Abstract
Th17 is a newly identified T-cell lineage that secretes proinflammatory cytokine IL-17. Th17 cells have been shown to play a critical role in mediating autoimmune diseases such as EAE, colitis, and arthritis, but their role in the pathogenesis of graft-versus-host disease (GVHD) is still unknown. Here we showed that, in an acute GVHD model of C57BL/6 (H-2b) donor to BALB/c (H-2d) recipient, IL-17−/− donor T cells manifested an augmented Th1 differentiation and IFN-γ production and induced exacerbated acute GVHD. Severe tissue damage mediated by IL-17−/− donor T cells was associated with increased Th1 infiltration, up-regulation of chemokine receptors by donor T cells, and enhanced tissue expression of inflammatory chemokines. Administration of recombinant IL-17 and neutralizing IFN-γ in the recipients given IL-17−/− donor cells ameliorated the acute GVHD. Furthermore, the regulation of Th1 differentiation by IL-17 or Th17 may be through its influence on host DCs. Our results indicate that donor Th17 cells can down-regulate Th1 differentiation and ameliorate acute GVHD in allogeneic recipients, and that treatments neutralizing proinflammatory cytokine IL-17 may augment acute GVHD as well as other inflammatory autoimmune diseases.
Introduction
Acute graft-versus-host disease (GVHD), the leading cause of morbidity and mortality of allogeneic hematopoietic cell transplantation (HCT), is a complex process involving dysregulation of inflammatory cytokine cascades and distorted donor cellular response against host alloantigens.1 Activation of alloreactive donor T cells is initiated by host antigen-presenting cells (APCs), especially dendritic cells (DCs).2-5 Much effort has been devoted to understand how the polarization of donor T cells to the Th1 or Th2 phenotype contributes to acute GVHD. In some experimental models, it has been shown that Th1 cells augment and Th2 cells ameliorate acute GVHD.1,6-8 However, it was also reported that the absence of Th1 cytokine interferon (IFN)-γ augments acute GVHD, but loss of the Th2 cytokine interleukin (IL)–4 reduces acute GVHD.9,10 Furthermore, donor T cells deficient in either Th1 or Th2 differentiation were shown to be able to mediate acute GVHD.11 Therefore, the role of donor T-cell subsets in GVHD pathogenesis is still controversial. It is likely that T-cell subsets other than Th1 or Th2 play a role in mediating acute GVHD.
Th17 is a newly identified T-cell lineage that secretes the proinflammatory cytokine IL-17.12 Naive CD4+ T cells differentiate into Th17 cells in the presence of IL-6 and transforming growth factor (TGF)–β.13-15 Th17 cells express IL-23 receptor, and IL-23, an IL-12 family member, is critical for their survival and proliferation.16-18 Orphan nuclear receptor RORγt is the key transcription factor that orchestrates differentiation of the Th17 lineage.19 Interestingly, it has been shown that naive CD8+ T cells can also differentiate into IL-17–producing T cells in the same culture condition as CD4+ T cells.20
One of the important functions of IL-17 is to coordinate local tissue inflammation through the up-regulation of proinflammatory cytokines and chemokines.21 Thus, IL-17 has been implicated in a critical role in the host defense against a series of extracellular pathogens, such as Klebsiella pneumoniae and Candida albicans.22,23 However, uncontrolled Th17 cells have been reported to be involved in autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, and experimental autoimmune encephalomyelitis (EAE).24-27 In contrast, IL-17 has also been shown to have some regulatory effect in mediating tissue inflammation. It was reported that IL-17 inhibited CD4+ T-cell activation,28 suppressed the tissue expression of chemokines (eg, CCL5 and CCL27),29,30 and down-regulated VCAM-1 expression on epithelial cells.31 Moreover, neutralization of IL-17 was reported to aggravate dextran sulfate sodium–induced colitis in mice,32 and absence of IL-17 augmented allergic asthma.33
The role of Th17 cells in GVHD pathogenesis is still unknown. In the current study, we demonstrated that IL-17−/− donor T cells showed an augmented Th1 differentiation and IFN-γ production and induced exacerbated acute GVHD. Our data also suggest that the IL-17 regulation of donor T-cell differentiation may be through its influence on host DCs. Our results indicate that donor Th17 cells can down-regulate donor Th1 differentiation and ameliorate acute GVHD, and that neutralizing IL-17 may augment acute GVHD.
Methods
Mice
IL-17−/− mice in C57BL/6 background were kindly provided by Dr Yoichiro Iwakura (Tokyo University, Japan). C57BL/6 (H-2b) and BALB/C (H-2d) mice at 6 to 8 weeks of age were purchased from National Cancer Institute Laboratories (Frederick, MD) and maintained in a pathogen-free room at City of Hope Research Animal Facilities (Duarte, CA). Mice at age 8 to 12 weeks were used in the current studies. Institutional Review Borad (IRB) approval was obtained from the research animal care committee of the Beckman Research Institute of City of Hope for these studies.
Induction and assessment of GVHD
HCT procedures for induction of acute GVHD were described previously.34,35 Briefly, BALB/c mice were given sublethally 800 rads total body irradiation (TBI) from a 137Cs souse on day −1, and C57BL/6 donor splenocytes with T cell–depleted bone marrow (TCD-BM) cells were injected intravenously on day 0. The experimental and control groups were always set up at the same time and experiments were always replicated 2 to 3 times. The recipients were monitored daily for survival and every 5 days for body weight changes and clinical signs of GVHD.
Cell preparation
Spleen T cells were purified by depletion of non–T cells with biotin labeled mAbs including anti-B220, Gr-1, Mac-1, CD11c, and Ter119, using a magnetic purification system from Miltenyi Biotec (Auburn, CA). The purity of CD3+ T cells was more than 95%. TCD-BM cells were collected by depleting CD4+, CD8+, and TCRβ+ cells using the magnetic purification system.
