Abstract
Gene targeting experiments have shown that Delta-like 4 (Dll4) is a vascular-specific Notch ligand critical to normal vascular development. Recent studies have demonstrated that inhibition of Dll4/Notch signaling in tumor-bearing mice resulted in excessive, yet nonproductive tumor neovascularization and unexpectedly reduced tumor growth. Because nonfunctional blood vessels have the potential to normalize, we explored the alternative approach of stimulating Notch signaling in the tumor vasculature to inhibit tumor growth. Here we show that retrovirus-induced over-expression of Dll4 in tumor cells activates Notch signaling in cocultured endothelial cells and limits vascular endothelial growth factor (VEGF)–induced endothelial cell growth. Tumors produced in mice by injection of human and murine tumor cells transduced with Dll4 were significantly smaller, less vascularized and more hypoxic than controls, and displayed evidence of Notch activation. In addition, tumor blood perfusion was reduced as documented by vascular imaging. These results demonstrate that Notch activation in the tumor microenvironment reduces tumor neovascularization and blood perfusion, and suggest that Dll4-induced Notch activation may represent an effective therapeutic approach for the treatment of solid tumors.
Introduction
Gene targeting experiments have shown that Delta-like 4 (Dll4) is a Notch ligand critical to normal vascular development.1-3 Mice lacking Dll4 expression displayed severe remodeling defects affecting the primary vascular plexus and died early in gestation.1-4 Remarkably, mice with heterozygous deletions of the Dll4 gene also died at the embryo stage with similar remodeling defects of the vascular systems, providing evidence for the dosage sensitivity of Dll4 developmental functions.1-4 Prenatally, expression of Dll4 is mostly confined to the vascular endothelium, a selectivity that is unique among Notch ligands.4 Postnatally, expression of Dll4 is observed in angiogenic endothelium, particularly in tumor vessels but is generally not detected in normal tissue vessels.5-7 Hypoxia and VEGF promote vascular expression of Dll4,5,7,8 which explains Dll4 expression in tumor vessels.
The biologic activities of Dll4 are mediated by the activation of Notch1 and Notch4 signaling.9,10 Notch1 and Notch4 double-mutant mice resemble phenotypically Dll4-null mice in displaying severe vascular defects and death early in gestation.4 Expression of activated Notch4 in the vasculature caused abnormal vascular development in the mouse, pointing to a requirement for a critical level of Notch activity during normal embryogenesis.11 Activation of Notch receptors triggers γ-secretase proteolytic cleavage of Notch intracellular domain, which translocates to the nucleous as a complex with the transcription factor RBP-J and activates the target HES and HEY family of genes.9,10,12 The Dll4/Notch pathway negatively regulates VEGF-induced endothelial cell proliferation, migration and sprouting, attributable to reduced expression of VEGFR2, Neuropilin-1, and CXCR4, and to modulation of tip cell function.6,12-18 Conversely, blocking Dll4/Notch signaling renders endothelial cells hyperproliferative, and promotes VEGF-dependent sprouting and neovascularization.6,16,19-21
Unexpectedly, different approaches to blocking Dll4/Notch signaling by use of Dll4 neutralizing antibodies or soluble recombinant Dll4-Fc protein reduced tumor growth despite promoting a considerably more expanded and highly branched tumor vasculature.6,19-21 Lectin-based perfusion studies suggested that the tumor vasculature induced by Dll4 blockade was nonfunctional resulting in reduced tumor blood perfusion and promoting tumor hypoxia.6,19
The vascular defects induced by Dll4 blockade are currently undefined, and their potential for self-correction is unclear. Structurally, such defects resemble the vascular defects observed in tumor vessels, which cause poor blood perfusion, reduce the effectiveness of chemotherapy and may select for more malignant cells.22,23 Tumor vessels display considerable plasticity and potential for rapid structural and functional normalization.23-26 For example, reduction of VEGF levels has been shown to produce vessel pruning and reduction of vessel diameter and permeability leading to structural and functional normalization of the tumor vasculature.24,26,27 This plasticity of tumor vessels raises concerns as to the long-term safety of Dll4 blockade-based therapies, which promote an expansion of the tumor vasculature.
Because Dll4/Notch signaling can reduce VEGF-dependent endothelial cell responses14 and VEGF controls critical aspects of endothelial cell function,28,29 we explored the alternative approach of stimulating Dll4/Notch signaling in the tumor microenvironment to reduce tumor neovascularization and inhibit tumor growth. Here we show that retrovirus-induced over-expression of Dll4 in tumor cells activates Notch signaling in cocultured endothelial cells and limits VEGF-induced endothelial cell growth. Tumors produced in mice by injection of human and murine tumor cells transduced with Dll4 were significantly smaller than controls, tumor vascularity was reduced and tissue hypoxia was increased.
Methods
Cells and reagents
Human umbilical vein endothelial cells (HUVECs) were prepared and maintained as described14 ; cells were used up to passage 5. The human BL41 cell line (from Burkitt lymphoma) was a gift of Dr E. Kieff (Harvard University, Boston, MA); the murine (BALB/c) plasmacytoma MOPC315 cell line was a gift of Dr M. Potter (National Institutes of Health [NIH], Bethesda, MD); the murine (BALB/c) myelomonocytic WEHI3 cell line was a gift of Dr J. D. Griffin (Harvard University, Boston, MA). Purified rabbit antibody to human Dll4 was a gift of Dr X. Zhang (Cell Signaling Technology, Danvers, MA); rabbit monoclonal antibody to VEGFR2 (55B11), mouse monoclonal antibody to cleaved Notch4 (NICD4), and rabbit polyclonal antibody to cleaved Notch1 (NICD1) were from Cell Signaling Technology. Bevacizumab (Avastin; Genentech, South San Francisco, CA) was purchased from the NIH Pharmacy (Bethesda, MD).
Engineered retroviruses
Retroviruses were engineered to express enhanced green fluorescence (EGFP; vector) and EGFP plus full-length Dll4 (Dll4), as described14 and used to transduce human BL41 and mouse MOPC315 and WEHI3 cell lines. EGFP/Dll4-expressing cells were sort-purified to more than 90% purity using the FACSVantage SE cell sorter (BD Biosciences, San Jose, CA). Dll4 expression was evaluated by flow cytometry, reverse-transcriptase–polymerase chain reaction (RT-PCR) analysis with human Dll4-specific primers, and immunoblotting with specific human Dll4 antibodies.
