Abstract
Inhibitory killer Ig–like receptors (KIR), expressed by human natural killer cells and effector memory CD8+ T-cell subsets, bind HLA-C molecules and suppress cell activation through recruitment of the Src homology 2 domain-containing protein tyrosine phosphatase 1 (SHP-1). To further analyze the still largely unclear role of inhibitory KIR receptors on CD4+ T cells, KIR2DL1 transfectants were obtained from a CD4+ T-cell line and primary cells. Transfection of CD4+ T cells with KIR2DL1 dramatically increased the T-cell receptor (TCR)–induced production of interleukin-2 independently of ligand binding but inhibited TCR-induced activation after ligation. KIR-mediated costimulation of TCR activation involves intact KIR2DL1-ITIM phosphorylation, SHP-2 recruitment, and PKC-θ phosphorylation. Synapses leading to activation were characterized by an increase in the recruitment of p-Tyr, SHP-2, and p-PKC-θ, but not of SHP-1. Interaction of KIR2DL1 with its ligand led to a strong synaptic accumulation of KIR2DL1 and the recruitment of SHP-1/2, inhibiting TCR-induced interleukin-2 production. KIR2DL1 may induce 2 opposite signaling outputs in CD4+ T cells, depending on whether the KIR receptor is bound to its ligand. These data highlight unexpected aspects of the regulation of T cells by KIR2DL1 receptors, the therapeutic manipulation of which is currently being evaluated.
Introduction
Killer immunoglobulin-like inhibitory receptors (KIRs) are the most potent inhibitory receptors on human natural killer (NK) cells. They recognize classic self-major histocompatibility complex (MHC) class I molecules and efficiently block NK-cell activation. During virus infection or malignant transformation, MHC class I molecules are down-regulated, thus triggering target cell lysis and secretion of cytokines by NK cells.1 KIRs are type I transmembrane glycoproteins of the immunoglobulin-like receptor family. KIR2D receptors have 2 extracellular Ig-like domains (KIR2DL) and recognize different HLA-C alleles: KIR2DL1 and KIR2DL2/3 recognize HLA-Cw4 and HLA-Cw3, respectively. KIR molecules with 3 extracellular Ig-like domains (KIR3DL) recognize HLA-A and HLA-B molecules.2
Inhibitory receptor signal transduction depends on at least one immunoreceptor tyrosine-based inhibitory motif (ITIM: I/V/L/S-x-T-x-x-L/V) in the cytoplasmic tail. After KIR engagement, tyrosine residues of ITIMs are phosphorylated, leading to recruitment of Src homology 2 (SH2) domain-containing protein tyrosine phosphatases, such as SHP-1, SHP-2, and inositol-phosphatase SHIP and the generation of inhibition signals.3-5 The KIR2DL1 C-terminal ITIM can recruit both SHP-1 and SHP-2, whereas the N-terminal ITIM recruits only SHP-2, although the role of SHP-2 in KIR function remains unclear.6 The recruitment of SHP-1 and SHP-2 is required to abolish activating receptor signal transduction by dephosphorylating protein tyrosine kinases.
However, the specificity of inhibition by ITIM and activation by the immunoreceptor tyrosine-based activation motif (ITAM) is not absolute. Indeed, recent studies have shown that the ITAM may, in some circumstances, mediate inhibition. Conversely, the ITIM may also mediate the activation of different signaling pathways.7 A recent report also showed that SHP-2 positively regulates the extracellular signal-regulated kinase (ERK) pathway in both a Ras-dependent and a Ras-independent manner,8 promoting Src family kinase activation by dephophorylation of the C-src kinase (Csk) binding protein (Cbp).9
KIR expression has also been reported on peripheral blood T-cell subsets, including γ/δ T cells10,11 and effector memory CD8+, CD28−, T-cell receptor (TCR) α/β+ lymphocytes.12,13 The mechanisms governing the induction of these receptors on T cells remain unknown. KIR may be induced during the peripheral differentiation of antigen-specific T cells, and KIR+ CD3+ T cells may correspond to T cells chronically stimulated in response to self-antigen.14,15 KIR+ CTL were rapidly identified as potentially involved in the tumor immune response because of KIR expression on memory effector T cells and their specificity for HLA-I molecules, which are frequently down-regulated by tumor cells.16 We have shown that inhibitory KIRs are expressed on tumor-specific CD8+ T cells derived from patients with renal carcinoma, contributing to the functional anergy of TILs in these tumors. After triggering by HLA-C molecules, KIR2DL recruits SHP-1, which down-regulates the signaling cascade of the activating TCR/CD3 complex.17 Thus, on CD8+ T cells, KIR increases the activation threshold, reducing lytic and secretory functions after CD3-mediated activation.
Little is known about expression and function of KIR molecules in CD4+ T cells. A few studies have shown memory KIR+ CD4+ T cells to be present in healthy persons, with the KIR repertoire expressed on these cells depending on CD28 expression.18 CD4+ CD28− KIR+ T cells are frequently detected in patients with acute coronary syndrome or rheumatoid arthritis.19,20 In these patients as in healthy donors, KIR2DL1 and KIR2DS1 were infrequently expressed by CD4+ CD28− T cells, and these cells displayed preferentially KIR2DL2, KIR2DL3, and KIR2DS2.18,20,21 Moreover, CD4+ T lymphocytes expressing KIR3DL2 have been also characterized in cutaneous T-cell lymphomas.22 Finally, KIR2DL1 and KIR2DL2 have been detected in the peripheral blood mononuclear cells (PBMCs) of patients with Sezary syndrome.23
We investigated KIR function in CD4+ T cells through the generation of stable transfectants of Jurkat T cells expressing KIR2DL1. We found that TCR-mediated signaling was inhibited when KIR2DL1 was bound to its ligand, whereas, in the absence of ligand binding, the KIR2DL1 receptor in CD4+ T cells acted as a costimulator for interleukin-2 (IL-2) production after TCR stimulation, through the recruitment of SHP-2.
Methods
Plasmid constructs
The cDNA encoding the full-length KIR2DL1 was obtained by polymerase chain reaction (PCR) from a construct provided by Dr R. Biassoni (University of Genoa, Genoa, Italy). The PCR product was introduced into the TOPO cloning vector (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions, and was then subcloned into the pEGFP-N1 vector in frame with the sequence corresponding to the N-terminus of green fluorescent protein (GFP). The pEGFP-N1 vector used was produced by Clontech (Mountain View, CA) and contained the CMV promoter, the neomycin-resistant gene, and the GFP gene. The construct encoding GFP fused to KIR2DL1 was obtained by inserting the PCR-amplified complete KIR2DL1 sequence between the EcoR and BamHI sites of pEGFP-N1, resulting in the production of KIR2DL1-GFP protein. Correct insertion was verified by DNA sequencing. Mutagenesis was performed with sets of primers. The tyrosine residue of the 2 cytoplasmic KIR2DL1-ITIMs was mutated to a phenylalanine residue using the oligonucleotide 5′-CCTCAGGAGGTGACATTCACACAGTTGAATCAC-3′ and 5′-CAACAGATATCATCGTGTTCACGGAACTTCCAAATGC-3′ and their complements with a QuickChange Site-directed mutagenesis kit (Stratagene, La Jolla, CA). The mutations were confirmed by sequencing and cloned into the plasmid pEGFP-N1.
Cell culture, CD4+ T-cell immunoselection, and transfection
The MHC-deficient human lymphoblastoid B-cell line 721.221 and its derivative clones stably transfected with HLA-Cw4 or HLA-Cw7 were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum and penicillin/streptomycin antibiotics. CD4+ leukemic human Jurkat E6.1 T cells were grown in the same medium.
Parental Jurkat T cells did not stain with any anti-KIR monoclonal antibodies (mAbs). We obtained stable Jurkat transfectants, by electroporating 107 Jurkat T cells in 0.4 mL RPMI 1640, 960 μF/260 V, using an Easyjet Plus-Electroporator (Equibio, Ashton, United Kingdom) with 10 μg of pEGFP-N1/KIR2DL1, pEGFP-N1/KIR2DL1*2ΔY, or pEGFP-N1 (empty vector). After 48 hours, cells were cloned by limiting dilution into 96-well culture plates in selective RPMI 1640 medium containing 1 mg/mL G418 (PAA). Geneticin-resistant transfectants were tested for KIR2DL1/GFP protein production by flow cytometric analyses and confocal microscopy.