Monoclonal antibodies and flow cytometric analysis
The fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, allophycocyanin-, biotin-, cyanin 7 (Cy7)-, or allophycocyanin-CY7–conjugated mAbs to mouse CD4, CD8, TCRβ, Mac-1, B220, Gr-1, H-2b, CD11c, IL-17, and IFN-γ were all purchased from BD Pharmingen (San Diego, CA). Anti-CCR5 was purchased from Biolegend (San Diego, CA). Intracellular cytokine staining was performed following the vendor's instruction (Intracellular cytokine staining kits; BD Biosciences, San Jose, CA), as previously described.38 Briefly, cells were stimulated with 1 μg/mL inomycin plus 0.1 μg/mL PMA in the presence of Golgi-Stop for 5 hours. Cells were harvested, washed, and stained with anti-CD4 or CD8 in the presence of FcR-Block, anti-CD16/32. After wash, cells were then fixed using CytoFix/CytoPerm buffer and stained with antibodies against intracellular cytokines or isotype control on ice for 30 minutes. Multiple-color fluorescence-activated cell sorter (FACS) analyses were performed at City of Hope FACS facility using a 3-laser CyAn immunocytometry system (Dako Cytomation, Fort Collins, CO), and data were analyzed using FlowJo software (TreeStar, San Carlos, CA), as previously described.35,38,39 In some experiments, dead cells were excluded by fixable aqua dead cell stain kit (Invitrogen, Carlsbad, CA), as indicated in figure legends.
Administration of IL-17 and in vivo neutralization of IFN-γ
Recipients were injected with mouse recombinant IL-17 at a dose of 0.3 μg/mouse on days 0, 3, and 7 after HCT. Anti–IFN-γ mAbs (R4-6A2) were purified from the culture supernatant using protein G columns as described in our previous publication.35 Control rat IgG was obtained from Jackson ImmunoResearch Laboratory (West Grove, PA). Recipients were injected with anti–IFN-γ or rat IgG (0.5 mg/mouse) on days 0, 5, 10, and 15 after HCT.
Quantification of chemokine expression by real-time RT-PCR
Isolation of total liver RNA and synthesis of first-strand cDNA were described previously.40 mRNA was quantified by real-time quantitative polymerase chain reaction (PCR) using 7300 Fast Real-Time PCR System (Applied Biosystems, Forest City, CA). The following specific forward (F) and reverse (R) primers were used: mCCL3F, AAGGATACAAGCAGCAGCGAGTA; mCCL3R, TGCAGAGTGTCATGGTACAGAGAA; mCCL4F, TGCAGAGTGTCATGGTACAGAGAA; mCCL4R, CAACTCCAAGTCACTCATGTACTCAG; mCCL5F, CATATGGCTCGGACACCACT; mCCL5R, ACACACTTGGCGGTTCCTTC; mGAPDHF, TCACCACCATGGAGAAGGC; mGAPHDR, GCTAAGCAGTTGGTGGTGCA. Relative expression levels of genes were normalized within each sample to the house keeping gene GAPDH and were presented relative to the expression in syngeneic transplantation recipients.
Cell culture and measurement of cytokines in serum and culture supernatants
Sorted donor C57BL/6 CD4+ T cells (2 × 105) were cultured with CD11c+ DCs (105) from the spleen of BABL/c mice, which had been irradiated with 800 rad 2 hours before, in a U-bottom 96-well plate for 4 days. CD4+ T cells were purified by magnetic sorting system as described above. Cytokines (ie, IFN-γ, tumor necrosis factor [TNF]-α) in serum and culture supernatants were measured using enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems (Minneapolis, MN).
Histopathology and scoring
Liver, gut, and lung specimens of recipients were fixed in formalin before embedding in paraffin blocks. Tissue sections were stained with H&E as previously described.35,39 Assessment of tissue damage was peformed based on scoring systems previously described.36,41 In brief, liver GVHD was scored on the number of involved tracts and severity of liver cell necrosis; the maximum score is 10. Gut GVHD was scored on the basis of crypt apoptosis and lamina propria inflammation; the maximum score is 8. Lung GVHD was scored on the periluminal infiltrates, pneumonitis, and the severity of lung tissues involved; the maximum score is 9. Means plus or minus standard error of the scores of 6 recipients are shown for each group.
Statistical analysis
Body weight and survival in different groups were compared using the log-rank test with program GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA). Comparison of 2 means was analyzed using unpaired 2-tailed Student t test.
Results
IL-17−/− donor T cells induced more severe acute GVHD than wild-type donor T cells
Because Th17 cells mediate autoimmune diseases, we studied the role of IL-17 in the pathogenesis of acute GVHD, using an MHC-mismatched HCT model of C57BL/6 (H-2b) donor to BALB/c (H-2d) recipient. IL-17−/− (referred to IL-17A−/−)12 mice have been used to study the role of IL-17 in various diseases models such as contact hypersensitivity response, experimental autoimmune encephalomyelitis, and airway hypersensitivity response.26,42 In addition, we observed that, compared with wild-type (WT) C57BL/6 mice, IL-17−/− C57BL/6 showed no significant difference in total spleen mononuclear cells, percentage of T cells, ratio of CD4+ T versus CD8+ T, percentage of CD62LhiCD44lonaive CD4+ or CD8+ T cells, or percentage of Foxp3+CD25hiCD4+ regulatory T cells as well as their suppressor function (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Therefore, graded numbers (1.25-2.5 × 106) of spleen cells and TCD-BM cells (2.5 × 106) from IL-17−/− or WT control C57BL/6 donors were injected into sublethally irradiated BALB/c recipients. The spleen and the TCD-BM cells were from same donors. GVHD was assessed by clinical signs of GVHD such as weight loss, diarrhea, and death, as well as histopathology of GVHD target tissues including liver, colon, and lung.
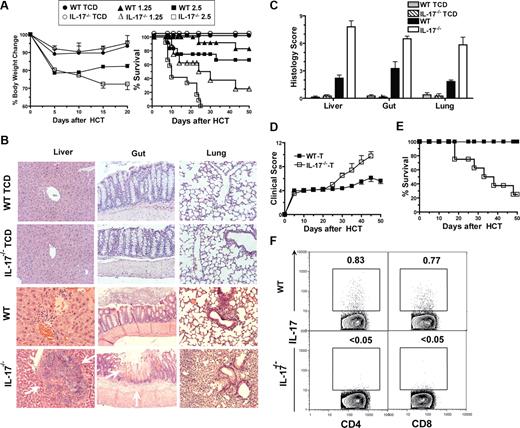
Surprisingly, we observed that the recipients given 1.25 or 2.5 × 106 IL-17−/− donor spleen cells showed more severe diarrhea and weight loss compared with the recipients given the same number of WT donor cells (P < .01; Figure 1A). Whereas 75% or 100% of the recipients given 1.25 or 2.5 × 106 IL-17−/− donor spleen cells died before day 30, more than 70% of the recipients given the same number of WT donor spleen cells survived for more than 50 days (P < .01; Figure 1). The recipients given TCD-BM cells alone from IL-17−/− or WT donors all survived for more than 50 days without any signs of GVHD (Figure 1A).
