Abstract
We have explored the potential role of genetics in the development of osteonecrosis of the jaw (ONJ) in multiple myeloma (MM) patients under bisphosphonate therapy. A genome-wide association study was performed using 500 568 single nucleotide polymorphisms (SNPs) in 2 series of homogeneously treated MM patients, one with ONJ (22 MM cases) and another without ONJ (65 matched MM controls). Four SNPs (rs1934951, rs1934980, rs1341162, and rs17110453) mapped within the cytochrome P450-2C gene (CYP2C8) showed a different distribution between cases and controls with statistically significant differences (P = 1.07 × 10−6, P = 4.231 × 10−6, P = 6.22 × 10−6, and P = 2.15 × 10−6, respectively). SNP rs1934951 was significantly associated with a higher risk of ONJ development even after Bonferroni correction (P corrected value = .02). Genotyping results displayed an overrepresentation of the T allele in cases compared with controls (48% vs 12%). Thus, individuals homozygous for the T allele had an increased likelihood of developing ONJ (odds ratio 12.75, 95% confidence interval 3.7-43.5).
Introduction
Impaired bone remodeling and bone destruction is a typical hallmark in multiple myeloma (MM).1,2 Bisphosphonates (BPs) are antiresorptive agents currently used to treat MM bone disease, but they are probably involved in the development of osteonecrosis of the jaw (ONJ).3,4 How this process occurs is unclear but it has been attributed to a reduction or loss of vascular supply, or to impaired bone resorption associated with specific circumstances at the oral cavity, such as bone remodeling in the maxillae, frequent trauma, and the presence of oral saprophytic flora.5
Risk factors for ONJ development include dental extractions, thalidomide therapy, zoledronic versus pamidronate use, and long term exposure to BP therapy.6-8 However, like many other complex trait diseases, ONJ can be caused by a combination of environmental and genetic risk factors. Genetic susceptibility may be conferred by multiple genes with small variations.9 Genome-wide association studies are an attractive tool that enable the screening of thousands of single nucleotide polymorphisms (SNP).10,11 We conducted a genome-wide association study, typing 500 568 SNPs in 2 groups of MM patients who had received the same antimyeloma therapy, but who presented a significant difference: the presence or absence of ONJ.
Methods
The study was approved by the Institutional Review Board of the University Hospital of Salamanca and the Scientific and Ethics Committee of the Programa para el Estudio de la Terapéutica en Hemopatías Malignas (PETHEMA) group. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. We studied 22 cases (MM with ONJ) and 65 controls (MM without ONJ), matched for age, sex, and ethnicity. All patients were enrolled in the Grupo Español de Mieloma (GEM)–2000 protocol, which consists of polychemotherapy and autologous transplantation.12 All received BP therapy, either pamidronate (16 cases, 57 controls) or zoledronic acid (6 cases, 8 controls) planned for 2 years (median 22 months, range 9-24 months). Clinical characteristics were similar between controls and cases. Study protocols were approved by the ethics committee and written informed consent was obtained from all participants.
Each patient was genotyped using the Affymetrix GeneChip Mapping 500K set of microarrays (Affymetrix, Santa Clara, CA) according to the manufacturer's recommendations. Genotypes were determined using the BRLMM (Bayesian Robust Linear Model with Mahalanobis distance classifier) algorithm13 with cases and controls undergoing joint cluster analysis,14 after ensuring a robust association test through quality filtering tests. Based on stringent quality control criteria, a total of 339 972 SNPs were selected for subsequent analyses. Criteria for exclusion were (1) call rate less than 90%, (2) minor allele frequency less than 5%, and (3) deviations from Hardy-Weinberg equilibrium with a P less than 10−5. Sexual chromosomes were excluded for analysis.15 The microarray data were deposited into Gene Expression Omnibus (GEO) under accession number GSE11948.16
To test for allelic associations between SNPs and ONJ, we constructed 2×2 contingency tables and compared using the 2-sided Fisher exact or χ2 tests through SPSS software (SPSS 14.0, Chicago, IL). P values were corrected (Pc) using the Bonferroni correction. The strength of association was estimated by the odds ratio (OR), and their 95% confidence intervals (CI) were calculated by Cornfield methods. Linkage disequilibrium between SNPs was analyzed using Arlequin software (http://anthro.unige.ch/arlequin).
Results and discussion
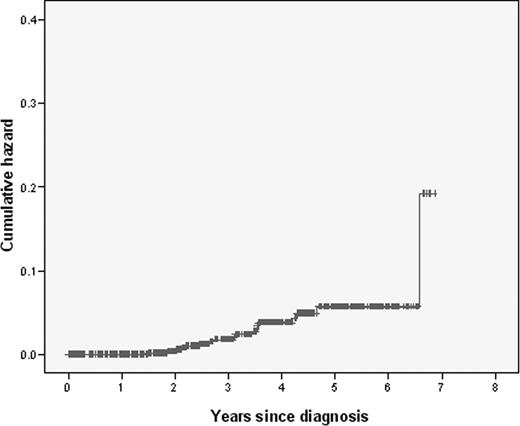
A total of 675 of the patients included in the Spanish GEM-2000 protocol had bone disease and received BP therapy.12 With a median follow-up of 64 months, 24 patients (3.6%) had developed ONJ and 651 had not, providing an accumulation incidence of 5.6% at 5 years (Figure 1). All clinical and biologic characteristics of these patients at diagnosis and their response to therapy were homogeneous. The 5-year overall survival (OS) and progression free survival (PFS) were similar among cases and controls (82% and 42% vs 87% and 51%, respectively).
Cumulative hazard of developing osteonecrosis of the jaw in this series of MM patients (N = 675).
Cumulative hazard of developing osteonecrosis of the jaw in this series of MM patients (N = 675).