PBMCs from healthy donors were isolated by centrifugation on Ficoll-Hypaque (GE Healthcare, Little Chalfont, United Kingdom). CD4+ T cells were immunoselected with a CD4+ isolation kit (Invitrogen), according to manufacturer's protocol. T-cell purity, as determined by flow cytometry, exceeded 90%. Transient transfectants were obtained from CD4+ T cells by electroporation (Amaxa Biosystems, Gaithersburg, MD), according to the manufacturer's guidelines, and were used 24 hours after transfection. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Antibodies and flow cytometric analysis
Anti-CD3 (UCHT1) and phycoerythrin-conjugated anti-KIR2DL1 (EB6) mAbs were purchased from Immunotech (Marseille, France). Anti–SH-PTP1, anti–SH-PTP2, antiactin (c-11 clone) (for immunoblotting), and anti–phospho-PKC-θ (for microscopy analysis) mAbs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antiphosphotyrosine antibody (4G10) was provided by Upstate Biotechnology (Charlottesville, VA). Anti–phospho-PKC-θ and anti–PKC-θ mAbs (for immunoblotting), and anti–phospho-ZAP70 mAbs (Tyr493) for confocal microscopy were purchased from Cell Signaling Technology (Danvers, MA). Anti-GFP (ab1218) mAbs were purchased from Abcam (Cambridge, United Kingdom). KIR2DL1 mAb (NKVSF) was kindly provided by François Romagne (Innate Pharma, Marseille, France).
Phenotypic analyses were performed on 3 × 105 cells stained with fluorescent mAbs, using a FACS CaliburTM flow cytometer. Data were processed using CellQuest software (BD Biosciences, San Jose, CA). For indirect immunofluorescence assays, cells were incubated with unlabeled primary antibodies and then stained with a phycoerythrin-conjugated goat anti–mouse Ig from Immunotech.
T-cell activation
For enzyme-linked immunosorbent assay (ELISA) and Western blots, CD4+ T cells, Jurkat cells, KIR2DL1 transfectants, and KIR2DL1 mutants were treated with anti-CD3 (1 μg/mL) or incubated with anti-CD3 and anti-CD28 antibody-coated beads (Dynal Biotech, Oslo, Norway) in plates for 6 hours. In other experiments, these cells were mixed in a 1:1 ratio (in a total volume of 300 μL), for 6 hours with 721.221, 721.221/HLA-Cw7, or 721.221/HLA-Cw4 cells pulsed with the staphylococcal enterotoxin E (SEE) superantigen. Activated cells were harvested for IL-2 quantitation, RNA isolation, or confocal microscopic analysis. In some experiments, NKVSF mAb (1.5 μg/mL) was coated on plates with anti-CD3 mAb.
IL-2 analysis
IL-2 concentration in cell supernatants was determined by ELISA (BD Biosciences PharMingen, San Diego, CA), according to the manufacturer's instructions. Alternatively, IL-2 was analyzed by intracellular staining. Briefly, cells were incubated with 10 μg/mL brefeldin A (Sigma-Aldrich, St Louis, MO) for 2 hours. Then, 107 cells were fixed by incubation for 10 minutes with 4% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized by incubation with 0.5% bovine serum albumin and 0.2% saponin in PBS. Cells were stained by incubation with allophycocyanin-labeled anti–IL-2 mAb for 30 minutes at room temperature. Cells were washed 3 times between steps.
Purification of RNA and TaqMan real-time quantitative reverse-transcription PCR analysis
JK32, EV, and Jurkat T cells were stimulated for 1 to 4 hours with immobilized mouse IgG or anti-CD3 mAbs. Total RNA was extracted using the TRIzol reagent (Invitrogen), and cDNA was synthesized with oligo-dT primers. PCR primers and probes for the IL-2 gene were designed by Applied Biosystems (Foster City, CA) and used according to the manufacturer's recommendations. The amounts of RNA present in samples were normalized by amplifying an endogenous control (18S). The relative quantification of transcripts was achieved by the standard curve method (Applied Biosystems User Bulletin 2, ABI PRISM 7700 Sequence Detection system).
Immunoblotting
Cells were lysed in a buffer containing 20 mM Tris-HCl (pH 7.5), 1% CHAPS (Sigma-Aldrich), 150 mM NaCl, 10% glycerol, 1 mM of Na3VO4, and protease inhibitors (Complete Protease Inhibitor Mixture; Roche Molecular Biochemicals, Basel, Switzerland). Samples were placed on ice for 30 minutes and then centrifuged at 10 000g for 30 minutes at 4°C. The supernatants were collected and proteins concentration determined with BCA protein assay reagent (Pierce Chemical, Cramlington, United Kingdom). Equivalent protein extracts (15 μg) for each sample were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were probed with primary antibodies (1:1000 dilution) and then incubated with peroxide-conjugated secondary antibodies (1:2000 dilution). Finally, membranes were washed and signals visualized by enhanced chemiluminescence (Western Blotting Kit, Pierce Chemical).
Immunoprecipitation
JK32 and EV cells were left unstimulated or were stimulated in a ratio of 1:1 with SEE-pulsed 721.221 cells, 721.221/HLA-Cw7, or 721.221/HLA-Cw4 cells for 20 minutes at 37°C. Cells were lysed by incubation in 1 mL lysis buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na3VO4 plus 1% Nonidet P-40 and protease inhibitor for 30 minutes at 4°C. After centrifugation (10 000g, 30 minutes at 4°C), lysates were precleared by incubation with Protein G-Sepharose beads (Sigma-Aldrich). Antibodies or isotype controls were added at optimal concentrations, and the mixture was incubated for 2 hours at 4°C. Protein G-Sepharose (25 μL) was added and the mixture was incubated for another hour at 4°C. Samples were washed 4 times in lysis buffer, and the immunoprecipitate was dissolved in 1× Laemmli buffer and resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Samples were subjected to electrophoresis and processed for immunoblot analysis as described in “Immunoblotting.”
Confocal microscopy
JK32 or EV cells were incubated at a 1:1 ratio with SEE-pulsed 721.221/HLA-Cw7 or 721.221/HLA-Cw4 cells at 37°C for 30 minutes and plated on poly-(L-lysine)-coated coverslips (Sigma-Aldrich). The cells were washed with PBS, fixed by incubation with 4% paraformaldehyde in PBS for 60 minutes, and rinsed 3 times with PBS. Cells were permeabilized by incubation with dodecyl sulfate (0.1% in PBS) for 10 minutes and then washed 3 times with PBS. Nonspecific binding sites were blocked by incubation with 10% FCS in PBS for 20 minutes. Cells were then incubated for 60 minutes with antibodies against p-Tyr (4G10), p-ZAP70, p-PKC-θ, SHP-1, or SHP-2 and stained with Alexa 546-conjugated goat antimouse or antirabbit antibody (Invitrogen). Nuclei were stained with TO-PRO-3 iodide (Invitrogen). Coverslips were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and conjugates analyzed with a fluorescence microscope (LSM-510, Zeiss, Jena, Germany). A Z-projection of slices was generated, using LSM Image Examiner software (Zeiss). The percentages of JK32 and EV cells forming conjugates with B cells were calculated: 3 × 104 cells of the 721.221 cell lines were grown in Petri dishes for 48 hours, and 7 × 104 EV or JK32 cells were then added. After 20 minutes of coculture, nonconjugated T cells were removed by gentle washing, and the remaining cells were fixed, permeabilized, and stained with TO-PRO-3 iodide. Ten confocal microscopy fields were analyzed for each set of conditions, and percentages of fluoresecent cells (with SD) were determined.