In vitro assays
To evaluate the effects of Dll4 expressed by tumor cells on proliferation, HUVECs (4 × 103/well) were added to 0.2% gelatin-coated 96-well tissue culture plates (Corning, Corning NY) with or without VEGF165 (25 ng/mL; R&D Systems, Minneapolis, MN) in RPMI 1640 culture medium (Invitrogen, Carlsbad, CA) with 18% fetal bovine serum (FBS; Biofluids, Rockville, MD) and 18U/mL porcine heparin (Sigma-Aldrich, St Louis, MO). After HUVECs had attached (50% confluent), Dll4 or control tumor cells (2 × 103/well) were added in triplicate and incubation continued for 72 hours. After removing the nonadherent cells by gently washing with PBS, cell proliferation was measured by 3H-thymidine deoxyribose uptake (0.5 μCi/well; New England Nuclear, Boston, MA) during the last 18 to 22 hours of culture. To determine the effects of Dll4-Fc on HUVEC proliferation, 96-well plates were coated (50 μL/well) with Dll4-Fc (R&D Systems; 1 μg/mL PBS mL). Wells were washed with PBS before 4 × 103 HUVECs per well were seeded in triplicate. Cell proliferation was measured after 72 hours. To evaluate the effects of Dll4 expressed by tumor cells on Notch signaling, Dll4 or control cells (2 × 106 cells) were cocultured for 6 hours onto HUVEC monolayers (60%-70% confluence) established in 0.2% gelatin-coated 100 × 20 mm tissue culture plates (Falcon 353003; Becton Dickinson, Franklin Lakes, NJ) in M199 culture medium (Invitrogen) supplemented with 2% FBS (Biofluids). After removal of nonadherent cells, HUVEC cell lysates and RNAs were prepared.
Gene expression analysis
Total RNA from HUVECs or mouse tumor tissue was extracted using TRIzol (Invitrogen). Total RNA (1 μg) was reverse-transcribed using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). Human Dll4, HEY1, HEY2 and VEGFR2, and murine CD31, HEY1, HEY2, and VEGFR2 mRNAs were measured by real-time PCR using Assay-on-Demand Taqman Gene expression probes (Applied Biosystems). PCR reactions were performed with 1 μL cDNA, Taqman PCR Universal Master Mix (Applied Biosystems). PCR reaction conditions were those recommended by the manufacturer. Fluorescence signals were monitored after each PCR cycle with ABI Prism 7900 sequence detection system (Applied Biosystems). CT values (cycle number where fluorescence exceeded a fixed threshold) were obtained for each target probe and normalized with the corresponding CT values for the internal control (human or mouse GADPH). mRNA levels were expressed as relative units.
Tumor models
All mouse studies were approved by the NCI-Bethesda Animal Care and Use Committee. Human BL41, mouse MOPC315 and mouse WEHI3 tumor cells (10 × 106 in 0.2 mL PBS) were implanted subcutaneously in the left abdominal quadrant of 6- to10-week-old female nonobese diabetic severe combined immunodeficiency (NOD/SCID) or BALB/c mice. Tumors were harvested, weighed, and processed for histology and/or gene expression studies.
Assays for vascular perfusion and hypoxia
Mice were anesthetized with isofluorane. Texas Red–labeled lycopersicon esculentum lectin (150 μg in 150 mL of PBS; Vector Laboratories) was injected intravenously and allowed to circulate 20 minutes before the mice were killed. Tumors were removed and fixed by immersion in 4% paraformaldehyde (PFA) in PBS for 1 to 2 hours at 4°C followed by overnight incubation at 4°C in 15% sucrose in PBS, soaking in 30% sucrose in PBS for 48h at 4°C and embedding in OCT (Optimal Cutting Temperature) matrix (Sakura Finetek, Torrance, CA). Lectin bound to the endothelial cell surface (identified by CD31 immunostaining) was visualized in tissue section (20μm thickness). To measure hypoxia, HypoxyProbe-1 (Chemicon International, Temecula, CA; 60 mg kg−1) was injected intraperitoneally (150 μL) 1 hour before death. Tumors were removed, and tissue sections embedded in OCT were fixed 5 minutes in 4% PFA and immunostained with anti–HypoxyProbe-1 monoclonal antibody (Chemicon).
Imaging studies
Animal imaging was performed on a GE Signa Excite 3.0 T clinical scanner (GE Healthcare, Piscataway, NJ) using a mouse receiver coil of the modified Alderman-Grant resonator design (38 mm diameter × 70 mm length). A 30G catheter attached to a tygon tubing (0.25 mm ID × 15 cm length) was inserted under isofluorane (1.5%-5% in oxygen) anesthesia administered through a nose cone. The mouse was positioned on a holder equipped with a respiratory monitoring pad and fiber-optic temperature sensor to maintain constant physiologic conditions. The catheter line was then spliced to another line, which had been preloaded with the contrast agent, Magnevist (50 μL × 80 mM gadolinium) and then the bed assembly was positioned in the mouse coil and placed in the magnet. After acquiring a 3-plane survey scan, a multislice fat-suppressed Short Time Inversion Recovery Fast Spin Echo (STIR) in the sagittal orientation (TR = 5000 ms, TE ∼ 50 ms, TI = 175 ms, ST = 2 mm) was acquired to locate the tumor. This scan was used to plan a multislice coronal STIR image (ST = 1.5 mm) that included the tumor and the jugular vein to allow for the measurement of a contrast input function. Dynamic Contrast-Enhanced Magnetic Resonance Imaging (DCE-MRI) data were acquired in 2 scans: 3-dimensional T1-weighted Spoiled Gradient Echo (T1w SPGR) acquired before injection with a low flip angle (typical parameters: TR = 23 ms, TE = 2.3, FA = 6, ST = 2 mm, 256 × 128 × 12), and a dynamic scan with the same parameters using a larger flip angle (FA = 30, dynamic time = 30 s) acquired for 30 minutes where the contrast agent was injected at 150 μL/min after the third dynamic scan.
Tumor immunohistochemistry
Tumors dissected from the mice were fixed in 4% PFA and cryopreserved. Tissue sections were stained with purified rabbit antibody to human Dll4 (1:100; Cell Signaling Technology) followed by Alexa Fluor750 goat anti–rabbit IgG (1:500; Invitrogen); with rat anti–mouse CD31 monoclonal antibody (1:100; BD Pharmingen, San Diego, CA) followed by Alexa Fluor647 goat anti–rat IgG (1:500; Invitrogen); with rabbit anti–VEGFR2 monoclonal antibody (1:200; Cell Signaling Technology) followed by Alexa Fluor750 goat anti–rabbit IgG (1:500; Invitrogen); with mouse antiproliferating cell nuclear antigen (PCNA) monoclonal antibody (clone PC10, 1:200; Chemicon) followed by Alexa Fluor546 goat anti–mouse IgG (1:500; Invitrogen). Nuclei were visualized with DAPI (1:2000; Invitrogen).