IL-17−/− donor cells induced exacerbated acute GVHD. (A) Sublethally irradiated BALB/c recipients were transplanted with graded numbers (1.25∼2.5 × 106) spleen cells and 2.5 × 106 TCD-BM cells from wild-type (WT) or IL-17−/− C57BL/6 donors. The recipients were checked for clinical signs of GVHD and body weight every 5 days and monitored for survival daily. Body weight change and survival curves are shown. There were 12 mice in each group, and 3 replicated experiments were combined, except the group given TCD-BM alone had 4 recipients. (B,C) Histopathology of liver, gut, and lung of recipients given TCD-BM alone or TCD-BM and spleen cells (2.5 × 106) from WT or IL-17−/− donor 13 days after HCT. One representative histopathology and mean (± SE) of histologic score of 6 examined recipients in each group are shown. (D,E) Sublethally irradiated BALB/c recipients were transplanted with sorted T cells (0.5 × 106) from WT or IL-17−/− donors. Clinical score and survival curves are shown. There were 8 recipients in each group; 2 replicated experiments were combined. (F) Liver mononuclear cells of the recipients were stained for H-2b (donor marker), CD4, CD8, and intracellular IL-17 on day 10 after HCT. Gated H-2b+ cells are shown in IL-17 versus CD4 or CD8. The percentage of IL-17 CD4+ or CD8+ T cells is shown beside the gating boxes. Mean (± SE) of the IL-17+ CD4+ or CD8+ T cells in the liver of recipients given WT donor T cells are 0.69 (± 0.02) or 0.77 (± 0.08). IL-17+ T cells in the spleen of the recipients given IL-17−/− donor T cells were not detectable (< 0.05%).
IL-17−/− donor cells induced exacerbated acute GVHD. (A) Sublethally irradiated BALB/c recipients were transplanted with graded numbers (1.25∼2.5 × 106) spleen cells and 2.5 × 106 TCD-BM cells from wild-type (WT) or IL-17−/− C57BL/6 donors. The recipients were checked for clinical signs of GVHD and body weight every 5 days and monitored for survival daily. Body weight change and survival curves are shown. There were 12 mice in each group, and 3 replicated experiments were combined, except the group given TCD-BM alone had 4 recipients. (B,C) Histopathology of liver, gut, and lung of recipients given TCD-BM alone or TCD-BM and spleen cells (2.5 × 106) from WT or IL-17−/− donor 13 days after HCT. One representative histopathology and mean (± SE) of histologic score of 6 examined recipients in each group are shown. (D,E) Sublethally irradiated BALB/c recipients were transplanted with sorted T cells (0.5 × 106) from WT or IL-17−/− donors. Clinical score and survival curves are shown. There were 8 recipients in each group; 2 replicated experiments were combined. (F) Liver mononuclear cells of the recipients were stained for H-2b (donor marker), CD4, CD8, and intracellular IL-17 on day 10 after HCT. Gated H-2b+ cells are shown in IL-17 versus CD4 or CD8. The percentage of IL-17 CD4+ or CD8+ T cells is shown beside the gating boxes. Mean (± SE) of the IL-17+ CD4+ or CD8+ T cells in the liver of recipients given WT donor T cells are 0.69 (± 0.02) or 0.77 (± 0.08). IL-17+ T cells in the spleen of the recipients given IL-17−/− donor T cells were not detectable (< 0.05%).
Additionally, we compared the histopathology of GVHD target tissues 13 days after HCT. We observed much more severe tissue damage and higher histopathology scores in the liver, colon, and lung tissues of the recipients given 2.5 × 106 IL-17−/− donor spleen cells compared with recipients given 2.5 × 106 WT donor spleen cells (P < .05; Figure 1B,C). In addition, areas of necrosis in liver tissues and inflammatory ulcers in colon tissues were with the former recipients. The more severe tissue damage was correlated with more severe clinical GVHD and higher mortality in recipients given IL-17−/− donor cells (Figure 1A-C). These results indicate that IL-17−/− donor cells induce exacerbated acute GVHD.
To address whether IL-17 from donor T cells plays an important role in regulating acute GVHD, purified T cells including CD4+ and CD8+ T cells (0.5 × 106) from IL-17−/− or WT donors were coinjected with WT TCD-BM cells into BALB/c recipients. The purity of T cells (∼96%) and the ratio of CD4+ T/CD8+ T (∼1.6) was not significantly different between WT and IL-17−/− donors (P > .5; Figure S1B). We found that recipients given T cells from IL-17−/− donors showed a much higher clinical score of acute GVHD by 20 days after HCT than the recipients given WT donor T cells (P < .01; Figure 1D). Whereas 80% of the former recipients died of acute GVHD within 50 days after HCT, all of the latter recipients survived for more than 50 days (P < .01; Figure 1E). Furthermore, we observed that a small percentage (0.5%-1%) of the donor CD4+ and CD8+ T cells in the recipients given WT T cells were IL-17–secreting T cells, but IL-17–secreting T cells were not detectable in the recipients given IL-17−/− T cells (Figure 1F). Taken together, our results indicate that IL-17 deficiency in donor T cells leads to exacerbated acute GVHD, and that IL-17 from donor T cells may play a negative feedback role in donor T cell–mediated acute GVHD.
IL-17−/− donor T cells showed enhanced differentiation into Th1 cells in GVHD recipients
Next, we explored how IL-17−/− donor spleen cells exacerbated acute GVHD. Because inflammatory cytokines (eg, IFN-γ and TNF-α) were shown to play a critical role in mediating acute GVHD,1,43 we compared serum levels of IFN-γ and TNF-α in the recipients. Five days after HCT, we found that the serum levels of both IFN-γ and TNF-α were significantly elevated in the recipients given IL-17−/− donor cells compared with recipients given WT donor cells (P < .05; Figure 2A,B). Furthermore, we observed that the percentage of donor IFN-γ–secreting CD4+ and CD8+ T cells increased more than 10-fold in mesenteric lymph nodes (MLN) and increased approximately 2-fold in spleen of recipients given IL-17−/− donor cells, as compared with that of recipients given WT donor cells (P < .01; Figure 2C-E). These results indicate that the accelerated acute GVHD mediated by IL-17−/− donor T cells is associated with enhanced Th1 differentiation.
