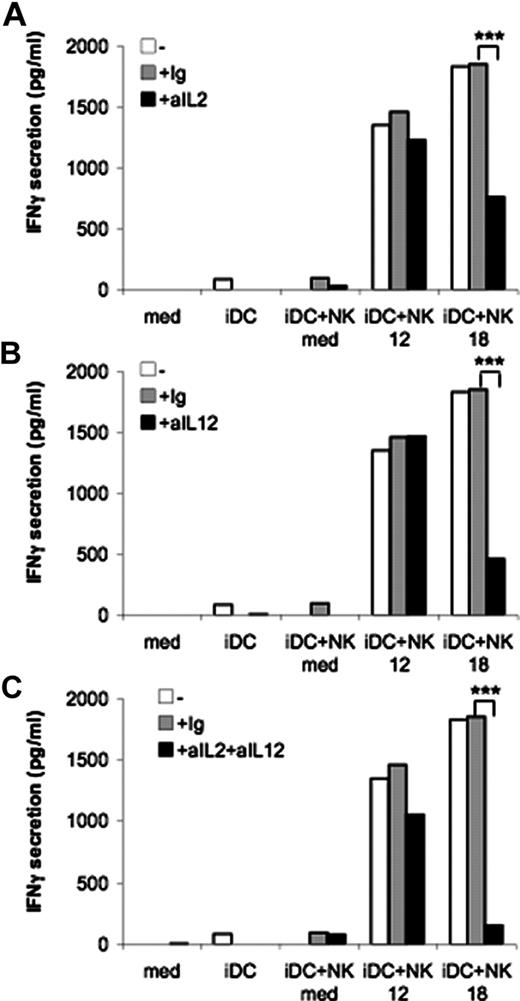
Thus, we asked whether IL-2 produced by T cells or IL-12 produced by DCs could represent the second signal needed for IL-18–conditioned NK cells to secrete IFN-γ. Even though the amounts of cytokines produced were very low, they were sufficient to serve as second signal for IL-18–conditioned NK cells (Figure 2). As shown in Figure 5A,B, neutralizing Ab against IL-2 or IL-12 in MLR performed in the presence of IL-18–conditioned NK cells partially reduced the secretion of IFN-γ, whereas the combined use of these mAbs virtually abrogated the production of this cytokine (Figure 5C). In contrast, the effect mediated by IL-12–conditioned NK cells was not significantly inhibited by the same Ab used alone or in combination.
The helper effect of IL-18–conditioned NK cells is dependent on IL-2 and IL-12. MLR were carried out with DC, cytokine-conditioned NK cells and allogeneic naive CD4+ T cells in the presence of a neutralizing Ab against IL-2 (A), IL-12 (B), or a combination of both (C). IFN-γ was measured in MLR supernatants by ELISA after 5 days. Data are from 1 representative experiment of 3. ***P < .001.
The helper effect of IL-18–conditioned NK cells is dependent on IL-2 and IL-12. MLR were carried out with DC, cytokine-conditioned NK cells and allogeneic naive CD4+ T cells in the presence of a neutralizing Ab against IL-2 (A), IL-12 (B), or a combination of both (C). IFN-γ was measured in MLR supernatants by ELISA after 5 days. Data are from 1 representative experiment of 3. ***P < .001.
Discussion
Exposure of NK cells either to cytokines released during innate immune responses, in inflamed peripheral tissues, or to T cell–derived cytokines in secondary lymphoid organs may deeply impact the outcome of naive CD4+ T-cell priming. In the present study we show that innate cytokines such as IL-12 and IL-18 (derived from APCs including MoDCs), released at early stages of an immune response, can differentially modulate NK-cell function. Indeed, such “cytokine-conditioned” NK cells promote 2 distinct pathways of T cell priming, both characterized by a sharp polarization toward Th1. In contrast, exposure of NK cells to IL-4 (released from other cells of innate immunity such as mastocytes and eosinophils) leads to nonpolarized T-cell responses. In addition, IL-2 produced by T cells within lymph nodes or after their recruitment at the sites of an inflammatory response may also favor Th1 responses by acting on NK cells. However, this function is strongly counterbalanced by IL-4. Thus, the influence of cytokine-conditioned NK cells on T-cell priming can take place in either peripheral tissues or secondary lymphoid organs, or in both compartments.