Sections were imaged with an Axiovert 200 fluorescence microscope (Carl Zeiss, Thornwood, NY) using optimized excitation/emission filter sets (Omega Optical, Brattleboro, VT). Each labeling reaction was captured using light filtered through an appropriate filter set and the images digitized using the OpenLab imaging program (Improvision, Lexington, MA). An appropriate color table was applied to each image to either match its emission spectrum or to set a distinguishing color balance. The pseudocolored images were then converted into .tif files, exported to Adobe (San Jose, CA) Photoshop, and overlaid as individual layers to create multicolored merged composites.
VEGF concentration
Mouse VEGF was measured in mouse sera by a specific ELISA (R&D Systems, Minneapolis, MN).
Image quantification
Images from fluorescence staining were analyzed using NIH ImageJ software (http://rsb.info.nih.gov/ij/download.html). Pixel values for CD31 and DAPI fluorescence were separately obtained from individual tumor sections to compose the entire section. A ratio of CD31 pixel values/DAPI pixel values was calculated from each section to derive a relative mean CD31 pixel count. Relative mean CD31 pixel counts from tumor sections of the same group were averaged. Images from the DCE-MRI were analyzed with the 2-compartment general kinetic model30 using Interactive Data Language computer software (Research Systems, Boulder, CO).
Statistical analysis
Group differences were evaluated by 2-tailed Student t test. P values less than .05 were considered significant. Variables were correlated by 2-tailed Spearman rho correlation coefficients.
Results
Retroviral transduction of Dll4 in tumor cells activates Notch signaling and inhibits proliferation in endothelial cells
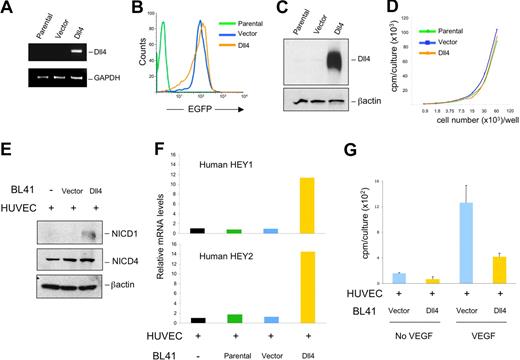
To investigate the activities of Dll4 in tumors, we used a retroviral vector encoding full-length EGFP-human Dll414 to induce Dll4 expression in human Burkitt lymphoma BL41 cells (Figure 1A-C). Dll4 mRNA could be amplified from Dll4-transduced BL41 tumor cells but not from the parental or vector-only transduced cells (Figure 1A). EGFP expression was detected in most (> 90%) BL41 cells transduced with Dll4 or vector-only but not from parental BL41 cells (Figure 1B). Dll4 protein was identified in cell lysates of Dll4-transduced BL41 cells but not from parental and control cells transduced with vector only (Figure 1C). Dll4-expressing BL41 cells did not have different growth characteristics in vitro from parental or vector-transduced BL41 (Figure 1D). We did not detect mRNAs and protein for the Dll4 receptors Notch1 and Notch4 in BL41 cells (parental, vector-, or Dll4-transduced) by RT-PCR with specific primers (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) and immunoblotting with specific antibodies to NICD1 (Notch1 intracellular domain) and NICD4 (Notch4 intracellular domain, Figure S1B).
Dll4 transduced in tumor cells activates Notch signaling and inhibits endothelial cell proliferation. Delta-like 4 (Dll4) transduction in BL41 cells induced increased expression of Dll4 mRNA (A). Dll4 and vector-only transduced BL41 express EGFP detected by flow cytometry (B). Dll4 transduction induced increased expression of Dll4 protein detected by immunoblotting in BL41 lymphoma cells (C), without affecting BL41 proliferation (D). Dll4-BL41 cells induced Notch activation (E), HEY1/HEY2 expression (F), and inhibited VEGF (25 ng/mL)-induced proliferation (G) in cocultured HUVECs. Bars represent the mean plus or minus SEM (n = 3).
Dll4 transduced in tumor cells activates Notch signaling and inhibits endothelial cell proliferation. Delta-like 4 (Dll4) transduction in BL41 cells induced increased expression of Dll4 mRNA (A). Dll4 and vector-only transduced BL41 express EGFP detected by flow cytometry (B). Dll4 transduction induced increased expression of Dll4 protein detected by immunoblotting in BL41 lymphoma cells (C), without affecting BL41 proliferation (D). Dll4-BL41 cells induced Notch activation (E), HEY1/HEY2 expression (F), and inhibited VEGF (25 ng/mL)-induced proliferation (G) in cocultured HUVECs. Bars represent the mean plus or minus SEM (n = 3).
To evaluate whether the transduced Dll4 was functional, we cocultured BL41 tumor cells growing in suspension with HUVECs growing as a semiconfluent monolayer, and tested for Notch activation after removal of the nonadherent cells. When Notch is activated, proteolytic cleavage by γ-secretase generates the NICD, which translocates to the nucleus and promotes the expression of target genes, including HEY1 and HEY2.9,10,12,31 We found that Dll4-BL41, but not control cells, induced Notch1 cleavage in HUVECs and modestly enhanced levels of NICD4 in HUVECs (Figure 1E). By quantitative real-time analyses of mRNAs, we detected increases in expression of the Notch target genes HEY1 and HEY2 in HUVECs cocultured with Dll4-expressing BL41 cells compared with controls (Figure 1F). Because expression of Notch1 and Notch4 is undetectable in BL41 cells (Figure S1A,B), these results indicate that Dll4-BL41 can promote Notch activation in endothelial cells. Previously, we showed that immobilized Dll4 significantly inhibits VEGF-induced endothelial cell growth.14 We now tested whether Dll4 overexpressed in tumor cells could also inhibit VEGF-induced endothelial cell proliferation. After incubation with Dll4-overexpressing tumor cells and subsequent removal of the nonadherent tumor cells, HUVEC proliferation to VEGF165 was reduced compared with control cocultures (Figure 1G). This reduction was similar in magnitude to that induced by immobilized Dll4-Fc (Figure S2A), and was associated with a reduction in VEGFR2 expression in HUVECs (Figure S2B). In addition, compared with control tumor cells, Dll4-tumor cells induced a reduction of VEGFR2 mRNA (Figure S2C) and protein (Figure S2D) expression in HUVECs. These results indicate that tumor cell–derived Dll4 is an effective Notch activator, and confirm that Notch signaling inhibits VEGF-induced growth in endothelial cells.