Augmented Th1 differentiation in recipients given IL-17−/− donor cells. (A,B) Serum levels of IFN-γ and TNF-α of recipients given WT or IL-17−/− donor spleen cells 5 days after HCT. (C) Mesenteric lymph nodes (MLN) and spleen cells of the recipients given WT or IL-17−/− donor cells were stained for H-2b, CD4, CD8, and intracellular IFN-γ, 13 days after HCT. Gated H-2b+ cells are shown in IFN-γ versus CD4 or CD8. The percentage of IFN-γ+ cells is shown beside the gating boxes. One representative of 4 recipients in each group is shown. (D,E) Means (± SE) of the percentages of IFN-γ+ CD4+ or CD8+ donor T cells in MLN and spleen.
Augmented Th1 differentiation in recipients given IL-17−/− donor cells. (A,B) Serum levels of IFN-γ and TNF-α of recipients given WT or IL-17−/− donor spleen cells 5 days after HCT. (C) Mesenteric lymph nodes (MLN) and spleen cells of the recipients given WT or IL-17−/− donor cells were stained for H-2b, CD4, CD8, and intracellular IFN-γ, 13 days after HCT. Gated H-2b+ cells are shown in IFN-γ versus CD4 or CD8. The percentage of IFN-γ+ cells is shown beside the gating boxes. One representative of 4 recipients in each group is shown. (D,E) Means (± SE) of the percentages of IFN-γ+ CD4+ or CD8+ donor T cells in MLN and spleen.
Severe GVHD target tissue damage mediated by IL-17−/− donor cells was associated with increased Th1-cell infiltration, up-regulation of chemokine receptors by donor T cells, and enhanced tissue expression of inflammatory chemokines
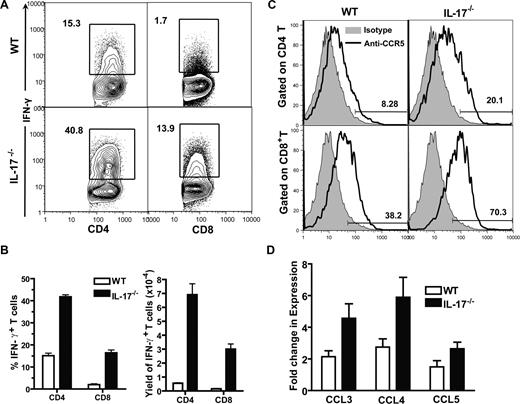
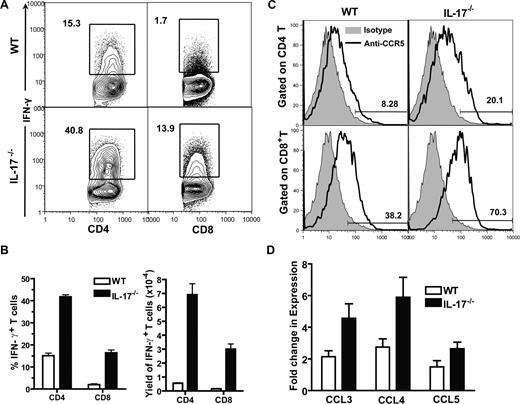
Since we observed enhanced Th1 differentiation of donor T cells in host lymphoid tissues and much more severe GVHD target tissue infiltration and tissue damage in the recipients given IL-17−/− donor cells, as compared with recipients given WT donor cells (Figures 1,2), we tested whether more severe GVHD target tissue damage was associated with increased Th1 infiltration. Taking liver as an example, we found that the percentage of IFN-γ–secreting CD4+ and CD8+ donor T cells in the liver of recipients given IL-17−/− donor cells was more than 2-fold higher than that in recipients given WT donor cells; the yield of IFN-γ+ CD4+ or CD8+ T cells in the liver of the former recipients was more than 6-fold higher than that in latter recipients (P < .01; Figure 3A,B).
Increased infiltration of Th1 cells in liver of recipients given IL-17−/− donor cells. (A) Liver mononuclear cells from recipients given WT or IL-17−/− donor cells were stained for H-2b, CD4, CD8, and intracellular IFN-γ, 13 days after HCT. Gated H-2b+ cells are shown in IFN-γ versus CD4 or CD8. The percentage of IFN-γ+CD4+ or CD8+ T cells is shown beside the gating boxes. One representative of 4 recipients in each group is shown. (B) Means (± SE) of the percentages of donor IFN-γ+ CD4+ or CD8+ T cells of the 4 recipients and of the yield of IFN-γ+ CD4+ or CD8+ T cells of the 4 recipients. (C) MLN cells of the recipients were stained for H-2b, CD4, CD8, and CCR5. Histogram of CCR5 (solid line) versus isotype control (shaded area) of H-2b+ CD4+ or CD8+ cells are shown. One representative of 4 recipients is shown. Means (± SE) of the percentage of CCR5+ cells among CD4+ or CD8+ donor T cells are 43.1% (± 3.9%) or 10.8% (± 2.0%) in recipients given WT donor cells and 67.6% (± 2.8%) or 23.3% (± 1.6%) in recipients given IL-17−/− donor cells. (D) Means (± SE) of chemokine expression levels by liver of the recipients given WT or IL-17−/− donor cells 5 days after HCT. Relative gene expression levels were normalized within each sample to the house keeping gene GAPDH and were presented relative to the expression in liver tissue of syngeneic HCT recipients. There were 4 mice in each group.
Increased infiltration of Th1 cells in liver of recipients given IL-17−/− donor cells. (A) Liver mononuclear cells from recipients given WT or IL-17−/− donor cells were stained for H-2b, CD4, CD8, and intracellular IFN-γ, 13 days after HCT. Gated H-2b+ cells are shown in IFN-γ versus CD4 or CD8. The percentage of IFN-γ+CD4+ or CD8+ T cells is shown beside the gating boxes. One representative of 4 recipients in each group is shown. (B) Means (± SE) of the percentages of donor IFN-γ+ CD4+ or CD8+ T cells of the 4 recipients and of the yield of IFN-γ+ CD4+ or CD8+ T cells of the 4 recipients. (C) MLN cells of the recipients were stained for H-2b, CD4, CD8, and CCR5. Histogram of CCR5 (solid line) versus isotype control (shaded area) of H-2b+ CD4+ or CD8+ cells are shown. One representative of 4 recipients is shown. Means (± SE) of the percentage of CCR5+ cells among CD4+ or CD8+ donor T cells are 43.1% (± 3.9%) or 10.8% (± 2.0%) in recipients given WT donor cells and 67.6% (± 2.8%) or 23.3% (± 1.6%) in recipients given IL-17−/− donor cells. (D) Means (± SE) of chemokine expression levels by liver of the recipients given WT or IL-17−/− donor cells 5 days after HCT. Relative gene expression levels were normalized within each sample to the house keeping gene GAPDH and were presented relative to the expression in liver tissue of syngeneic HCT recipients. There were 4 mice in each group.