Four SNPs showed an association with ONJ: rs1934951, rs1934980, rs1341162, and rs17110453. All of them mapped within the cytochrome P450, subfamily 2C polypeptide 8 gene (CYP2C8) which spans a 31 Kb region sited at the cytochrome P450 gene cluster, chromosome 10q23.
The SNP rs1934951 (intron 8) was significantly associated with ONJ (overall P value = 1.07 × 10−6; OR 12.75, 95% CI: 3.7-43.5) even after Bonferroni correction (Pc = .02; Table 1). This SNP was successfully genotyped in 85 of 87 individuals (21 cases and 64 controls) and the results showed an overrepresentation of the T allele among cases (48% vs 12%). Furthermore, the genotype frequency of this SNP varied drastically between the case and control populations (Table 1). Thus, the heterozygous genotype CT was present in 66% of the cases versus 25% of the controls, whereas the homozygous genotype CC was more frequent in the control population: 75% versus 20%. Individuals homozygous for the T allele were exclusively present within the ONJ group (14% vs 0%).
The SNPs rs1934980 and rs1341162 (intron 5), and rs17110453 (5′ gene region), showed a significant correlation with ONJ presentation (P = 4.23 × 10−6, P = 6.22 × 10−6 and P = 2.15 × 10−6, respectively), although the Bonferroni correction was not statistically significant (Pc = .09, Pc = .13 and Pc = .46, respectively; Table 1).
Haplotype analysis (Table 2) revealed a linkage disequilibrium across CYP2C8 locus between intron 8 and intron 5 (data not shown). When considering the rs1934951, rs1934980, and rs1341162 SNPs, there was a different distribution between cases and controls for the 2 main haplotypes: CAC (in 50% vs 83% of cases and controls, respectively) and TGT (in 45% vs 10%, respectively; Table 2).
Overall, our results demonstrate differences in the allele and genotype frequencies between cases and controls, especially in the SNP rs1934951 in CYP2C8 gene (intron 8). Furthermore, we found differences in 3 additional SNPs mapped within this gene: rs1934980 and rs1341162 (intron 5) and rs17110453 (promoter region). Although all these SNPs are placed in intronic regions, it should be noted that not only polymorphisms in the open reading frame are capable of modifying the function of a given gene. Intronic polymorphisms could also disturb the function by affecting the promoter region,17 splicing sites,18 or intronic microRNAs.19
CYP2C8 is responsible for the metabolism of several drugs (paclitaxel, cerivastatin, or repaglinide).20 Recent reports have shown that variability in genes encoding CYP2C8 may affect drug pharmacokinetics.21 However, because BPs do not suffer any physical-chemical modification, polymorphisms on CYP2C8 would not play a role in their metabolism. CYP2C8 gene polymorphisms can affect several biologic pathways,20 which could be involved in the development of ONJ in patients treated with BPs. CYP2C8 metabolizes arachidonic acid (AA) to epoxyeicosatrienoic acids,22 which play a key role in the regulation of vascular tone and cardiovascular homeostasis.23 Because ONJ is an avascular necrosis of the jawbone, the alteration of this pathway due to a variant of CYP2C8 could make development of osteonecrosis more likely. Moreover, CYP2C8 is also involved in the initiation of the 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoAR) pathway, a biologic key metabolic cascade for cholesterol synthesis which apparently plays a relevant role in osteoblastic differentiation.24 In addition, BPs taken up by osteoclasts inhibit farnesyl pyrophosphate synthase, another enzyme of the cholesterol synthesis. This enzyme also prevents lipid modification of signaling proteins, which ultimately induces osteoclast apoptosis.25 This will lead to a disturbance in the delicate and continuous physiologic balance between destruction and formation of bone, which could contribute to the development of osteonecrosis in areas such as maxilla and mandible, characterized by very high bone remodeling activity.26
In conclusion, this is the first report that analyses half a million SNPs in a group of homogenously treated MM patients in order to determine the involvement of genetics in the pathogenesis of jaw osteonecrosis after BP treatment. Our data suggest that the rs1934951 polymorphism on CYP2C8 gene is a risk factor for ONJ. This information could help us to exclude patients at high risk from BP therapy or to take specific preventive measures. Nevertheless, these results need further confirmation in independent series of patients.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Anton, M. Hernandez, and F. Garcia for their technical assistance. A. Alegre, E. Monzo, and J. Garcia-Frade also contributed by providing samples.
This work has been partially supported by grants PI06-1354 from the Spanish Fondo de Investigaciones Sanitarias de la Seguridad Social, Red Española de Cancer RD06/0020/0006, and with the support of the Hemato-Oncology Institut, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Hospital Clinic, Barcelona, Spain.
Authorship
Contribution: M.E.S. participated in the design of the study, carried out all molecular studies, prepared the database for the final analysis, and prepared the initial version of the paper; R.G.-S. conceived and designed most of the work, reviewed the database, made the statistical analysis, rewrote the paper, and provided preapproval of the final version of the paper; L.M. participated in the initial conception and design of the study and made the first statistical analyses; M.A., M.C.C., A.B., and C.S. participated in the generation of the molecular results; L.R., J.d.l.R., M.T.H., I.G.-N., and J.J.L. are clinicians who were responsible for the patients and who collected samples and clinical data; M.G. and J.F.S.M. were the initial persons promoting the study and were responsible for getting the financial support; J.F.S.M. was responsible for the overall group and for the most important revision of the draft, as well as the person who gave the final approval of the version to be published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ramón García-Sanz, MD, PhD, Department of Haematology, University Hospital of Salamanca, Paseo de San Vicente, 58-182, Salamanca, 37007, Spain; e-mail: rgarcias@usal.es.
References
Author notes
*M.E.S. and R.G.-S. contributed equally to this article.