Dll4 overexpression in tumor cells inhibits tumor growth
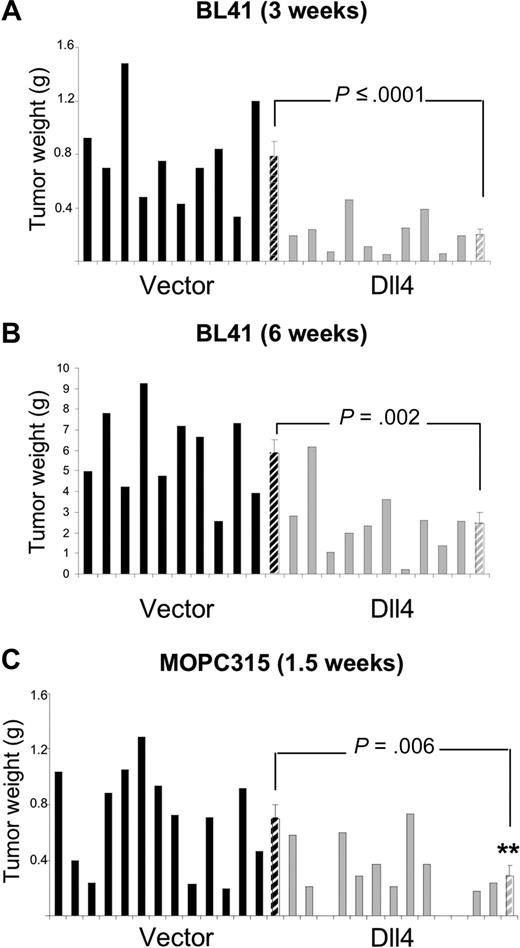
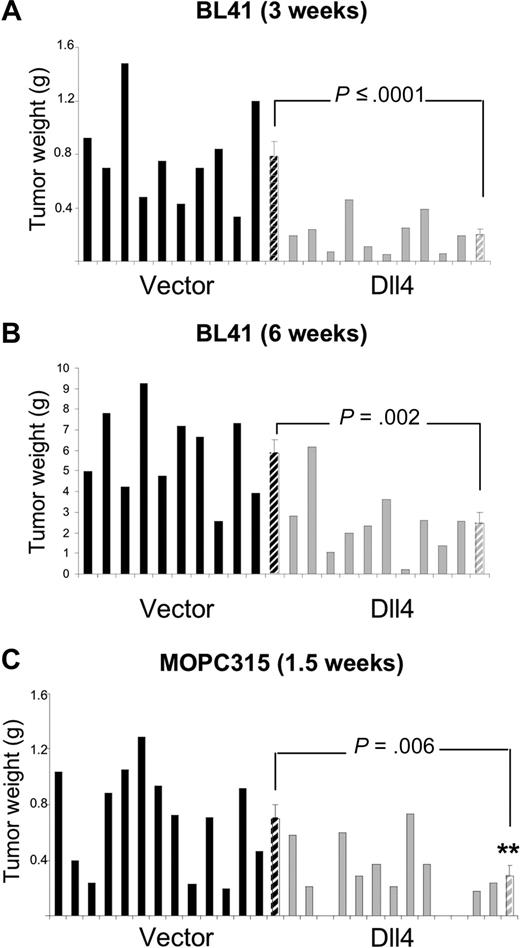
We used preclinical mouse models to test for the possibility that tumor-derived Dll4 might reduce tumor angiogenesis and tumor growth. In the BL41 xenograft tumor model using NOD/SCID mice, we inoculated subcutaneously equal numbers of control or Dll4-transduced human lymphoma cells, and evaluated the tumors after 3 weeks (Figure 2A). The mean tumor weight (measured after tumor excision) was 74.3% lower in the Dll4-tumor group than in the control group (Figure 2A); this is a significant difference (P ≤ .001). In a separate experiment that extended over 6 weeks, the tumor weight was 58% lower in the Dll4-group than in the control group (P = .002; Figure 2B), suggesting a persistent effect of Dll4 in vivo.
Dll4 overexpression in tumor cells inhibits tumor growth. Weight of Dll4 and control BL41 tumors removed 3 weeks (A) and 6 weeks (B) after subcutaneous injection of BL41 tumor cells in NOD/SCID mice. Weight of Dll4 and control MOPC315 tumors removed 12 days after subcutaneous injection of MOPC315 tumor cells in BALB/c mice (C). Solid bars represent individual tumor weights and striped bars represent group means plus or minus SEM (A,B: n = 10; C: n = 13).
Dll4 overexpression in tumor cells inhibits tumor growth. Weight of Dll4 and control BL41 tumors removed 3 weeks (A) and 6 weeks (B) after subcutaneous injection of BL41 tumor cells in NOD/SCID mice. Weight of Dll4 and control MOPC315 tumors removed 12 days after subcutaneous injection of MOPC315 tumor cells in BALB/c mice (C). Solid bars represent individual tumor weights and striped bars represent group means plus or minus SEM (A,B: n = 10; C: n = 13).
To confirm these effects of Dll4 in a different tumor model and test a highly vascularized tumor, we transduced the murine MOPC315 plasmacytoma cells with the retroviruses encoding full-length Dll4 or empty vector. By RT-PCR, we detected human Dll4 mRNA in the Dll4-transduced MOPC315 cells, but not in the parental or vector-only transduced cells (Figure S3A). By FACS analysis, EGFP-expressing cells were detected in both the Dll4- and vector-only transduced MOPC315 cells, but not in the untransduced parental cells (Figure S3B). By Western blotting, human Dll4 protein was detected in the cell lysates from Dll4-transduced MOPC315 cells but not in the vector-only transduced or parental cells (Figure S3C). Dll4 transduction minimally changed the growth characteristics of MOPC315 cells (Figure S3D). Dll4 transduced in the MOPC315 tumor cells was functional as judged by the ability of Dll4-MOPC315 cells but not control MOPC315 cells to activate Notch signaling in cocultured HUVECs (Figure S3E,F). By Western blotting, we detected increased levels of NICD1 protein in HUVECs cocultured with Dll4-MOPC315, but not control cells, indicative of Notch1 cleavage (Figure S3E). By real-time PCR, we detected increased levels of the Notch target genes HEY1 and HEY2 in HUVECs cocultured with Dll4-MOPC315, but not control cells (Figure S3F). In addition, Dll4-MOPC315 but not control MOPC315 reduced VEGF-induced proliferation in cocultured HUVECs (Figure S3G). Because MOPC315 cells, including the parental, vector-only, and Dll4-transduced cells, express similar levels of Notch1 mRNA and NICD1 protein (Figure S4), the increased NICD1 and HEY1/2 mRNA levels (detected with human-specific probes) provides evidence of specific activation in the cocultured HUVECs.