It was reported that IFN-γ plays an important role in up-regulating chemokine and chemokine receptor expression, and chemokines from GVHD target tissues and chemokine receptors expressed by donor T cells mediate donor T-cell migration to GVHD target tissues.35,44-47 CCR5 and its chemokine ligands CCL3-5 were shown to play an important role in donor T-cell migration to the liver.48 Thus, we compared CCR5 expression by donor T cells in the spleen and CCL3-5 expression in the liver tissue of recipients given IL-17−/− or WT donor cells. We found that, as compared with recipients given WT donor cells, CCR5 expression by donor CD4+ and CD8+ T cells in recipients given IL-17−/− donor cells increased approximately 2-fold (P < .05; Figure 3C); the liver tissue expression of CCL3(MIP-1α) and CCL4(MIP-1β) in those recipients also increased approximately 2-fold (P < .05; Figure 3D), although no significant difference was found in CCL5 (RANTES) expression (Figure 3D). These results indicate that enhanced Th1 response of donor T cells in recipients given IL-17−/− donor cells results in up-regulation of chemokine receptors on donor T cells and increased chemokine expression by GVHD target tissues, which leads to more severe donor T-cell infiltration in GVHD target tissues.
Neutralizing IFN-γ reversed exacerbated acute GVHD in recipients given IL-17−/− donor cells
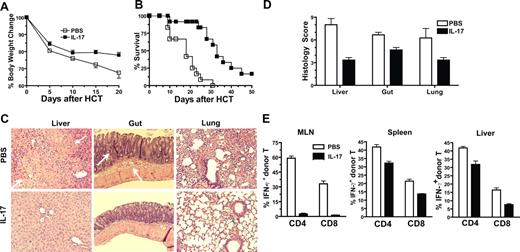
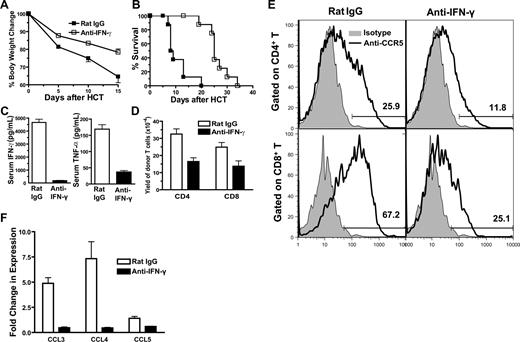
To further test whether augmented donor Th1 differentiation and IFN-γ production in recipients given IL-17−/− donor cells was responsible for the exacerbated acute GVHD, anti–IFN-γ mAb was used to neutralize IFN-γ in the recipients given IL-17−/− donor cells, as described previously.9 The control recipients were treated with rat IgG. We observed that anti–IFN-γ treatment markedly reduced clinical signs of acute GVHD early after HCT, and none of the recipients developed diarrhea. Anti–IFN-γ treatment also significantly increased body weight and prolonged survival of the recipients as compared with control recipients (P < .01; Figure 4A,B). In addition, anti–IFN-γ treatment reduced serum levels of IFN-γ by 20-fold and TNF-α by 3-fold (P < .01; Figure 4C) and reduced donor T-cell infiltration in liver tissues by approximately 2-fold (P < .05; Figure 4D). The reduced infiltration after anti-IFN-γ treatment was associated with significantly reduced expression of CCR5 by donor T cells and reduced expression of chemokine CCL3, CCL4 and CCL5 by liver tissues (P < .05; Figure 4E,F). These results indicate that augmented IFN-γ production plays an important role in the exacerbation of acute GVHD mediated by IL-17−/− donor T cells.
Neutralizing IFN-γ in recipients given IL-17−/− donor cells ameliorated acute GVHD. (A,B) BALB/c recipients given spleen cells (2.5 × 106) and TCD-BM cells (2.5 × 106) from IL-17−/− donors were injected intraperitooneally with anti–IFN-γ mAb or control rat IgG (0.5 mg/mouse) on days 0, 5, 10, and 15 after HCT. Body weight change and survival curves are shown. There were 8 mice in each group; 2 replicated experiments were combined. (C) Means (± SE) of serum levels of IFN-γ and TNF-α of the above recipients 5 days after HCT. (D) Means (± SE) of donor T-cell yield in liver of recipients treated with anti–IFN-γ or rat IgG 5 days after HCT. There were 4 recipients in each group. (E) MLN cells of the recipients treated with anti–IFN-γ or rat IgG were stained for H-2b (donor marker), CD4, CD8, and CCR5 or isotype control 5 days after HCT. Gated on H-2b+CD4+or CD8+ T cells, a histogram of anti-CCR5 (solid line) and isotype control (shaded area) is shown. One representative of 4 recipients in each group is shown. Means (± SE) of the CCR5+CD4+ T cells in recipients treated with anti–IFN-γ or rat IgG are 15.4% (± 1.5%) or 32.3% (± 1.4%). Means (± SE) of the CCR5+CD8+ T cells in recipients treated with anti–IFN-γ or rat IgG are 28.4% (± 1.1%) or 65.3% (± 5.2%). (F) Means (± SE) of chemokine expression levels in liver of the recipients treated with anti–IFN-γ or rat IgG 5 days after HCT. Relative gene expression levels were normalized within each sample to the house keeping gene GAPDH and were presented relative to the expression in synergic HCT recipients. There were 4 mice in each group.
Neutralizing IFN-γ in recipients given IL-17−/− donor cells ameliorated acute GVHD. (A,B) BALB/c recipients given spleen cells (2.5 × 106) and TCD-BM cells (2.5 × 106) from IL-17−/− donors were injected intraperitooneally with anti–IFN-γ mAb or control rat IgG (0.5 mg/mouse) on days 0, 5, 10, and 15 after HCT. Body weight change and survival curves are shown. There were 8 mice in each group; 2 replicated experiments were combined. (C) Means (± SE) of serum levels of IFN-γ and TNF-α of the above recipients 5 days after HCT. (D) Means (± SE) of donor T-cell yield in liver of recipients treated with anti–IFN-γ or rat IgG 5 days after HCT. There were 4 recipients in each group. (E) MLN cells of the recipients treated with anti–IFN-γ or rat IgG were stained for H-2b (donor marker), CD4, CD8, and CCR5 or isotype control 5 days after HCT. Gated on H-2b+CD4+or CD8+ T cells, a histogram of anti-CCR5 (solid line) and isotype control (shaded area) is shown. One representative of 4 recipients in each group is shown. Means (± SE) of the CCR5+CD4+ T cells in recipients treated with anti–IFN-γ or rat IgG are 15.4% (± 1.5%) or 32.3% (± 1.4%). Means (± SE) of the CCR5+CD8+ T cells in recipients treated with anti–IFN-γ or rat IgG are 28.4% (± 1.1%) or 65.3% (± 5.2%). (F) Means (± SE) of chemokine expression levels in liver of the recipients treated with anti–IFN-γ or rat IgG 5 days after HCT. Relative gene expression levels were normalized within each sample to the house keeping gene GAPDH and were presented relative to the expression in synergic HCT recipients. There were 4 mice in each group.