We show that pretreatment of NK cells with IL-12 resulted in up-regulation of IFN-γ release when they were cocultured with iDCs (Figure 2). In agreement with previous data, we show that such IL-12–conditioned NK cells acquire important effector functions such as the ability to mediate killing of iDCs (data not shown) and to induce substantial DC maturation as assessed by the expression of CD86 costimulatory molecules (Figure 1D).21 More importantly, DCs that had been cocultured with IL-12–treated NK cells acquired the ability to induce polarization of naive CD4+ T cells toward IFN-γ–producing Th1 cells (Figure 3). In line with this finding, we also found that these T cells were characterized by the up-regulation of T-bet expression (data not shown).
On the contrary, IL-18–treated NK cells did not release substantial IFN-γ in cocultures with iDCs nor induce their maturation (Figures 1 and 2). In agreement with these data, DCs cocultured with IL-18–conditioned NK cells could neither prime naive CD4+ T cells toward Th1 responses nor induce up-regulation of T-bet expression (Figure 3 and data not shown).
Based on these results, it is possible to conclude that exposure of NK cells to IL-12 (but not to IL-18) is important to induce mature DCs that are capable of driving polarized Th1 responses. However, as previously reported, NK cells may also participate directly in the process of T-cell priming.20,24,25 In mice, NK cells recruited from the periphery into lymph nodes were shown to represent a crucial source of IFN-γ necessary to induce Th1 polarization.26,27 In humans, tonsillar NK cells have been shown to produce IFN-γ in response to cytokines or after interaction with mature DCs. In turn, IFN-γ favors allogeneic CD4+ Th1 priming.28 We therefore analyzed whether human NK cells pretreated with cytokines, particularly IL-18, may directly influence the type of T-cell priming in the presence of DCs. Indeed, the presence of NK cells together with DCs and naive CD4+ T cells could greatly amplify T-cell polarization. In particular, we show that production of IFN-γ by naive CD4+ T cells was strongly enhanced when cultured with DCs that were previously exposed to IL-2– or IL-12–conditioned NK cells. Remarkably, although no Th1 polarization was detected when T cells were cultured with DC that were pre-exposed to IL-18–conditioned NK cells (Figure 3), direct addition of such NK cells to cocultures induced maximal Th1 polarization (Figure 4A). Thus IL-18–primed NK cells, after recruitment into lymph nodes, may directly influence the DC-induced polarization of naive T cells.
But how might NK cells be recruited to these sites? In mice, in addition to the CCR7-dependent migration, NK cells may be recruited to secondary lymphoid compartments by a process dependent primarily on CXCR3. Indeed, in CCR7-deficient animals, NK cells could migrate to lymph nodes as in normal animals.26 This is due to the ability of murine CXCR3 to bind to CCL21 produced by DCs in the T-cell areas of the lymph nodes. Nonetheless, the CCL21-CXCR3 interaction may not be applicable to the human system.29,30 Indeed, in humans, the ability of NK cells to migrate toward lymph nodes has always been associated with their expression of CCR7.31,32 Thus, lymph node human NK cells under steady-state conditions are characterized by the expression of this chemokine receptor.6,33-35 On the contrary, the remaining peripheral blood NK cells express chemokine receptors such as CXCR1, CX3CR1, and Chem23R that allow their migration toward inflamed peripheral tissues rather than lymph nodes.2,16,31,35,36 Because these NK cells recruited by chemokine gradients into inflamed peripheral tissues are CCR7−, they cannot migrate from these sites to lymph nodes along with mature DC. However, Maillard et al recently reported that peripheral NK cells exposed for a short time interval to exogenous IL-18 may express CCR7 de novo at their surface and acquire the ability to migrate in response to CCL19/CCL21.20 Thus, peripheral NK cells could recirculate from peripheral tissues to lymph nodes in a manner similar to mature DCs. It is important to underline that CCR7 expression was selectively detected in the presence of IL-18 but not with other cytokines, including IL-2, IL-4, IL-12, and IL-15. These concepts may provide some insight in the interpretation of our present data indicating that IL-18–treated NK cells do not participate in the mechanisms of DC editing or maturation, nor in promoting the capability of DCs to prime naive CD4+ T cells. Thus, NK cells exposed to IL-18 would first acquire the potential to release high amounts of IFN-γ and then, after the surface expression of CCR7, migrate to lymph nodes. There, in the presence of T cells and DCs, they may release abundant IFN-γ that has a major impact in Th1 polarization.