Results
Generation of KIR2DL1 transfectants
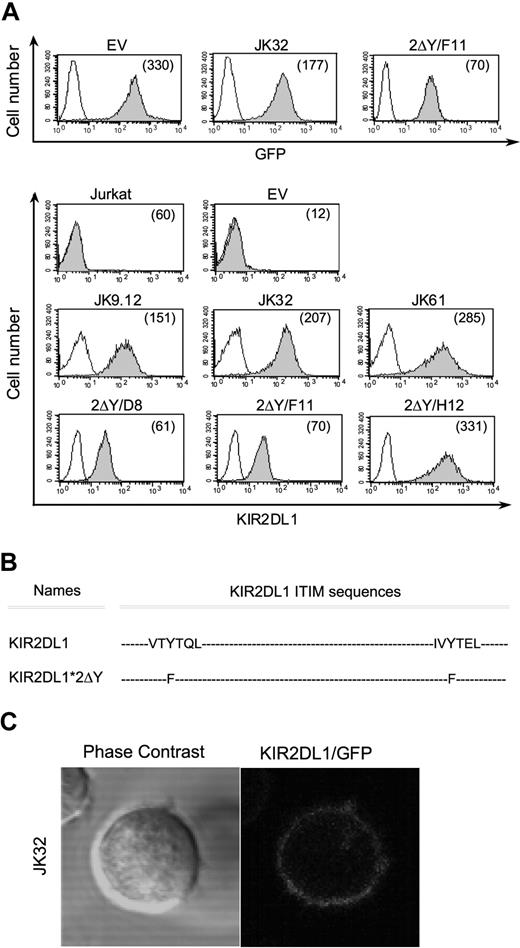
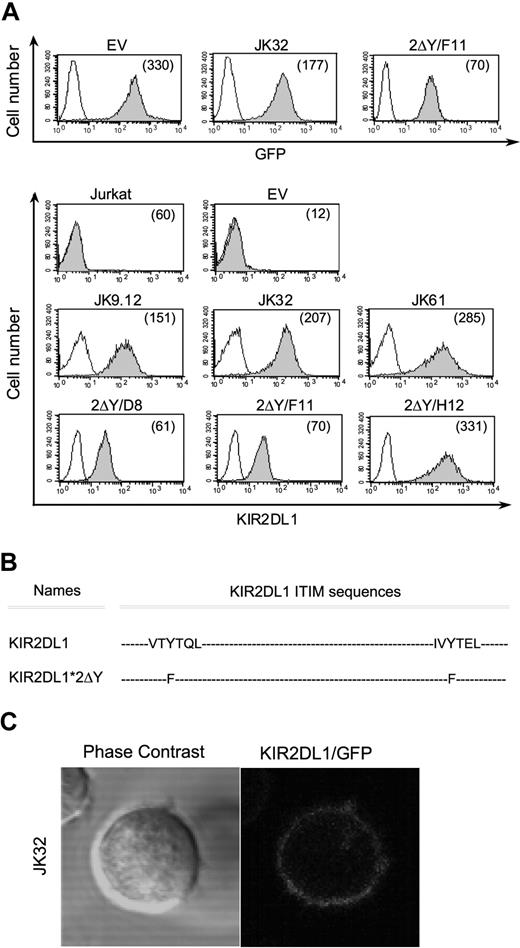
We investigated the role of KIR in T-cell activation, by inserting a full-length or a mutated cDNA encoding KIR2DL1 into pEGFP-N1. Jurkat CD4+ T cells were stably transfected with the empty pEGFP-N1 vector (EV), pEGFP-N1/KIR2DL1+ vector, or pEGFP-N1/KIR2DL1*2ΔY. Positive clones were isolated by limiting dilution methods. Three representative clones (JK9.12, JK32, JK61) expressing pEGFP-N1/KIR2DL1+ and 3 representative clones (2ΔY/D8, 2ΔY/F11, 2ΔY/H12) expressing pEGFP-N1/KIR2DL1*2ΔY are shown in Figure 1A. To test the need for cytoplasmic ITIMs in KIR2DL1-signaling, a tyrosine to phenylalanine substitution (Y-F) was introduced in both ITIM sequences (Figure 1B). All transfectants displayed homogenous high levels of KIR2DL1 and GFP expression at the membrane (Figure 1A). They had levels of CD3 and TCRα/β similar to the parental Jurkat cells (data not shown). Confocal microscopy also showed KIR2DL1 to be evenly distributed on the plasma membrane (Figure 1C).
KIR2DL1 and GFP membrane expression on Jurkat transfectants and KIR2DL1 mutants. (A) The protein-coding region of KIR2DL1 or mutated KIR2DL1 was inserted into pEGFP-N1, and Jurkat T cells were stably transfected with the recombinant DNA and pEGFP-N1 empty vector. Three of the geneticin-resistant transfectants expressing KIR2DL1 at the membrane (JK9.12, JK32, JK61) and mutated KIR2DL1 (2ΔY/D8, 2ΔY/F11, 2ΔY/H12) were selected. GFP and KIR2DL1 (shaded histograms) or isotypic control (open histograms) levels were determined by flow cytometry. Numbers in parentheses are mean fluorescence intensities (MFI). (B) KIR2DL1-ITIMs sequences of wild-type KIR2DL1 and with amino acid substitutions in double Tyr mutant (2ΔY) are shown. (C) Confocal microscopic analysis of KIR2DL1/GFP distribution in Jurkat KIR2DL1-GFP transfectants.
KIR2DL1 and GFP membrane expression on Jurkat transfectants and KIR2DL1 mutants. (A) The protein-coding region of KIR2DL1 or mutated KIR2DL1 was inserted into pEGFP-N1, and Jurkat T cells were stably transfected with the recombinant DNA and pEGFP-N1 empty vector. Three of the geneticin-resistant transfectants expressing KIR2DL1 at the membrane (JK9.12, JK32, JK61) and mutated KIR2DL1 (2ΔY/D8, 2ΔY/F11, 2ΔY/H12) were selected. GFP and KIR2DL1 (shaded histograms) or isotypic control (open histograms) levels were determined by flow cytometry. Numbers in parentheses are mean fluorescence intensities (MFI). (B) KIR2DL1-ITIMs sequences of wild-type KIR2DL1 and with amino acid substitutions in double Tyr mutant (2ΔY) are shown. (C) Confocal microscopic analysis of KIR2DL1/GFP distribution in Jurkat KIR2DL1-GFP transfectants.
KIR2DL1 triggers IL-2 secretion after TCR activation
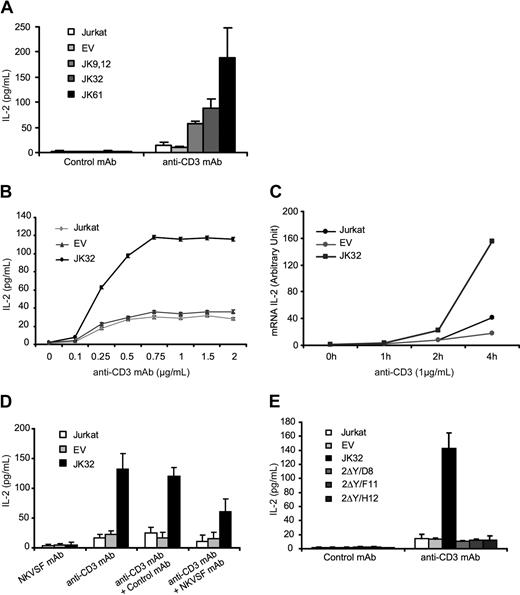
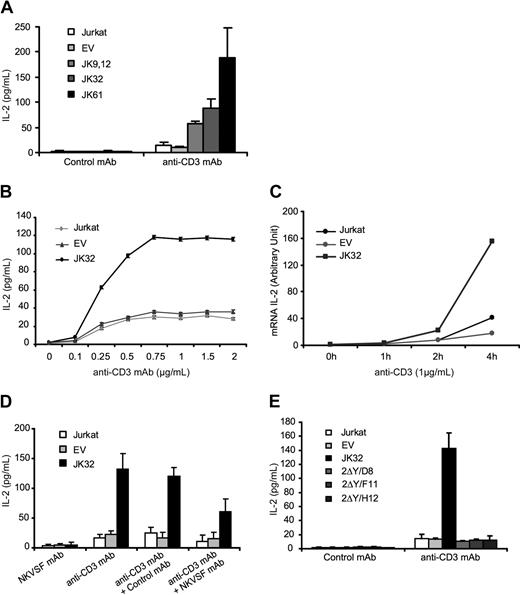
Jurkat cells or EV control cells produced small amounts of IL-2 (< 20 pg/mL) after TCR triggering with optimal concentrations of anti-CD3 mAbs (1 μg/mL). KIR2DL1 transfectants (JK 9.12, JK32, and JK61) secreted 5 to 10 times more IL-2 than control cells when stimulated in identical conditions (Figure 2A). Dose-response curves were established, using the JK32 clone, which was used thereafter (Figure 2B). These curves indicated that these cells responded to suboptimal stimulation with anti-CD3 mAbs (< 250 ng/mL), reaching plateau of approximately 750 ng/mL. This amplification was also confirmed at the mRNA level by real-time PCR analysis, which showed the induction of IL-2 transcripts to be much stronger in JK32 cells stimulated by anti-CD3 mAb (Figure 2C). We assessed IL-2 production after anti-CD3 stimulation in the presence of plate-bound anti-KIR, NKVSF mAb, to confirm KIR2DL1 involvement. This mAb significantly decreased IL-2 secretion by JK32 (Figure 2D). Furthermore, no IL-2 secretion was detected for 2ΔY/D8, 2ΔY/F11, and 2ΔY/H12 clones (Figure 2E), indicating that intact ITIMs are required for IL-2 production after TCR stimulation. Thus, after TCR stimulation, KIR2DL1 expression in Jurkat T cells was correlated with higher levels of IL-2 production.