Administration of IL-17 early after HCT inhibited Th1 differentiation and ameliorated acute GVHD in recipients given IL-17−/− donor cells
To directly test whether IL-17 down-regulates acute GVHD, recipients given IL-17−/− donor cells were intraperitoneally injected with recombinant IL-17 at a dose of 0.3 μg/mouse or phosphate-buffered saline (PBS) on days 0, 3, and 7 after HCT. We found that IL-17 treatment ameliorated clinical signs of GVHD such as diarrhea, significantly increased body weight 10 to 20 days after HCT (P < .01; Figure 5A), and prolonged survival (P < .01; Figure 5B). IL-17 treatment also reduced GVHD target tissue infiltration and histopathology scores (P < .05; Figure 5C,D) and significantly reduced the percentage of IFN-γ–secreting CD4+ and CD8+ T cells in host MLN, spleen, and liver tissues (Figure 5E). It is noteworthy that, in contrast to IL-17 treatment of recipients given IL-17−/− donor cells, this low-dose IL-17 treatment of recipients given WT donor cells showed no significant impact on GVHD severity (data not shown). These results indicate that administration of a low dose of IL-17 early after HCT to recipients given IL-17−/− donor cells inhibits donor Th1 cell differentiation and ameliorates acute GVHD. This is consistent with our observation that the presence of a small percentage of IL-17–secreting CD4+ and CD8+ donor T cells in recipients given WT donor cells was associated with reduced GVHD compared with recipients given IL-17−/− donor cells (Figure 1).
Adding back of IL-17 to recipients given IL-17−/− donor cells ameliorated acute GVHD. (A,B) Recipients given spleen (2.5 × 106) and TCD-BM cells (2.5 × 106) from IL-17−/− donor cells were injected intraperitoneally with recombinant IL-17 (0.3 μg/mouse) on days 0, 3, and 7 after HCT. Body weight change and survival curves are shown. There were 12 recipients in each group; 3 replicated experiments were combined. (C,D) Histopathology of liver, gut, and lung of recipients treated with PBS or IL-17 13 days after HCT. Arrow points to the necrosis area in liver and infiltrated epithelial and lamina propria areas in gut of recipients given PBS treatment only. One representative of histopathology and mean (± SE) of histology score of 6 examined recipients in each group is shown. (E) Mean (± SE) of the percentage of donor-type IFN-γ+CD4+ or CD8+ T cells in MLN, spleen, and liver of recipients treated with IL-17 or PBS 13 days after HCT. There were 4 recipients in each group.
Adding back of IL-17 to recipients given IL-17−/− donor cells ameliorated acute GVHD. (A,B) Recipients given spleen (2.5 × 106) and TCD-BM cells (2.5 × 106) from IL-17−/− donor cells were injected intraperitoneally with recombinant IL-17 (0.3 μg/mouse) on days 0, 3, and 7 after HCT. Body weight change and survival curves are shown. There were 12 recipients in each group; 3 replicated experiments were combined. (C,D) Histopathology of liver, gut, and lung of recipients treated with PBS or IL-17 13 days after HCT. Arrow points to the necrosis area in liver and infiltrated epithelial and lamina propria areas in gut of recipients given PBS treatment only. One representative of histopathology and mean (± SE) of histology score of 6 examined recipients in each group is shown. (E) Mean (± SE) of the percentage of donor-type IFN-γ+CD4+ or CD8+ T cells in MLN, spleen, and liver of recipients treated with IL-17 or PBS 13 days after HCT. There were 4 recipients in each group.
IL-17 inhibition of Th1 cell differentiation may be not through direct interaction with CD4+ T cells, but through its influence on host DCs
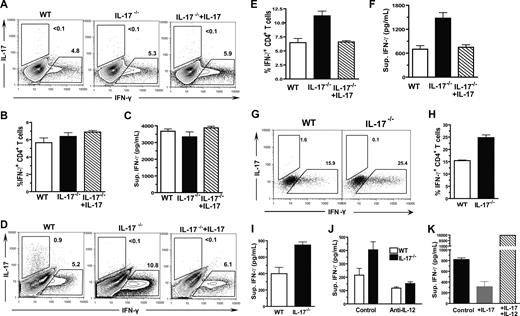
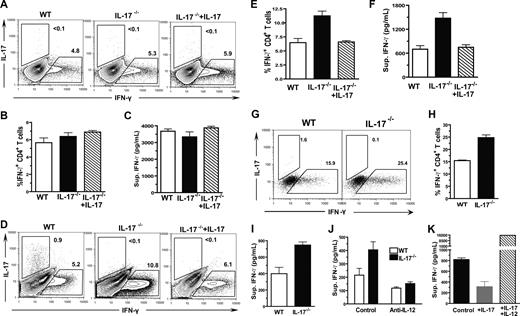
Since we observed that administration of low doses of IL-17 inhibited Th1 differentiation and ameliorated acute GVHD (Figure 5), we next explored the target cells of IL-17 regulation. It was reported that activated CD4+ T cells expressed IL-17 receptor,49 thus we first tested whether IL-17 could inhibit Th1 differentiation via direct interaction with CD4+ T cells. Accordingly, CD4+ T cells were induced to differentiate into IFN-γ–secreting Th1 cells via plate-bound anti-CD3/CD28 stimulation and IL-12 in culture medium, as described previously.26 We found that, a similar percentage of IFN-γ+ cells were present among WT CD4+ T cells and IL-17−/− CD4+ T cells, and the addition of IL-17 to the culture did not lead to significant changes in the percentage of IFN-γ+ cells (Figure 6A,B). Furthermore, the IFN-γ concentration in culture supernatants of the 3 groups was similar (Figure 6C). These results are consistent with a previous report and indicate that IL-17 may not directly inhibit Th1 differentiation.50
IL-17 down-regulation of Th1 differentiation required host DCs. (A-C) Sorted CD4+ T cells (2 × 105) from WT or IL-17−/− donors were stimulated with plate-bound anti-CD3 and anti-CD28 as well as IL-12 (10 ng/mL) in culture for 72 hours. The percentage of IFN-γ+ or IL-17+ cells among cultured donor CD4+ T cells were determined by intracellular staining, and the IFN-γ concentration in culture supernatant was measured by ELISA. One representative of 4 replicated intracellular staining experiments, the mean (± SE) percentage of IFN-γ+ cells among donor CD4+ T cells, and the mean (± SE) of the IFN-γ concentration in the culture supernatants are shown. (D-F) Sorted donor CD4+ T cells (2 × 105) from WT or IL-17−/− donors were cocultured with enriched DCs from irradiated host BALB/c mice for 96 hours. The percentage of IFN-γ+ or IL-17+ cells among cultured donor CD4+ T cells were determined by intracellular staining, and the IFN-γ concentration in culture supernatant was measured by ELISA. One representative of 4 replicated intracellular staining experiment, the mean (± SE) of the percentage of IFN-γ+ cells among donor CD4+ T cells, and the mean (± SE) of the IFN-γ concentration in the culture supernatants