In agreement with these concepts, we also provide evidence that in cocultures containing naive T cells, DCs, and IL-18–conditioned NK cells, IFN-γ is produced not only by polarized T cells but also by NK cells (Figure 4). The helper effect seems to be independent of cell-to-cell interactions involving HLA class I/inhibitory receptors, since HLA class I–specific mAb did not up- or down-regulate this function. In addition, different mAbs against various activating NK receptors including NCR, NKG2D, and 2B4 had no effect on this helper function (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). As previously reported, IL-18–conditioned NK cells require a second signal to release their high IFN-γ content.11,20 This signal is provided either by CD40/CD40L interactions or by low amounts of IL-2 produced by T cells. In addition, IL-12 produced by DCs upon interaction with IL-18–conditioned NK cells and with T cells could provide an effective second signal. Small amounts of IL-2 or IL-12 would be sufficient to induce the release of IFN-γ by IL-18–conditioned NK cells after their migration into lymph nodes. As mentioned above, low levels of IL-2 are released by naive CD4+ T cells upon culture with DCs that had been pre-exposed to IL-18–conditioned NK cells (Figure 3). In addition, these DCs released small amounts of IL-12 (Figure 2). In this regard, neutralizing both cytokines resulted in virtual abrogation of the IFN-γ released in MLR supernatants (Figure 5C).
Notably, the amount of IFN-γ released by cytokine–conditioned NK cells cultured with naive T cells and DCs was high in the case of IL-18–conditioned NK cells, but low when NK cells were exposed to other cytokines. For example, although IL-12–conditioned NK cells can induce the release of IFN-γ by naive T cells in MLR, they do not secrete relevant amounts of this cytokine (Figure 4B). These data are in agreement with the finding that IL-12–conditioned NK cells do not acquire CCR7 (as the IL-18–conditioned ones) and consequently they do not migrate into lymph nodes. Thus a role of IL-12–conditioned NK cells could be to promote DC maturation and editing by acting up-stream the migration of DCs into lymph nodes. Accordingly, release of IFN-γ by these cells would be limited to a time interval preceding DC migration. This is in line with the concept that NK cells exposed to IL-12 display an “effector” phenotype (ie, produce cytokines in the presence of DCs, mediate cytolytic activity against tumors or iDCs) that is remarkably different from the “helper” phenotype characteristic of IL-18–conditioned NK cells.20,21
The functional property of NK cells exposed to IL-4 is remarkably different from that of IL-12– or IL-18–conditioned NK cells. Indeed, IL-4–conditioned NK cells display very limited “effector” functions both in terms of DC editing and maturation.21 In addition, our present study reveals that they do not display any detectable “helper” activity. Moreover, DCs cultured in the presence of IL-4–conditioned NK cells do not acquire the capability of polarizing T cells toward Th1, Th2, Treg, or Th17. Thus, in an vivo scenario in which IL-4 is released at early stages of an innate immune response (eg, by mast cells), the microenvironment would deviate NK cells from their functional role of inducing Th1 polarization.37-39
The mode of action of IL-2 on NK cells appears similar to that of IL-12. Indeed, both IL-2– and IL-12–conditioned NK cells are able to promote DC maturation (Figure 1) and DC editing. Moreover, upon exposure to IL-2–conditioned NK cells, DCs induce polarization of naive CD4+ T cells toward Th1. However, during the early phases of an innate inflammatory response IL-2 is not available in most instances. In mice, secretion of IL-2 by Ag-pulsed DCs has been reported to occur at the very beginning stages of innate responses.40,41 On the other hand, in humans, production of IL-2 by DCs is