KIR2DL1 enhances TCR-mediated IL-2 production. (A) Jurkat, EV, and Jurkat transfectants (JK9.12, JK32, JK61) were stimulated with 1 μg/mL plate-bound anti-CD3 or control mAb for 6 hours. Culture supernatants were collected, and IL-2 production was determined by ELISA. Error bars represent the SDs of n = 10 experiments. (B) Dose-response curve for IL-2 secretion after TCR triggering with various concentrations of anti-CD3 mAb. (C) IL-2 gene expression in Jurkat, EV, and JK32 cells was assessed by quantitative RT-PCR. Results are representative of 3 independent experiments. Experimental values were normalized with respect to values for 18S RNA. (D) NKVSF mAb or control IgG was coated (or not) with anti-CD3 as in panel A, and IL-2 production was measured. (E) As in panel A, Jurkat, EV, JK32, and mutated KIR2DL1 transfectants (2ΔY/D8, 2ΔY/F11, 2ΔY/H12) were stimulated, and IL-2 production was measured. Error bars represent the SD of n = 3 experiments.
KIR2DL1 enhances TCR-mediated IL-2 production. (A) Jurkat, EV, and Jurkat transfectants (JK9.12, JK32, JK61) were stimulated with 1 μg/mL plate-bound anti-CD3 or control mAb for 6 hours. Culture supernatants were collected, and IL-2 production was determined by ELISA. Error bars represent the SDs of n = 10 experiments. (B) Dose-response curve for IL-2 secretion after TCR triggering with various concentrations of anti-CD3 mAb. (C) IL-2 gene expression in Jurkat, EV, and JK32 cells was assessed by quantitative RT-PCR. Results are representative of 3 independent experiments. Experimental values were normalized with respect to values for 18S RNA. (D) NKVSF mAb or control IgG was coated (or not) with anti-CD3 as in panel A, and IL-2 production was measured. (E) As in panel A, Jurkat, EV, JK32, and mutated KIR2DL1 transfectants (2ΔY/D8, 2ΔY/F11, 2ΔY/H12) were stimulated, and IL-2 production was measured. Error bars represent the SD of n = 3 experiments.
KIR2DL1 engagement inhibits TCR activation in JK32 transfectants
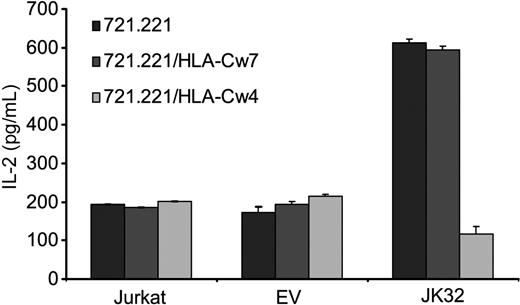
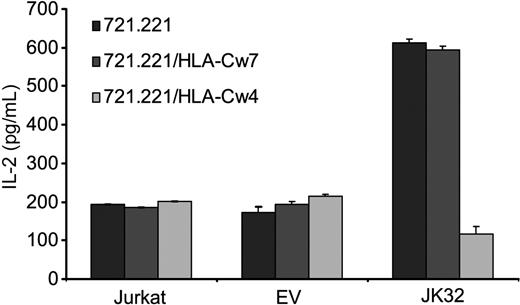
We assessed the effects of KIR2DL1 binding to its specific HLA-C ligand directly, using the 721.221 cell line and its derivative clones transfected with either HLA-Cw4 (721.221/HLA-Cw4) or HLA-Cw7 (721.221/HLA-Cw7), the ligands of KIR2DL1 and KIR2DL2/3, respectively. The 721.221 cell line expresses MHC class II molecules, but not MHC class I molecules, at the cell surface. TCR triggering was optimized by pulsing these cell lines with the superantigen SEE before incubating them for 6 hours with the various T cells. We then quantified IL-2 secretion. JK32 cells produced large amounts of IL-2 (> 550 pg/mL) after stimulation with SEE-pulsed 721.221 and 721.221/HLA-Cw7 cells, well above the values reached with Jurkat and EV control cells (Figure 3). By contrast, levels of IL-2 secretion were much lower (< 150 pg/mL) in response to 721.221/HLA-Cw4 cells expressing the KIR2DL1 ligand. Thus, KIR2DL1 increases TCR-mediated IL-2 production by CD4+ T cells in a ligand-independent manner. Our result also demonstrates that this process is inhibited by the binding of this receptor to its ligand, suggesting that opposite signaling outputs may be driven by KIR2DL1.
KIR2DL1 promotes TCR-induced IL-2 production in a ligand-independent manner. Jurkat, EV, and JK32 cells were incubated with SEE-pulsed 721.221, 721.221/HLA-Cw4, or 721.221/HLA-Cw7 cells for 6 hours. Culture supernatants were collected, and IL-2 production was determined by ELISA. Error bars represent the SDs of n = 3 experiments.
KIR2DL1 promotes TCR-induced IL-2 production in a ligand-independent manner. Jurkat, EV, and JK32 cells were incubated with SEE-pulsed 721.221, 721.221/HLA-Cw4, or 721.221/HLA-Cw7 cells for 6 hours. Culture supernatants were collected, and IL-2 production was determined by ELISA. Error bars represent the SDs of n = 3 experiments.