We used Dll4-MOPC315 in a syngeneic tumor model using BALB/c mice. We found that the weight of the subcutaneous tumors that developed 12 days after injection of Dll4-MOPC315 cells was 58.6% lower than that of controls (P = .006; Figure 2C). These results demonstrate that tumor overexpression of a functional Dll4 protein limits significantly tumor growth in immunodeficient and immunocompetent mice.
Dll4 overexpression in tumor cells reduces tumor vascularization and perfusion
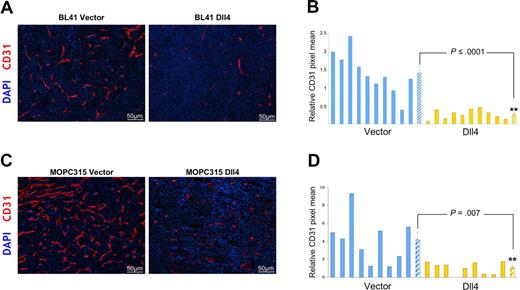
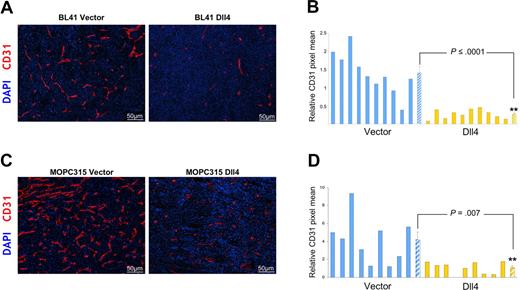
We evaluated vascular morphology and function in tumor tissues. We found that expression of Dll4 resulted in a significant reduction of endothelial cell density and vessel branching (evaluated by staining for mouse-specific CD31) without evidence of other morphologic change in the vasculature of BL41 and MOPC315 tumors (Figure 3A-D). Microscopic examination of the vasculature in the skin surrounding the tumors and in the spleen detected no appreciable morphologic differences in tissue sections from mice bearing control or Dll4 tumors (not shown).
Dll4 overexpression in tumor cells reduces tumor angiogenesis. Tumor vasculature identified by immunohistochemical staining of mouse CD31 in representative BL41 (A), MOPC315 (C) tumor sections, and quantification of CD31 staining in individual tumors and group means using NIH ImageJ software (B,D). Striped bars represent the mean plus or minus SEM (B: n = 10; D: n = 9).
Dll4 overexpression in tumor cells reduces tumor angiogenesis. Tumor vasculature identified by immunohistochemical staining of mouse CD31 in representative BL41 (A), MOPC315 (C) tumor sections, and quantification of CD31 staining in individual tumors and group means using NIH ImageJ software (B,D). Striped bars represent the mean plus or minus SEM (B: n = 10; D: n = 9).
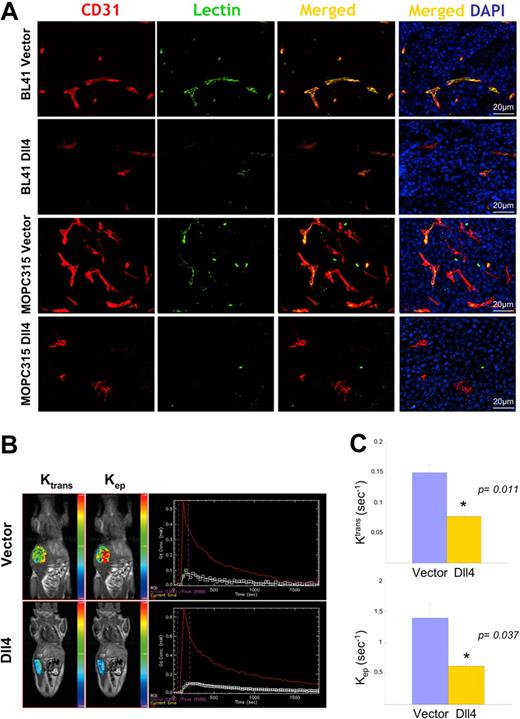
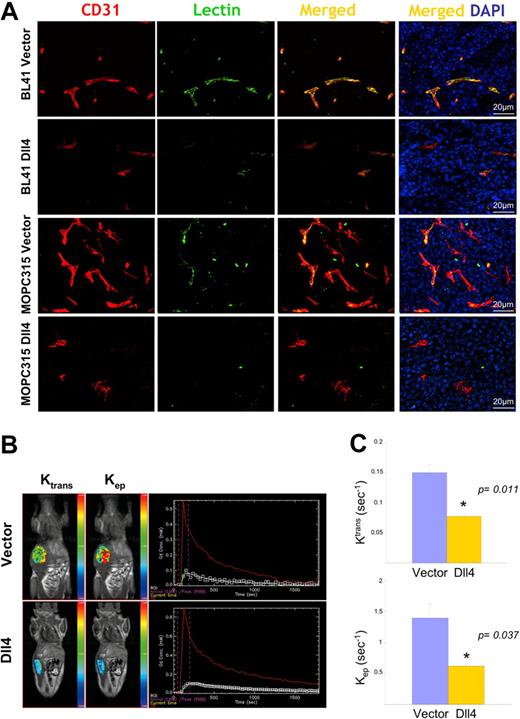
The reduction in vascular density would predict that blood perfusion would also be reduced in Dll4 tumors. Systemic perfusion with Texas Red–labeled tomato lectin revealed that Dll4 tumors displayed reduced lectin marking of the tumor vessels compared with controls (Figure 4A).