are shown. (G-I) Sorted naive CD44loCD4+ T cells from WT or IL-17−/− donors were cocultured with enriched DCs from irradiated host BALB/c mice for 5 days. The percentage of IFN-γ+ or IL-17+ cells among cultured donor CD4+ T cells were determined by intracellular staining, and the IFN-γ concentration in culture supernatant was measured by ELISA. Dead cells were excluded by fixable aqua dead cell stain kit. One representative of 3 replicated experiments, the mean (± SE) percentage of IFN-γ+ cells among donor CD4+ T cells, and the mean (± SE) of the IFN-γ concentration in the culture supernatants of triplicates are shown. (J) IFN-γ concentration in the culture supernatants of naive donor WT and IL-17−/− CD4+ T cells and host DCs with or without the presence of neutralizing anti–IL-12 Antibody. (K) IFN-γ concentration in the culture supernatants of IL-17−/− donor CD4+ T cells and host DCs with or without addition of IL-17 or IL-17 plus IL-12.
IL-17 down-regulation of Th1 differentiation required host DCs. (A-C) Sorted CD4+ T cells (2 × 105) from WT or IL-17−/− donors were stimulated with plate-bound anti-CD3 and anti-CD28 as well as IL-12 (10 ng/mL) in culture for 72 hours. The percentage of IFN-γ+ or IL-17+ cells among cultured donor CD4+ T cells were determined by intracellular staining, and the IFN-γ concentration in culture supernatant was measured by ELISA. One representative of 4 replicated intracellular staining experiments, the mean (± SE) percentage of IFN-γ+ cells among donor CD4+ T cells, and the mean (± SE) of the IFN-γ concentration in the culture supernatants are shown. (D-F) Sorted donor CD4+ T cells (2 × 105) from WT or IL-17−/− donors were cocultured with enriched DCs from irradiated host BALB/c mice for 96 hours. The percentage of IFN-γ+ or IL-17+ cells among cultured donor CD4+ T cells were determined by intracellular staining, and the IFN-γ concentration in culture supernatant was measured by ELISA. One representative of 4 replicated intracellular staining experiment, the mean (± SE) of the percentage of IFN-γ+ cells among donor CD4+ T cells, and the mean (± SE) of the IFN-γ concentration in the culture supernatants are shown. (G-I) Sorted naive CD44loCD4+ T cells from WT or IL-17−/− donors were cocultured with enriched DCs from irradiated host BALB/c mice for 5 days. The percentage of IFN-γ+ or IL-17+ cells among cultured donor CD4+ T cells were determined by intracellular staining, and the IFN-γ concentration in culture supernatant was measured by ELISA. Dead cells were excluded by fixable aqua dead cell stain kit. One representative of 3 replicated experiments, the mean (± SE) percentage of IFN-γ+ cells among donor CD4+ T cells, and the mean (± SE) of the IFN-γ concentration in the culture supernatants of triplicates are shown. (J) IFN-γ concentration in the culture supernatants of naive donor WT and IL-17−/− CD4+ T cells and host DCs with or without the presence of neutralizing anti–IL-12 Antibody. (K) IFN-γ concentration in the culture supernatants of IL-17−/− donor CD4+ T cells and host DCs with or without addition of IL-17 or IL-17 plus IL-12.
In contrast, when CD4+ T cells were stimulated with enriched host DCs, IL-17−/− CD4+ T cells had approximately a 2-fold higher percentage of IFN-γ+ cells compared with WT CD4+ T cells (P < .01), and adding IL-17 back to the culture reversed the augmentation (Figure 6D,E). The same difference was observed with the IFN-γ concentration in the culture supernatants of the 3 groups (P < .01; Figure 6F). To exclude the possible influence of memory CD4+ T cells, CD44loCD4+ naive T cells were sorted (as described in Figure S2) and used to replace the total CD4+ T cells in the culture. We found that the percentage of IFN-γ+ cells and the concentration of IFN-γ in supernatant in the culture of IL-17−/− CD4+ T cells was still significantly higher than that in the culture of WT CD4+ T cells (P < .05; Figure 6G-I). In addition, we found that, after neutralization of IL-12, the concentration of IFN-γ in the culture of WT or IL-17−/− naive CD4+ T cells become similar (Figure 6J). Furthermore, although IL-17 inhibited IFN-γ production of IL-17−/− CD4+ T cells (P < .05; Figure 6K), addition of mouse recombinant IL-12 completely reverse the inhibition by IL-17, and markedly increased the IFN-γ production (P < .01). These results indicate that IL-17 regulation of Th1 differentiation may be through its influence on host DCs early after HCT, and the effect is IL-12–dependent.
Discussion
We have demonstrated that IL-17−/− donor T cells induced exacerbated acute GVHD, which was associated with augmented Th1 differentiation and IFN-γ production of donor T cells, and that the enhanced Th1 differentiation resulted from a lack of IL-17–mediated down-regulation of host DCs.
First, the enhanced Th1 differentiation and IFN-γ production play a critical role in the augmentation of acute GVHD by IL-17−/− donor T cells. Acute GVHD is initiated by the interactions between host APCs (especially DCs) and naive alloreactive donor T cells.3,4 The subsequent Th1 differentiation leads to the generation of cytotoxic T cells and large amounts of inflammatory cytokines that damage host tissues.1 Although the role of IFN-γ in mediating acute GVHD is controversial, augmented Th1 differentiation and IFN-γ production was clearly associated with exacerbated acute GVHD.51,52 In the current study, we observed that, compared with WT donor T cells, IL-17−/− donor T cells induced more severe clinical signs of acute GVHD and earlier death of the recipients. The exacerbated GVHD was associated with augmented Th1 differentiation and IFN-γ production, which was manifested by elevated serum levels of IFN-γ and TNF-α, increased IFN-γ–secreting T cells in lymphoid tissues and GVHD target tissues, and up-regulation of proinflammatory chemokines in GVHD target tissues and chemokine receptors on donor T cells.