Tyrosine phosphorylation and synaptic accumulation of KIR2DL1 are affected by HLA-Cw4 recognition
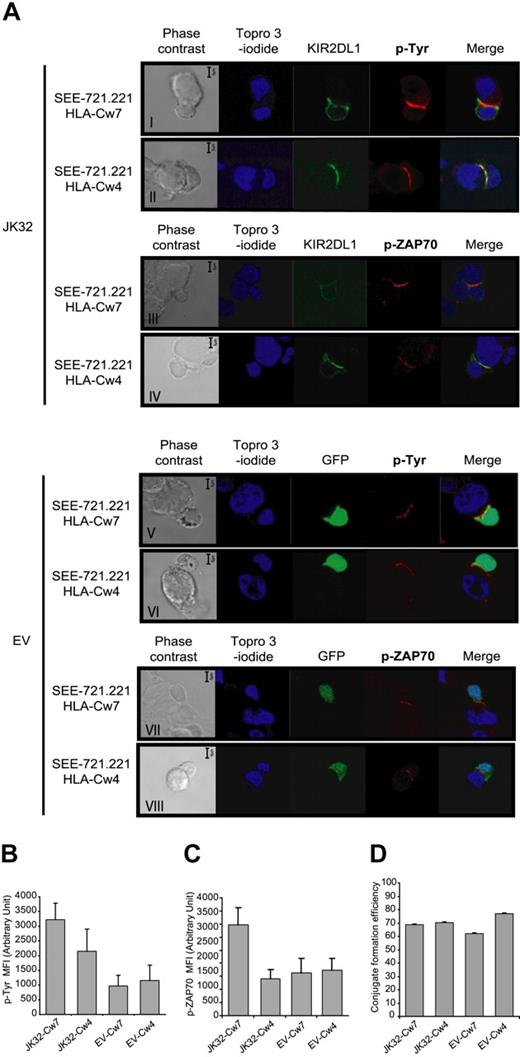
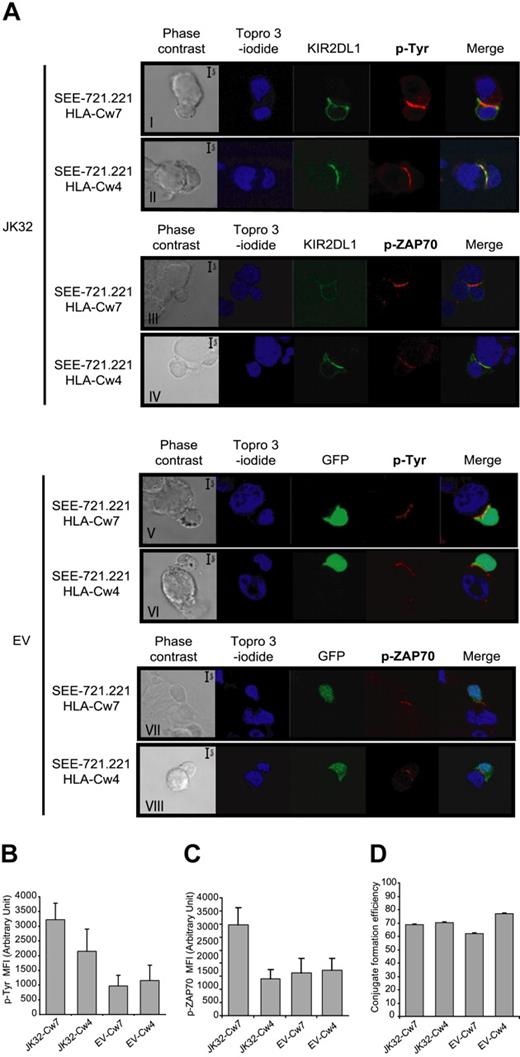
Confocal microscopic analysis of conjugates was used to follow synapse formation between KIR2DL1+ T cells and various SEE-pulsed 721.221 cell lines expressing or not the cognate ligand, HLA-Cw4. Tyrosine phosphorylation levels and KIR2DL1 distribution were followed in parallel (Figure 4A). KIR2DL1-GFP accumulated and was colocalized with p-Tyr at the immunologic synapse only if JK32 cells were incubated with 721.221/HLA-Cw4-expressing cells (Figure 4Aii). These conjugates displayed significant, but much lower, levels of p-Tyr accumulation at the synapse than conjugates between JK32 and 721.221/HLA-Cw7 cells, in which no recruitment of KIR2DL1 to the synapse was observed (Figure 4Ai). Conjugates between EV cells and the various 721.221 cell lines also displayed a weaker p-Tyr signal at the synapse than JK32–721.221/HLA-Cw7 conjugates (Figure 4Av). In addition, we investigated ZAP70 phosphorylation (p-ZAP70) (Figure 4A). A high level of p-ZAP70 was observed in JK32–721.221/HLA-Cw7 conjugate (Figure 4Aiii). Interestingly, we observed a weak level of p-ZAP70 in JK32–721.221/HLA-Cw4 conjugate as in EV conjugates (Figure 4Aiv-viii). Thus, the difference of p-Tyr level at the synapse of JK32–721.221/HLA-Cw4 was probably because of TCR-signal in addition to ITIMs phosphorylation. A quantitative analysis on numerous conjugates is shown for p-Tyr and p-ZAP70 in Figure 4B,C. Finally, similar numbers of conjugates formed between JK32 or EV and 721.221/HLA-Cw7 or 721.221/HLA-Cw4 cell lines, indicating that KIR2DL1 receptor had no impact on conjugate formation (Figure 4D). Thus, experimental conditions in which KIR2DL1 is expressed but does not bind to its cognate ligand seem to enhance the signals triggered at the immune synapse after antigen recognition.
Confocal microscopic analysis of the distribution of p-Tyr, p-ZAP70, and KIR2DL1 in EV and JK32 cells conjugated with SEE-pulsed 721.221/HLA-Cw7 or 721.221/HLA-Cw4 cells. (A) JK32 and EV cells were incubated in a 1:1 ratio with 721.221/HLA-Cw7 or 721.221/HLA-Cw4 cells for 20 minutes to ensure optimal conjugate formation. Conjugates were fixed and processed for immunofluorescence staining of p-Tyr with 4G10 or p-ZAP70 (red fluorescence) and nuclei with TO-PRO-3 iodide (blue fluorescence). KIR2DL1 and GFP appear as green fluorescence. All confocal pictures are representative of 3 experiments with at least 50 contact areas analyzed in each experiment. Bars represent 5 μm. (B,C) Cells were prepared and treated as described in panel A, and images of 10 fields were visualized with a 63×/1.4 oil-immersion objective. Signal intensity for p-Tyr and p-Zap70 was quantified with ImageJ software. (D) Efficiency of conjugate formation between 721.221/HLA-Cw7 or 721.221/HLA-Cw4 and EV or JK32 cells was calculated by determining the ET ratio × 100, as described in “Confocal microscopy.” Data are expressed as the mean plus or minus SD of 10 fields.
Confocal microscopic analysis of the distribution of p-Tyr, p-ZAP70, and KIR2DL1 in EV and JK32 cells conjugated with SEE-pulsed 721.221/HLA-Cw7 or 721.221/HLA-Cw4 cells. (A) JK32 and EV cells were incubated in a 1:1 ratio with 721.221/HLA-Cw7 or 721.221/HLA-Cw4 cells for 20 minutes to ensure optimal conjugate formation. Conjugates were fixed and processed for immunofluorescence staining of p-Tyr with 4G10 or p-ZAP70 (red fluorescence) and nuclei with TO-PRO-3 iodide (blue fluorescence). KIR2DL1 and GFP appear as green fluorescence. All confocal pictures are representative of 3 experiments with at least 50 contact areas analyzed in each experiment. Bars represent 5 μm. (B,C) Cells were prepared and treated as described in panel A, and images of 10 fields were visualized with a 63×/1.4 oil-immersion objective. Signal intensity for p-Tyr and p-Zap70 was quantified with ImageJ software. (D) Efficiency of conjugate formation between 721.221/HLA-Cw7 or 721.221/HLA-Cw4 and EV or JK32 cells was calculated by determining the ET ratio × 100, as described in “Confocal microscopy.” Data are expressed as the mean plus or minus SD of 10 fields.
The association of SHP-2 with KIR2DL1 does not require receptor engagement
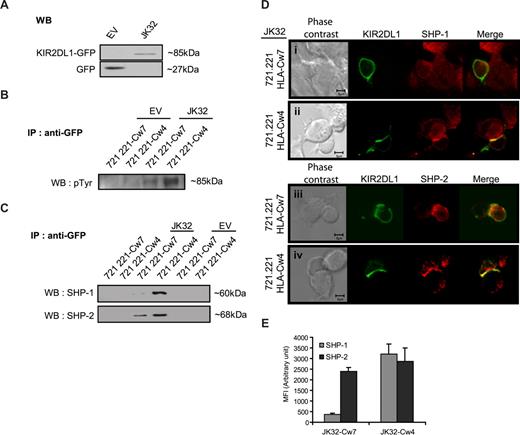
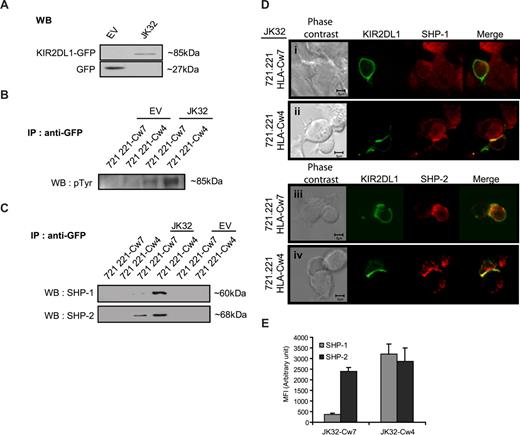
Immunoprecipitation experiments were then carried out to identify the signaling molecules recruited by KIR2DL1 cytoplasmic domains (ITIMs). An anti-GFP antibody was used to precipitate the KIR2DL1-GFP fusion protein (∼85 kDa) from JK32 cells and GFP (27 kDa) from EV control cells (Figure 5A). JK32 and EV cells were then stimulated with SEE-pulsed 721.221/HLA-Cw7 or 721.221/HLA-Cw4 cells, before GFP immunoprecipitation and Western blotting. The triggering of KIR2DL1 with HLA-Cw4 ligand induced strong tyrosyl-phosphorylation of KIR2DL1-ITIMs (Figure 5B). This phosphorylation was weak but detectable in JK32 cells stimulated with 721.221/HLA-Cw7 target cells (Figure 5B). The recruitment of SHP-1 and/or SHP-2 to KIR2DL1-ITIMs was also assessed. KIR2DL1 recruited both SHP-1 and SHP-2 when bound to its ligand (Figure 5C). However, SHP-2 was also recruited by KIR2DL1-ITIMs in a ligand-independent manner (Figure 5C). Confocal microscopy analyses were carried out for further analyses of the differential recruitment of SHP-1 and SHP-2 (Figure 5D). In JK32–721.221/HLA-Cw4 conjugates, SHP-1 and SHP-2 were redistributed at the synapse, whereas only SHP-2 was present in JK32 stimulated by SEE-pulsed 721.221/HLA-Cw7 targets (Figure 5D,E). Furthermore, we noticed a comparable amount of SHP-2 relocalized within the synapse between JK32-721.221/HLA-Cw7 or -Cw4 conjugates (Figure 5E). These results establish that KIR2DL1 differentially recruits SHP-1 and SHP-2, as a function of ligand binding. They further support the hypothesis that SHP-2 is required for activation of IL-2 production.