Reduced vascular perfusion in tumors overexpressing Dll4. CD31 immunostaining, lectin perfusion, and nuclear staining of DII4-overexpressing and control BL41 and MOPC315 tumors (A). Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in mice bearing Dll4 or control BL41 tumors: (B) Representative images showing tumor enhancement (blue = lowest, red = highest) and contrast kinetics (red line = arterial input; white line = kep, green line = Ktrans) of forward blood-plasma transfer to the tumor extravascular space (Ktrans) and reverse plasma transfer from the tumor extravascular space to the blood (kep); (C) Quantification of Ktrans and kep from DCE-MRI. Bars represent the mean plus or minus SEM (n = 3).
Reduced vascular perfusion in tumors overexpressing Dll4. CD31 immunostaining, lectin perfusion, and nuclear staining of DII4-overexpressing and control BL41 and MOPC315 tumors (A). Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in mice bearing Dll4 or control BL41 tumors: (B) Representative images showing tumor enhancement (blue = lowest, red = highest) and contrast kinetics (red line = arterial input; white line = kep, green line = Ktrans) of forward blood-plasma transfer to the tumor extravascular space (Ktrans) and reverse plasma transfer from the tumor extravascular space to the blood (kep); (C) Quantification of Ktrans and kep from DCE-MRI. Bars represent the mean plus or minus SEM (n = 3).
To assess blood perfusion in live tumor-bearing animals, we used a vascular MRI technique for evaluating the kinetics of gadolinium contrast enhancement within the tumor.32 A 2-compartment model30 was used to generate the kinetic parameter ktrans, a constant that measures forward volume transfer between blood plasma and the tumor extravascular-extracellular space, and kep, a rate constant that measures the reverse transfer from the tumor extravascular-extracellular space to blood plasma; both values were significantly reduced in Dll4 tumors compared with controls (Figure 4B,C), indicative of reduced blood flow.
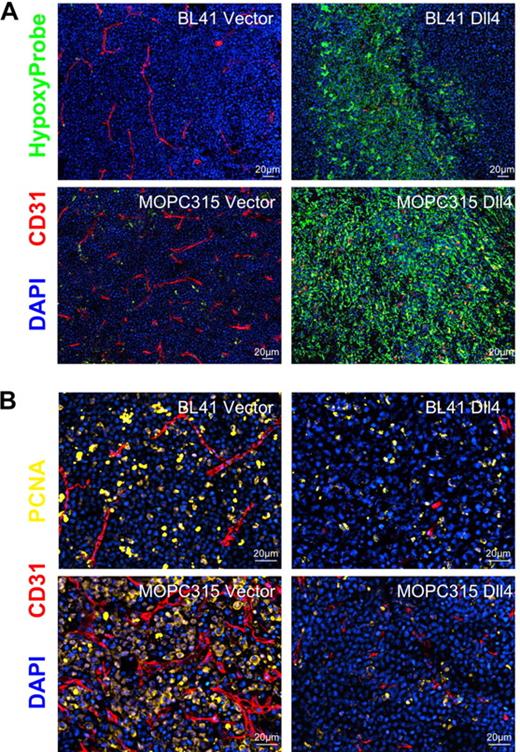
Consistent with blood perfusion being reduced, Dll4 tumor tissues displayed areas of intense hypoxia (marked by anti-HypoxyProbe antibodies) not seen in control tissues (Figure 5A), and a reduction in the proportion of dividing cells (marked by antibodies to the proliferating cell nuclear antigen, PCNA; Figure 5B). Together, these results support the conclusion that Dll4 expression in tumor cells reduces blood perfusion resulting in tumor-tissue hypoxia and reduced tumor growth.
Hypoxia and proliferative rate in Dll4 and control tumors. (A) Control BL41 and MOPC315 tumors display little hypoxia whereas Dll4-BL41 and Dll4-MOPC315 tumors display prominent hypoxia (HypoxyProbe, green). (B) PCNA immunostaining (yellow) shows reduced proliferation rates in Dll4-BL41 and Dll4-MOPC315 tumors compared with BL41 and MOPC315 controls. Tumor vessels (red, CD31 immunostaining) and nuclei (blue, DAPI) are also shown.
Hypoxia and proliferative rate in Dll4 and control tumors. (A) Control BL41 and MOPC315 tumors display little hypoxia whereas Dll4-BL41 and Dll4-MOPC315 tumors display prominent hypoxia (HypoxyProbe, green). (B) PCNA immunostaining (yellow) shows reduced proliferation rates in Dll4-BL41 and Dll4-MOPC315 tumors compared with BL41 and MOPC315 controls. Tumor vessels (red, CD31 immunostaining) and nuclei (blue, DAPI) are also shown.
Dll4 overexpression in tumor cells activates Notch signaling in the tumor microenvironment
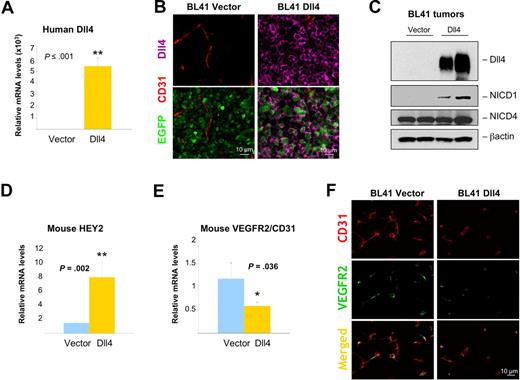
The reduced vascularity of Dll4 tumors compared with controls is consistent with Dll4 activating Notch in endothelial cells at the tumor site, and inhibiting tumor neovascularization. We tested whether this supposition was true. First, we documented persistent high-level human Dll4 expression in Dll4 tumors from the mice by quantitative messenger RNA analyses (Figure 6A; Figure S5A), immunohistochemistry (Figure 6B; Figure S5B) and immunoblotting (Figure 6C). Interestingly, we found that relative Dll4 mRNA levels inversely correlated with tumor weight (BL41 3-week experiment r = −0.737, P = .001; BL41 6-week experiment r = −0.739, P ≤ .001; MOPC315 experiment r = −0.643, P = .005). Analyses of Dll4 tumor tissues documented the increased activation of Notch1 (Figure 6C; Figure S5C), and the increased expression of the Notch target gene HEY2 in comparison to control tumor tissues (Figure 6D; Figure S5D). The relative mouse mRNA levels of HEY1 were similar in Dll4 and control tumors (not shown), possibly related to kinetic differences in induction of HEY1 compared with HEY2. Furthermore, the expected14 reductions of host VEGFR2 mRNAs (Figure 6E; real-time PCR results corrected for CD31 mRNA) and protein (Figure 6F, results from immunohistochemistry) were noted in Dll4-BL41 tumors and to a lower degree in MOPC315 tumors (not shown) in comparison to control tumors. Circulating levels of murine VEGF were similar in mice bearing Dll4-BL41 or vector-BL41 tumors (77.08 ± 6.85 pg/mL vs 76.33 ± 4.22 pg/mL, P > .05; n = 7). These results provide evidence that Dll4 overexpression in tumor cells leads to Notch signaling in the tumor microenvironment.