IFN-γ was reported to play an important role in the up-regulation of proinflammatory cytokines, chemokines, and chemokine receptors that mediate donor T-cell migration to inflammatory GVHD target tissues.53,54 We observed that neutralizing IFN-γ in recipients given IL-17−/− donor cells markedly reduced serum levels of TNF-α, donor T-cell expression of chemokine receptors such as CCR5, GVHD target tissue expression of chemokines such as CCL3 and CCL4, and infiltration in GVHD target tissues, resulting in markedly reduced GVHD severity. Therefore, augmented Th1 differentiation and IFN-γ production predominantly contribute to the exacerbation of acute GVHD in recipients given IL-17−/− donor cells. However, we should point out that neutralizing IFN-γ did not completely prevent acute GVHD in recipients given IL-17−/− donor cells. This could be due to the incomplete neutralization of IFN-γ by anti-IFN-γ mAb. T cells from IFN-γ−/− IL-17−/− donor will be used to address this question in the future studies.
Second, the augmented Th1 differentiation was due to the lack of negative feedback mediated by Th17. We observed that administration of a low dose of IL-17 to recipients given IL-17−/− donor cells markedly reduced IFN-γ–producing T cells in host lymphoid and GVHD target tissues, donor T-cell expression of inflammatory chemokine receptors, and GVHD target tissue expression of inflammatory chemokines, resulting in significantly ameliorated acute GVHD. Therefore, donor T-cell deficiency in IL-17 leads to augmentation of Th1 differentiation and exacerbation of acute GVHD.
Third, the negative regulation of Th1 differentiation by Th17 may not be through direct impact of IL-17 on donor CD4+ T cells but via IL-17 influence of host DCs. Host DCs were shown to initiate the activation and differentiation of alloreactive donor T cells after HCT,3-5 and cytokines from DCs dictate T-cell differentiation during T-DC interaction.55 IL-12 induces Th1; IL-4 induces Th2; and TGF-β and IL-6 induce Th17.12,55 We observed that WT and IL-17−/− donor CD4+ T cells showed similar levels of differentiation into IFN-γ–secreting Th1 cells in a culture with plate-bound anti-CD3/CD28 and IL-12, and addition of IL-17 to the culture had no impact on the Th1 differentiation. This is consistent with a previous report.50 In contrast, when stimulated with host DCs without exogenous IL-12, IL-17−/− donor CD4+ T cells showed markedly augmented differentiation into Th1 cells compared with WT CD4+ T cells, and adding back of IL-17 or addition of neutralizing anti-IL-12 to the culture of IL-17−/− donor CD4+ T cells inhibited the augmentation. These results indicate that, during the initial phase of donor T-cell activation and differentiation, donor Th17 may influence donor Th1 differentiation through IL-17 influence on host DCs. However, it is not yet clear how donor Th17 cells influence host DCs.
A reciprocal regulation among Th1 and Th17 has been proposed with autoimmune disease models.56 It was shown that IFN-γ inhibited the generation of Th17,57 and IL-23 down-regulated IL-12 by inhibition of IL-12 p35 and p40 expression,58 but it is unknown whether IL-17 can directly down-regulate DC secretion of IL-12. It is possible that IL-17 may decrease IL-12 secretion by direct interaction with DCs, since DCs expressed IL-17 receptors.49 Consistently, in acute GVHD, we observed that augmentation of donor Th1 differentiation resulted from loss of IL-17 was IL-12 dependent. Therefore, we also hypothesize that there is a reciprocal regulation of donor Th1 and Th17 in allogeneic recipients. When donor T cells interact with activated host APCs, donor T cells simultaneously differentiate into Th1 and Th17 cells, but Th1 is dominant. This is supported by our observation that there were15-fold higher percentage of IFN-γ–secreting donor T cells than IL-17–secreting ones in the liver of recipients given WT donor T cells early after HCT (Figures 1,2). IFN-γ from Th1 cells can down-regulate Th17 and IL-17 from Th17 cells can down-regulate Th1. Loss of IL-17 leads to augmented Th1 differentiation and exacerbated GVHD as described in the current report. Loss of IFN-γ may lead to augmented Th17 differentiation and exacerbated GVHD (Figure S3). Therefore, we theorize that both Th1 and Th17 can mediate acute GVHD, but they reciprocally regulate each other. We are aware that exacerbation of acute GVHD by IFN-γ−/− donor T cells has been found to be associated with reduced apoptosis of alloreactive donor T cells59 and augmented idiopathic pneumonia syndrome.60 Therefore, the role of augmented Th17 differentiation in the GVHD pathogenesis in the recipients given IFN-γ−/− donor T cells needs to be investigated further, as does the interaction between Th17 and Th2 in GVHD recipients.
In conclusion, this is the first demonstration that absence of Th17 or IL-17 leads to augmented Th1 differentiation and exacerbated GVHD. This is of clinical interest because our studies indicate that treatment neutralizing proinflammatory cytokine IL-17 may lead to exacerbation of acute GVHD as well as other inflammatory autoimmune diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Yoichiro Iwakura of Tokyo University (Tokyo, Japan) for kindly providing IL-17−/− C57BL/6 mice. We thank James Young for critical reading of the manuscript, Lucy Brown and her staff at City of Hope Flow Cytometry Facility, Sofia Loera and her staff at City of Hope Anatomic Pathology Laboratory for their excellent technical assistance, and Dr Richard Ermel and his staff at City of Hope Research Animal Facility for providing excellent animal care.
This research was supported by the National Institutes of Health (NIH, Bethesda, MD) grant R01-AI66008 (D. Zeng).
National Institutes of Health
Authorship
Contribution: T.Y. designed and performed research, analyzed data, and wrote the manuscript; D. Zhao performed research and reviewed histology scores; C.-L.L., C.Z., Y.C., and I.T. performed research; T.L., F.K., and S.F. reviewed the manuscript; and D. Zeng designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Defu Zeng, MD, The Beckman Research Institute, Gonda Building, R2017, City of Hope National Medical Center, 1500 East Duarte Road, Duarte, CA 91010; e-mail: dzeng@coh.org.