not detectable by conventional ELISA assays, but can be detected only by intracellular staining after differentiation in the presence of IL-15.42 At the present we cannot exclude that very small amounts of IL-2 released by Ag-pulsed DCs might act on NK cells and promote an efficient NK/DC interaction. This would eventually result in the selection of mature DCs capable of inducing Th1 polarization. Nevertheless, IL-2 appears to play a relevant role in the process of priming Th1 responses through the IL-18 pathway. This concept was substantiated by the inhibitory effect of anti–IL-2 Ab on the release of IFN-γ promoted by IL-18–conditioned NK cells during MLR (Figure 5A). This may suggest that small amounts of IL-2 released by naive CD4+ T cells upon interaction with DCs in the presence of IL-18–conditioned NK cells would be sufficient to induce the release of IFN-γ by such NK cells after their migration into lymph nodes.
Taken together, our data suggest that NK cells that have been recruited by chemokine gradients into inflamed peripheral tissues, upon exposure to different cytokines including IL-12, IL-18, IL-4, IFN-α, and IL-2 can exert different regulatory effects on the downstream adaptive immune responses via different routes. These cytokines are released by various cell types, including both resident and circulating cells recruited at inflammatory sites in response to chemokine gradients. Similar to DCs and NK cells, these cells, including mast cells, eosinophils, basophils, monocytes, and neutrophils, are equipped with receptors for pathogen-associated products and release cytokines upon engagement by their specific ligands.43-46 The prevalence of one or another cytokine within the inflammatory microenvironment will have a markedly different effect on the subsequent NK-cell interaction with DC in the periphery and/or with both DCs and naive T cells within lymph nodes. In this context various molecules were shown to differentially influence NK and/or DC functions. PGE2 was shown to counteract the effects of IL-18 or IL-15 on NK cells, whereas it is a key factor for MoDC maturation.20,47-49 Transforming growth factor-β was shown to down-regulate the ability of NK cells to kill iDCs by modulating the expression of the NKp30 receptor.50 Moreover, it inhibits Langerhans cell maturation induced by LPS or inflammatory cytokines, but not by CD40L.51 Finally, IL-10 by acting on MoDC maturation may induce suppressive T cells.52-55 By contrast, IL-10 has a stimulatory effect on human NK-cell cytotoxic activity.56 All these factors, in a manner similar to IL-4, may differentially contribute to the regulation of the Th1-polarizing pathways sustained by IL-12– and IL-18–conditioned NK cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro (AIRC; Milan, Italy), Istituto Superiore di Sanità (ISS; Rome, Italy), Ministero della Sanità, Ministero dell'Istruzione, dell'Università e della Ricerca Scientifica e Tecnologica (MIUR; Rome, Italy), Ministero della Salute-RF 2002/149 (Rome, Italy), FIRB-MIUR (Fondo per gli Investimenti della Ricerca di Base) progetto-codRBNE017B4 (Rome, Italy), and the European Union FP6, LSHB-CT-2004-503319-AlloStem. (The European Commission is not liable for any use that may be made of the information container.) S.A. is a recipient of a fellowship of the Istituto Giannina Gaslini (Genoa, Italy).
Authorship
Contribution: S.A. participated in designing and performing the research, in analyzing the data and in writing the article; E.M. and B.F. participated in performing the research; L.M. analyzed data and participated in writing the article; and A.M. participated in searching funds, designing the research, analyzing data, and writing the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandro Moretta, MD, Dipartimento di Medicina Sperimentale, Sezione di Istologia, Via G B Marsano, 10, 16132 Genoa, Italy; e-mail: alemoret@unige.it.