Notch activation and signaling in the tumor microenvironment of Dll4-overexpressing BL41 tumors. Persistent Dll4 overexpression in BL41 tumor tissues removed from the mice 6 weeks after injection: human Dll4 mRNA levels measured by real-time PCR (A) and human Dll4 protein (pink) detected by immunohistochemistry (B) and immunoblotting (C) with human-specific Dll4 antibodies; NICD1 and NICD4 were detected by immunoblotting (C). Mouse HEY2 (D) and VEGFR2 (E) mRNA levels measured by real-time PCR (mouse-specific probes) in tumor tissues of Dll4- and control-BL41 tumors. Mouse VEGFR2 mRNA levels are corrected for mouse CD31 mRNA levels. Bars represent the relative mean plus or minus SEM mRNA levels (n = 10 A,D,E). VEGFR2 (green) and CD31 (red) immunostaining in representative BL41 tumor sections (F).
Notch activation and signaling in the tumor microenvironment of Dll4-overexpressing BL41 tumors. Persistent Dll4 overexpression in BL41 tumor tissues removed from the mice 6 weeks after injection: human Dll4 mRNA levels measured by real-time PCR (A) and human Dll4 protein (pink) detected by immunohistochemistry (B) and immunoblotting (C) with human-specific Dll4 antibodies; NICD1 and NICD4 were detected by immunoblotting (C). Mouse HEY2 (D) and VEGFR2 (E) mRNA levels measured by real-time PCR (mouse-specific probes) in tumor tissues of Dll4- and control-BL41 tumors. Mouse VEGFR2 mRNA levels are corrected for mouse CD31 mRNA levels. Bars represent the relative mean plus or minus SEM mRNA levels (n = 10 A,D,E). VEGFR2 (green) and CD31 (red) immunostaining in representative BL41 tumor sections (F).
Relationship between antitumor effects of Dll4 overexpression and VEGF blockade
Because Dll4/Notch activation reduces VEGFR2 expression and VEGF-induced responses in endothelial cells, it was possible that the antitumor effects of Dll4/Notch activation would be similar to those derived from VEGF blockade, and that Dll4 overexpression would be ineffective in tumors resistant to VEGF blockade. We first examined whether the Dll4-sensitive BL41 tumors are responsive to anti-VEGF treatment. Of note, BL41 cells release VEGF in the culture medium (824 ± 18 pg/mL/106 cells, 24-hour incubation), which could contribute to tumor neovascularization. We injected subcutaneously NOD/SCID mice with vector-BL41 cells; the subcutaneous tumors that arose were randomized to treatment with antihuman VEGF antibodies (bevacizumab 5 mg/kg twice weekly) or no treatment. The weight of tumors removed after 4.5 weeks from the control mice (1.85 ± 0.26 g) and the anti–VEGF-treated mice (2.17 ± 0.341 g) was similar (P = .522; n = 6/group). These results indicate that BL41 tumors are resistant to anti-VEGF treatment, and provide evidence that the antitumor effects of Dll4 overexpression in BL41 tumors are not exclusively dependent upon a reduction of VEGF responses.
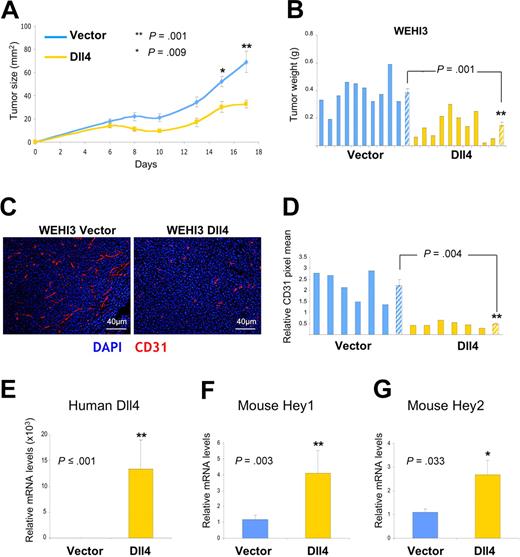
To test more directly whether Dll4 overexpression is effective in tumors that are resistant to anti-VEGF treatment, we used WEHI3 murine myelomonocytic tumors, which are known to be resistant to the antitumor effects of VEGF blockade.19 We retrovirally transduced WEHI3 with full-length Dll4 or empty vector. In a syngeneic BALB/c mouse model, we found that the growth rates (Figure 7A) and weight (Figure 7B) of the Dll4-WEHI3 subcutaneous tumors were significantly reduced compared with the vector-WEHI3 tumors. Immunohistochemical evaluation of the tumor tissues showed that Dll4 expression resulted in a significant reduction of endothelial cell density (Figure 7C,D). Quantitative mRNA analyses documented persistent human Dll4 expression (Figure 7E) in WEHI3 tumors, and showed higher levels of HEY1 and HEY2 mRNAs in Dll4-WEHI3 tumors compared with controls (Figure 7F,G). These results, together, indicate that the antitumor activities of Dll4/Notch activation are not simply attributable to a blockade of VEGF-induced responses.
Effects of Dll4 overexpression in tumors resistant to VEGF blockade. Growth curves (A) and weight (B) of Dll4 and control WEHI3 tumors; tumors were removed 17 days after subcutaneous injection of WEHI3 cells in BALB/c mice. Solid bars represent individual tumor weights and striped bars represent group means plus or minus SEM (n = 10). Vasculature identified by immunohistochemical staining of mouse CD31 in representative WEHI3 tumors (C), and quantification of CD31 staining in individual tumor sections and group means (D). Solid bars reflect individual tumor sections and striped bars represent the mean plus or minus SEM (n = 6). Human Dll4 (E), mouse HEY1 (F), and mouse HEY2 (G) mRNA levels measured by real-time PCR in WEHI3 tumor extracts. Bars represent the relative mean plus or minus SEM mRNA levels (n = 10, E-G).