KIR2DL1 recruits SHP-1 and/or SHP-2 differently, depending on whether it is bound to its ligand. (A) Total protein lysates from JK32 and EV cells were blotted with anti-GFP mAb: KIR2DL1-GFP fusion protein and GFP are identified at 85 and 27 kDa, respectively. (B) EV and JK32 T cells were stimulated with SEE-pulsed 721.221/HLA-Cw4 or 721.221/HLA-Cw7 cells for 20 minutes and lysed, and GFP was immunoprecipitated. GFP immunoprecipitates (IP) were analyzed by immunoblotting with antiphosphotyrosine (p-Tyr) antibodies, or (C) the membrane was probed with anti–SHP-1 and anti–SHP-2 mAbs. (D) The distribution of SHP-1 and SHP-2 (red fluorescence) was analyzed by confocal microscopy, as detailed in Figure 4. KIR2DL1 appears as green fluorescence. (E) Cells were treated as described in panel D, and images of 5 fields were visualized with a 63×/1.4 oil-immersion objective. Signal intensity was quantified with ImageJ software.
KIR2DL1 recruits SHP-1 and/or SHP-2 differently, depending on whether it is bound to its ligand. (A) Total protein lysates from JK32 and EV cells were blotted with anti-GFP mAb: KIR2DL1-GFP fusion protein and GFP are identified at 85 and 27 kDa, respectively. (B) EV and JK32 T cells were stimulated with SEE-pulsed 721.221/HLA-Cw4 or 721.221/HLA-Cw7 cells for 20 minutes and lysed, and GFP was immunoprecipitated. GFP immunoprecipitates (IP) were analyzed by immunoblotting with antiphosphotyrosine (p-Tyr) antibodies, or (C) the membrane was probed with anti–SHP-1 and anti–SHP-2 mAbs. (D) The distribution of SHP-1 and SHP-2 (red fluorescence) was analyzed by confocal microscopy, as detailed in Figure 4. KIR2DL1 appears as green fluorescence. (E) Cells were treated as described in panel D, and images of 5 fields were visualized with a 63×/1.4 oil-immersion objective. Signal intensity was quantified with ImageJ software.
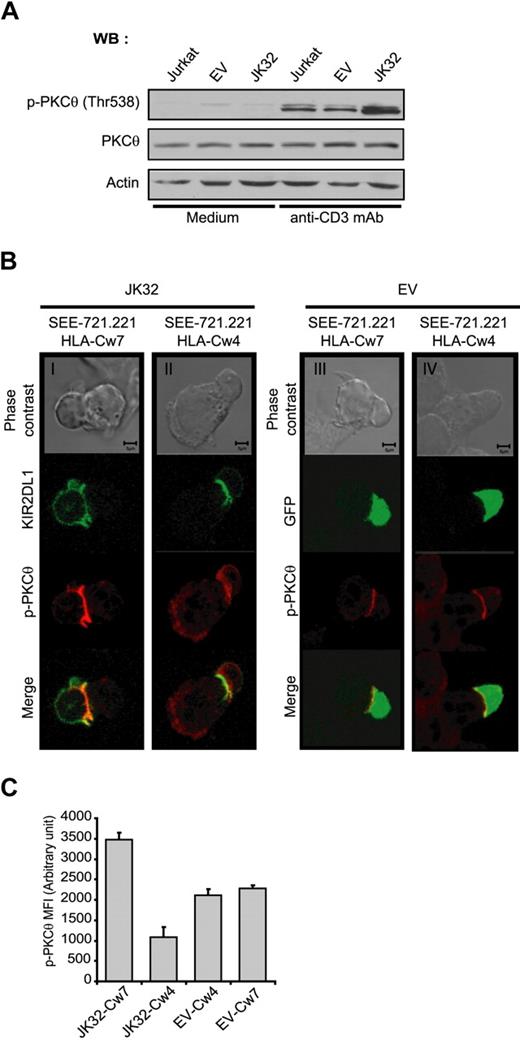
TCR-activation in JK32 enhances PKC-θ phosphorylation and recruitment to the synapse
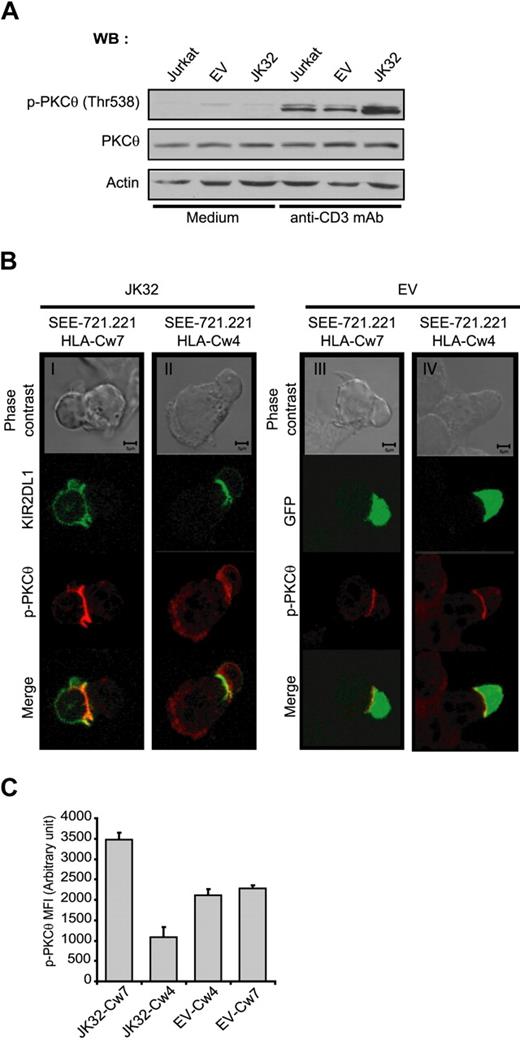
PKC-θ is a key signaling molecule in TCR-mediated activation for IL-2 production. We investigated the involvement of this molecule in the costimulatory effect of KIR2DL1. We assessed the phosphorylation of PKC-θ in EV and JK32 cells stimulated with anti-CD3 mAbs. Phospho-PKC-θ (p-PKC-θ) level was more strongly detected after TCR activation in JK32 cells than in EV cells (Figure 6A). Strong synaptic accumulation of p-PKC-θ was also observed in JK32 cells conjugated with SEE-pulsed 721.221/HLA-Cw7 (Figure 6Bi). This selective accumulation was not observed when JK32 cells were stimulated with 721.221/HLA-Cw4; instead, diffuse cytoplasmic labeling was detected (Figure 6Bii). A weak but significant p-PKC-θ signal was detected at the synapse in EV transfectants stimulated with 721.221/HLA-Cw7 and 721.221/HLA-Cw4 cell lines (Figure 6Biii,iv). This result was confirmed by a quantitative analysis on conjugates for synaptic level of PKC-θ in Figure 6C. These data suggest that PKC-θ activation plays a key role in the ligand-independent increase in IL-2 production induced by KIR2DL1 after TCR triggering.
