To the editor:
We read with interest the recent article by Hoster and colleagues from the German Low Grade Lymphoma Study Group (GLSG) and the European Mantle Cell Lymphoma Network regarding a new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma.1
The MIPI was generated via a complex mathematical model. A simplified MIPI was subsequently generated with a point score system (low risk: 0-3; intermediate risk: 4-5; high risk: 6-11). Although the authors stated a high concordance between the MIPI and simplified MIPI(weighted kappa 0.79), no overall survival curves nor median time of overall survival were provided for the simplified MIPI.1
The MIPI score was also generated based on 455 patients from 3 randomized trials receiving heterogenous treatment regimens including an inferior regimen of CHOP (cyclophosphamide, daunorubicin, vincristine, and prednisone), MCP (mitoxantrone, chlorambucil, prednisone), Rituximab-CHOP, autologous stem cell transplant, and interferon-alpha maintenance therapy in some patients.
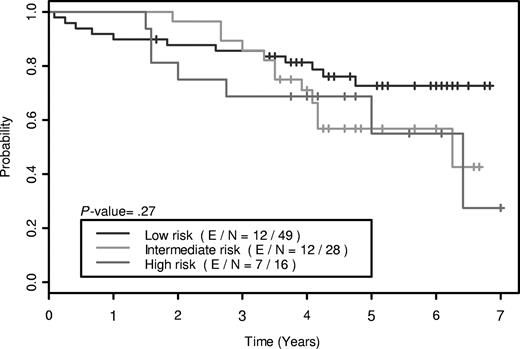
We have previously reported on a phase 2 trial of rituximab plus fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (R-hyper-CVAD) alternating every 21 days with rituximab plus high-dose methotrexate-cytarabine(MTX-AraC) and a recent update with 5 years of follow-up.2,3 Among the 97 assessable patients (blastic variant, n = 14), the overall response rate was 97%, and a complete response (CR)/unconfirmed CR rate of 87%. We attempted to validate the simplified MIPI in these cohorts of patients treated homogenously with this regimen in the clinical trial.
Using the simplified MIPI prognostic index, the Kaplan-Meier method was used to generate the overall survival curves, and the log-rank test was used to test the difference in overall survival (OS) between patient groups. The OS curves are shown in Figure 1, and demonstrate no significant difference in OS between patients with low-risk (0-3 points), intermediate-risk (4-5 points), and high-risk (6-11 points) scores (P = .27). We attempted to combine patients with intermediate- and high-risk scores into a single group and compare with the low-risk group to improve the ability of the model to differentiate patients with low-risk as opposed to high-risk features. This also did not demonstrate a significant difference between the 2 cohorts (P = .11).
A major limitation in the generation of the simplified MIPI index by Hoster et al was the use of a heterogenous group of patients. The second major limitation was the lack of a second separate cohort to prospectively validate the index that was not described in the article. We were not able to validate the simplified MIPI in a homogenous group of patients treated with a more intensive regimen of R-Hyper-CVAD alternating with MTX-AraC, suggesting a more intensive treatment may overcome the high risk features described by Hoster and the MIPI is not applicable for the majority of patients treated with this current standard of care regimen.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jatin J. Shah, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 429, Houston, TX 77030; e-mail: jjshah@mdanderson.org.