Effects of Dll4 overexpression in tumors resistant to VEGF blockade. Growth curves (A) and weight (B) of Dll4 and control WEHI3 tumors; tumors were removed 17 days after subcutaneous injection of WEHI3 cells in BALB/c mice. Solid bars represent individual tumor weights and striped bars represent group means plus or minus SEM (n = 10). Vasculature identified by immunohistochemical staining of mouse CD31 in representative WEHI3 tumors (C), and quantification of CD31 staining in individual tumor sections and group means (D). Solid bars reflect individual tumor sections and striped bars represent the mean plus or minus SEM (n = 6). Human Dll4 (E), mouse HEY1 (F), and mouse HEY2 (G) mRNA levels measured by real-time PCR in WEHI3 tumor extracts. Bars represent the relative mean plus or minus SEM mRNA levels (n = 10, E-G).
Discussion
A humanized antibody blocking VEGF and a synthetic inhibitor of VEGF receptors tyrosine phosphorylation with activity against PDGF receptors and cKit are the only antiangiogenic agents approved for the treatment of cancer in conjunction with chemotherapy.33 Such VEGF-targeted therapies have shown benefit in some cancer types, but other tumors are resistant or acquire resistance to VEGF blockade.33,34 Benefits may be transient, as regrowth of tumor vessels rapidly follows suspension of therapy.23,26 Therefore, new approaches to reducing tumor blood perfusion are currently under investigation, including the blockade of Dll4/Notch.6,19 Here we show that activation of the Dll4/Notch pathway in tumor tissues reduces vascularity and responsiveness to VEGF, providing a novel approach to inhibition of tumor angiogenesis. It was not previously reported that Dll4/Notch activation is effective at reducing tumor angiogenesis and tumor growth in any system. Besides inhibiting expression of VEGFR2, the VEGF coreceptor NRP-1, and the chemokine receptor CXCR4,14 Dll4/Notch signaling regulates other genes important to endothelial cell function and angiogenesis,35 suggesting that the antiangiogenic activities of Dll4 are more complex than previously suspected and need further investigation. Consistent with this notion, the current experiments show that Dll4 overexpression produced significant tumor size reductions even in tumors that are resistant to VEGF blockade.
Previously, attempts at reducing tumor growth by overexpressing Dll4 in tumor cells of glioma (C6), glioblastoma (U87), prostate adenocarcinoma (PC3), breast carcinoma (MDA231), melanoma (B16), and fibrosarcoma (HT1080) lineages were unsuccessful.6,20,21 Consistent with these observations, we found no tumor size reduction by overexpressing Dll4 in the human fibrosarcoma HT1080 (results not shown). Several reasons might account for the absence of Dll4 antitumor efficacy against these tumors, including distinctive features of the tumor types that were investigated, particularly the relative tumor dependency on neovascularization, and potential differences in the nature of the pro-angiogenic factors sustaining tumor angiogenesis in various settings. Future studies will be necessary to identify the critical determinants of tumor responsiveness to Dll4/Notch activation. An important variable worth investigating is the level of Dll4 expression and Notch activation that are achieved. In the Dll4-unresponsive Dll4-HT1080 tumors, we did not detect evidence of Notch activation (not shown). In several other tumor types that were reported unresponsive to Dll4/Notch activation, little or no evidence of Notch activation was noted as well.20,21 A critical dosage sensitivity of Notch activation is suggested by mouse genetic studies showing the importance of Dll4 levels and Notch activation during vascular development.1-3,11 Tumor vessels express Dll4,5,6 but most tumors grow progressively and new tumor vessels are formed, providing evidence that such endogenous Dll4 expression is insufficient at halting tumor angiogenesis and tumor growth. Yet, when endothelial cells are transduced with Dll4, they grow poorly in the presence of VEGF,14 and when tumor cells are transduced with Dll4 -as we show here- tumor neovascularization and tumor growth are restrained.
As expected from many observations in vitro, different approaches to blocking Dll4/Notch interactions resulted in increased VEGF-dependent tumor neovascularization in vivo.6,19 Paradoxically, however, this expanded tumor vasculature was not functional and resulted in tumor hypoxia and reduced tumor growth.6,19 Thus, the 2 opposite approaches of stimulating Notch signaling (as reported here) and blocking Notch signaling (as reported by others6,19 ) have shown effectiveness in distinct tumor models: lymphoma, plasmacytoma, and myelomonocytic tumors responded to Dll4/Notch activation whereas carcinomas, gliomas, and melanomas responded to Dll4/Notch blockade. It will be interesting to further investigate whether the opposite approaches of stimulating or inhibiting Notch activation are best suited for the treatment of distinct tumor types. It would not be surprising, however, that both approaches may be effective in the same tumor type based on the observation that both overexpression14 and silencing7 Dll4 impaired endothelial cell responses to VEGF.
An important question is how to develop Dll4 into a cancer therapeutic. Gene delivery systems are under intense investigation, and could be used to express full-length Dll4 at the tumor site. Dll4 could be engineered (as a complex or particle) for delivery of Notch-activating signals in vivo. A soluble jagged1 (another Notch ligand) peptide with Notch agonistic activity was effective at activating Notch signaling,36 and when given systemically to mice reduced tip cell function in retinal endothelial cells.16 Repeated injections of the soluble jagged1 peptide showed continued efficacy at suppressing the tip-cell phenotype in mouse retinas, and no unwanted side effects were reported.16 There is, however, a concern about the potential for side effects derived from generalized activation of Notch1/4 given the multitude of effects derived from Notch signaling, and further studies will be needed. Nonetheless, our results suggest that activation of Notch in the tumor microenvironment might be a novel and very effective means of inhibiting tumor angiogenesis and tumor growth.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Adrian Harris, Steve Jay, Drs Douglas Lowy, Mark Williams, Karen J. Wong, Paola Gasperini, Ombretta Salvucci, and Maria C. Cid for their help on various aspects of this work.
This research was supported by the Intramural Research Program at NIH, NCI, Center for Cancer Research. M.S. is the recipient of a Ministerio de Educación y Ciencia (MEC)-Fulbright Fellowship. M.B. is supported by NCI contract N01-CO-12400.
National Institutes of Health
Authorship
Contribution: M.S. and G.T. designed and performed research, analyzed the results, and wrote the paper; C.K.W., M.D.L.L.S., and P.J.M. performed research; D.M. performed microscopic analyses; and M.B., C.R., and P.C. performed imaging studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marta Segarra, 37 Center Drive, Building 37, Room 4124, NCI, NIH, Bethesda, MD 20892; e-mail: segarram@mail.nih.gov.