Involvement of PKC-θ in the costimulatory effect of KIR2DL1 in human T cells. (A) Jurkat, EV, and JK32 cells were stimulated with mAb for 15 minutes. Cell lysates were analyzed by immunoblotting with mAbs against p-PKC-θ, PKC-θ, and actin. (B) Confocal microscopic analysis of the distribution of p-PKC-θ and KIR2DL1 in EV and JK32 cells conjugated with SEE-pulsed 721.221/HLA-Cw7 or 721.221/HLA-Cw4 cells. The distribution of p-PKC-θ (in red) was analyzed as in Figure 4. KIR2DL1 appears as green fluorescence. (C) Cells were treated as described in panel B, and images of 5 fields were visualized with a 63×/1.4 oil-immersion objective. Signal intensity was quantified with ImageJ software.
Involvement of PKC-θ in the costimulatory effect of KIR2DL1 in human T cells. (A) Jurkat, EV, and JK32 cells were stimulated with mAb for 15 minutes. Cell lysates were analyzed by immunoblotting with mAbs against p-PKC-θ, PKC-θ, and actin. (B) Confocal microscopic analysis of the distribution of p-PKC-θ and KIR2DL1 in EV and JK32 cells conjugated with SEE-pulsed 721.221/HLA-Cw7 or 721.221/HLA-Cw4 cells. The distribution of p-PKC-θ (in red) was analyzed as in Figure 4. KIR2DL1 appears as green fluorescence. (C) Cells were treated as described in panel B, and images of 5 fields were visualized with a 63×/1.4 oil-immersion objective. Signal intensity was quantified with ImageJ software.
IL-2 production is also increased in KIR2DL1-transfected CD4+ T cells
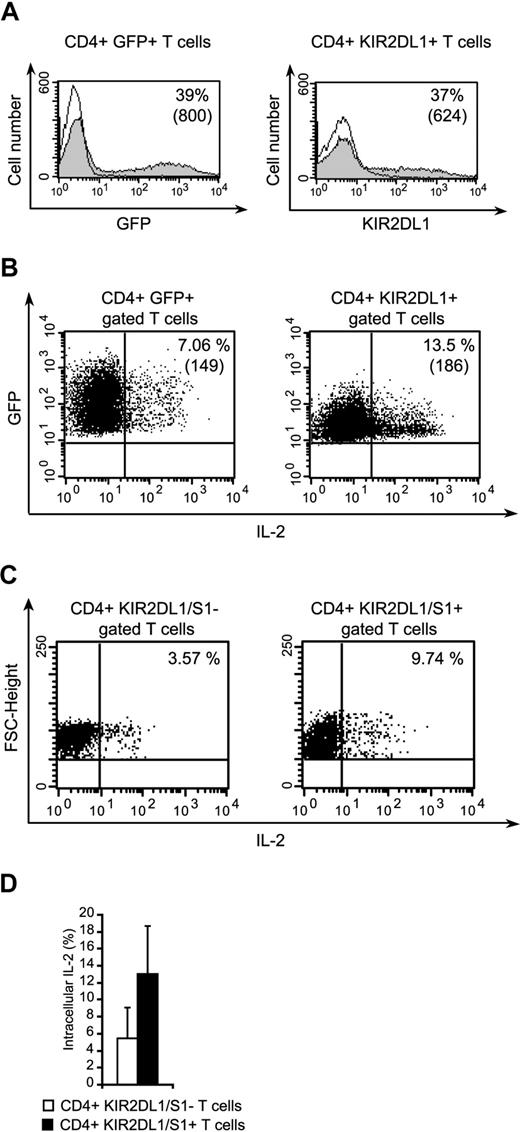
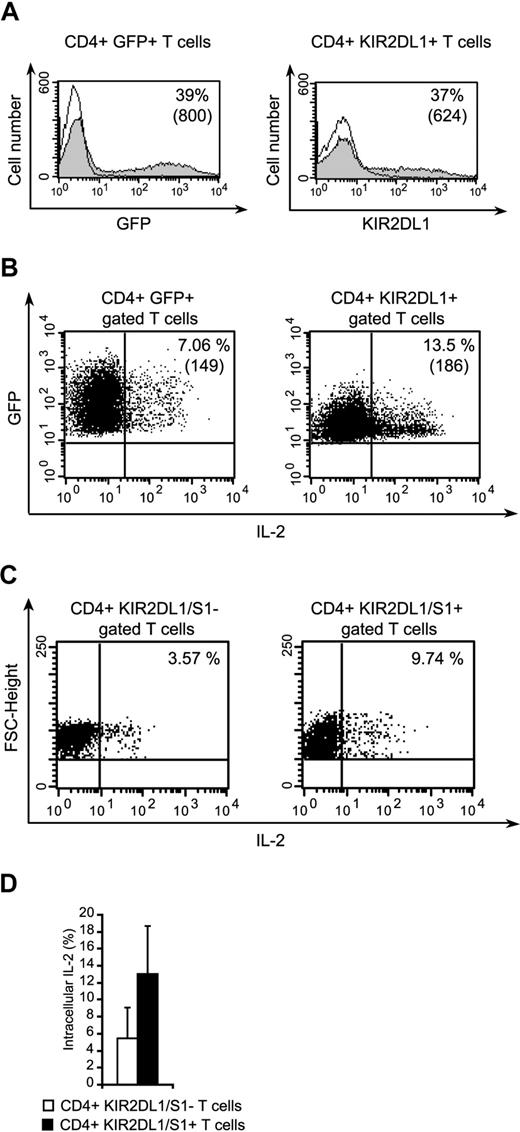
We investigated whether the costimulatory role of KIR2DL1 extended to T cells, by isolating peripheral blood CD4+ T cells and transfecting them with KIR2DL1. Transfection efficiency for the KIR2DL1 vector, GFP empty vectors ranged from 25% to 40% in 3 independent experiments (Figure 7A). IL-2 production by the transfected cells stimulated with anti-CD3 and anti-CD28 mAb-coated beads was evaluated by flow cytometry. A significantly higher percentage of CD4+ KIR2DL1+ T cells than that of CD4+ GFP+ T cells produced IL-2 (Figure 7B). In 3 independent experiments, the ratio of IL-2–producing CD4+ KIR2DL1+ cells to IL-2–producing CD4+ GFP+ cells was 2.5 plus or minus 0.44. These findings confirm that KIR2DL1 plays a costimulatory role in CD4+ T cells. Furthermore, we analyzed peripheral CD4+ T cells naturally expressing KIR2DL1/S1 for IL-2 production after anti-CD3 and anti-CD28 mAb-coated beads (Figure 7C). These cells produced twice more IL-2 than CD4+ KIR2L/S1− T cells (Figure 7D, n = 2).
The transfection of primary CD4+ T cells with KIR2DL1 enhances IL-2 production. (A) Expression of GFP and KIR2DL1 by naive CD4+ T cells transfected with pEGFP-N1/KIR2DL1 or pEGFP-N1. The percentages of positive cells are indicated. Numbers in parentheses correspond to mean fluorescence intensities. (B) Detection of IL-2 in CD3+CD28+stimulated CD4+/KIR2DL1+ or CD4+/GFP+ T cells. The percentages of positive cells are indicated, and mean fluorescence intensities are indicated in parentheses. (C) Immunoselected CD4+ T cells were stimulated as in panel B, stained with KIR2DL1/S1 mAbs and anti–IL-2 mAb. (D) Percentages of CD4+ KIR2DL1/S1+ or CD4+ KIR2DL1/S1− secreting IL-2 after CD3+CD8+ stimulation are shown (mean ± SD, n = 2).
The transfection of primary CD4+ T cells with KIR2DL1 enhances IL-2 production. (A) Expression of GFP and KIR2DL1 by naive CD4+ T cells transfected with pEGFP-N1/KIR2DL1 or pEGFP-N1. The percentages of positive cells are indicated. Numbers in parentheses correspond to mean fluorescence intensities. (B) Detection of IL-2 in CD3+CD28+stimulated CD4+/KIR2DL1+ or CD4+/GFP+ T cells. The percentages of positive cells are indicated, and mean fluorescence intensities are indicated in parentheses. (C) Immunoselected CD4+ T cells were stimulated as in panel B, stained with KIR2DL1/S1 mAbs and anti–IL-2 mAb. (D) Percentages of CD4+ KIR2DL1/S1+ or CD4+ KIR2DL1/S1− secreting IL-2 after CD3+CD8+ stimulation are shown (mean ± SD, n = 2).
Discussion
The inhibitory KIRs recognize MHC class I molecules and negatively regulate the activation of NK cells and CD8+ T-cell memory effectors. Their role in human CD4+ T cells is largely unknown. Our study provides new insight into the regulation of CD4+ T cells by KIR2DL1. Because only a small subset of CD4+ KIR+ T cells is present in healthy persons, we obtained stable KIR2DL1 transfectants from Jurkat T cells and studied their signaling in response to TCR activation. We found that TCR activation greatly increased IL-2 production in KIR2DL1-transfected T cells, requiring intact ITIMs independently of KIR2DL1 engagement. The TCR-induced overproduction of IL-2 is inhibited by the binding of KIR2DL1 to its ligand, consistent with the expected decrease in TCR signaling after KIR2DL1 engagement. Thus, the KIR2DL1 expressed by CD4+ T cells acts as a costimulatory receptor in a ligand-independent manner. These results are original and differ from the previous reports concerning KIR2DL CD8+ or CD4+ T cells. Indeed, NK receptors clearly inhibit the TCR activation of CD8+ T cells24-26 and contribute to the anergy of tumor-infiltrating lymphocytes.17 A few studies have reported the transfection of CD8+ T cells with inhibitory KIRs. Bakker et al showed that the transfection of T cells with KIR3DL1 led to strong inhibition of HLA-Bw4+ cell lysis but had no effect on HLA-Bw4–negative cells.27
We cannot rule out the possibility that our findings relate to CD4+ T cells and not to CD8+ T cells. Compared with NK and CD8+ T cells, CD4+ T cells underexpressed KIR2DL1.13,18 According to Anfossi et al,28 among CD3+ KIR+ cells, an average of 7% plus or minus 3% expressed CD4, whereas another study demonstrated a lower percentage (0.2%) of CD4+ T cells stained with KIR Abs in healthy donors.18 In addition, a recent functional study showed that KIR2DS2 costimulates TCR activation through the JNK pathway in CD4+ T cells, whereas it directly stimulates T cells via KARAP/DAP12 when bound to its ligand, indicating that the type of signaling induced by KIR depends on the adaptor molecule recruited in T cells.29 Henel et al recently demonstrated that KIR2DL2 is recruited at later stage to the central supramolecular activation cluster (cSMAC), indicating that this molecule inhibits late TCR signaling events.30 Furthermore, transfection of Jurkat T cells with KIR3DL1 resulted in the inhibition of Fas-mediated AICD independently of the binding of KIR to its ligand.31 Most studies have suggested that KIR2DL plays an inhibitory role in T cells, but a recent study in malignant CD4+ T cells from patients with Sezary syndrome established that KIR2DL1 and KIR2DL2 can activate the JNK pathway in a DAP12-independent manner.23 The data obtained suggested that stimulatory and inhibitory KIR receptors function differently in T and NK cells. KIR can recruit different molecules and transduce signals independently of their ligand-binding status. Our findings further demonstrate an intense costimulatory role of KIR2DL in CD4+ T cells and describe a mechanism that may be responsible for this effect. The TCR-mediated overproduction of IL-2 in CD4+ KIR2DL1+ T cells follows the amplification of TCR signaling by inhibitory KIR in a ligand-independent manner. We show that KIR2DL1-ITIMs are phosphorylated and recruit SHP-2, but not SHP-1 in the absence of KIR2DL1 binding to its ligand. In contrast, after TCR and KIR2DL1 coengagement, both SHP-1 and SHP-2 were recruited, leading to inhibitory signals. Our results confirm those of a recent study on another inhibitory receptor, programmed death 1 (PD-1), which has an ITIM and an immunoreceptor tyrosine-based switch motif (ITSM) in its cytoplasmic tail. In CD4+ T cells, the binding of PD-1 to its ligand blocks T-cell activation through the recruitment of SHP-1 and SHP-2. Interestingly, Chemnitz et al32 showed that SHP-2 was associated with PD-1 in TCR-activated T cells, even when PD-1 was not bound to its ligand. However, despite this association with SHP-2, the binding of PD-1 to its ligands was required to block T-cell activation.32 It was also shown that SHP-2 is recruited by KIR2DL4, whereas its ITIM is not phosphorylated.33 Thus, SHP-2 may be recruited in the absence of binding of the receptor to its ligand, and we show here that this may lead to T-cell activation rather than inhibition. The exact mechanism by which SHP-2 controls IL-2 production in CD4+ KIR+ T cells requires further investigations. One hypothesis is that SHP-2 could be sequestrated outside the synapse in absence of KIR ligation or enhances TCR signal.
Different protein tyrosine phosphatases have different functions, and the role of SHP-2 is not well characterized. Unlike SHP-1, SHP-2 does not always function as a negative regulator of TCR signaling. It has been reported to activate the Ras/Raf/MEK/ERK pathway via dephosphorylation of specific substrates.34,35 SHP-2 has been implicated in ERK-mediated activation by cytokine receptors and tyrosine kinase receptors.36,37 It also promotes Src family kinase (SFK) activation by regulating the phosphorylation of the Csk regulator PAG/cbp, thereby controlling the access of Csk to SFKs.9 These effects of SHP-2 are consistent with our results showing IL-2 overproduction after the recruitment of SHP-2 to KIR2DL1-ITIMs, as the Ras/ERK pathway induces IL-2 secretion via AP1/NFAT.38,39
T-cell activation requires the correct assembly and formation of the T-cell supramolecular activation cluster (SMAC) or synapse formation between immune effector and target cells. We found that extensive redistribution of KIR2DL1, p-Tyr, and SHP-1/SHP-2 at the synapse after TCR and KIR2DL1 coengagement inhibited IL-2 production. When not bound to its ligand, KIR remains uniformly distributed on the cell membrane, tyrosine and ZAP70 phosphorylation levels are high, and only SHP-2 is found at the synapse. We also found that and PKC-θ was activated and recruited to the synapse. PKC-θ is the only PKC isoform capable of stimulating AP-1 and JNK activity, which in turn activate the IL 2 gene.40,41 In addition, PKC-θ is translocated to the immunologic synapse, unlike any other PKC isoform.42 The observation that KIR2DL1 strongly affects p-Tyr and p-PKC-θ levels and polarization is consistent with the overproduction of IL-2 in JK32-721.221/HLA-Cw7 conjugates.
In conclusion, we have demonstrated that, by recruiting different phosphatases, KIR2DL1 may exert opposite functions in CD4+ T cells. KIR2DL1, in association with SHP-1 and SHP-2, after the coengagement of TCR and KIR2DL1, acts as an inhibitory receptor. In contrast, KIR2DL1 associated with SHP-2 only costimulates IL-2 production in CD4+ T cells. This model of KIR action should facilitate further dissection of the molecular mechanisms by which KIR2DL1 modulates T-cell activation in Jurkat cells and primary human CD4+ T cells. Such studies should increase our knowledge of the function of CD4+ T cells expressing KIR2DL1 and their pathogenic role in autoimmunity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Y. Lecluse for advice on FACS analyses and C. Richon for her help with quantitative RT-PCR analysis.
This work was supported by grants from Inserm, l'Institut National du Cancer (INCa), La Ligue Nationale contre le Cancer (comité de Paris), and l'Association Laurette Fugain.
Authorship
Contribution: E.F.-N. and A.C. designed and analyzed all experiments; E.F.-N., G.B., S.C., and A.C. wrote the paper; and S.D.R., A.J., and C.P. performed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Caignard, Inserm U567, Institut Cochin, 24, rue du Faubourg Saint Jacques, 75014, Paris, France; e-mail: anne.caignard@inserm.fr.