Abstract
The natural history of paroxysmal nocturnal hemoglobinuria (PNH) clinical subcategories (classic PNH and aplastic anemia [AA]/PNH syndrome) is still unknown. We retrospectively studied 460 PNH patients diagnosed in 58 French hematologic centers from 1950 to 2005. The median (SE) follow-up time was 6.8 (0.5) years. The median survival time (SE) was 22 (2.5) years. We identified 113 patients with classic PNH, 224 patients with AA-PNH syndrome, and 93 (22%) intermediate patients who did not fit within these 2 categories. At presentation, classic PNH patients were older, with more frequent abdominal pain and displayed higher levels of GPI-AP–deficient granulocytes. A time-dependent improved survival was observed. In classic PNH, diagnoses before 1986 (hazard ratio [HR]: 3.6; P = .01) and increasing age (P < .001) were associated with worse survival prognoses, whereas use of androgens within the first year after diagnosis was protective (HR, 0.17; P = .01). Transfusions before 1996 (HR, 2.7; P = .007) led to lower survival rates in patients with AA-PNH syndrome, whereas immunosuppressive treatment was associated with better outcomes (HR, 0.33; P = .03). Evolution to thrombosis affected survival in both subcategories (classic PNH: HR, 7.8 [P < .001]; AA-PNH syndrome: HR, 33.0 [P < .001]). Evolution to bicytopenia or pancytopenia for classic PNH (HR, 7.3, P < .001) and malignancies for AA-PNH syndrome (HR, 48.8; P < .001) were associated with worse outcomes. Although clinical presenta-tion and prognosis factors are different, classic PNH and AA-PNH syndrome present roughly similar outcomes, affected mainly by complications.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired disorder of hematopoietic stem cells. The disease is diagnosed with hemolytic anemia, marrow failure, or episodes of venous thrombosis.1 PNH is related to a somatic mutation in the phosphatidylinositol glycan class A (PIG-A), X-linked gene, responsible for a deficiency in glycosyl phosphatidylinositol–anchored proteins (GPI-APs).2 The lack of one of the GPI-AP complement regulatory proteins (CD59) leads to hemolysis.3 Recent studies have focused on inhibiting the complement cascade, with encouraging clinical results using eculizumab, a C5-inhibitor humanized monoclonal antibody.4,5
PNH diagnosis has evolved over time. Prior to the past 2 decades, Ham test was used. It was based on the increased sensitivity of PNH-affected erythrocytes to complement-mediated lysis.6 In the late 1980s, flow cytometry (FC) allowed direct quantification of the GPI-AP–deficient cells. FC, the current standard for diagnosis,1 is a reliable quantitative test, and is more sensitive than Ham test for the identification of small populations of GPI-AP–deficient cells.7
Although significant advances have been reported in the detection of the GPI-AP–deficient cells, as well as in the pathophysiology of the disease, the natural history and the identification of survival prognostic factors have been rarely described.8-10 From earlier descriptions, the clinical polymorphism of PNH has been recognized by 2 presentations: one form, predominantly hemolytic without overt marrow failure, was referred to as classic PNH,1 and the other one, with marrow failure, was often described as the aplastic anemia PNH syndrome (AA-PNH).11 This dichotomy is used as a working classification, but clinical presentations and evolutions of these 2 clinical entities have not yet been elucidated. The aim of this study was to better determine, in a sizeable cohort of patients, the presentation at time of diagnosis, the development of complications, the overall survival rate, the risk factors for thrombosis, and prognostic factors that affect survival in both classic PNH and AA-PNH syndrome.
Methods
Patients and study design
A previous report in 1996 contained data from 220 patients.9 The present questionnaire was intended to replicate the information collected in that study carried out in 1995,9 with more detailed information on successive treatments (Document S2, available on the Blood website; see the Supplemental Materials link at the top of the online article). The study protocol was approved by the review board of the French Society of Hematology. The study was carried out in accordance with the Declaration of Helsinki. The questionnaire was sent to all members of the French Society of Hematology (SFH) and/or the French Association of Young Hematologists (AIH) in January 2005, which requested information from diagnosis to latest news on all patients with PNH who were diagnosed or followed up in their center. The data were carefully checked for duplicates by the first 3 letters of the patient's name, surname, or maiden name; sex; and date of birth. Of the 220 patients previously reported, 71 had died by the time of the preceding report. Among the latter, 42 patients were completely documented again from diagnosis to death. Among the 149 patients who were still alive, 107 patients were documented again from diagnosis and consequently updated. Thus, the data of 149 patients belonging to the first study were entirely checked and 107 were updated. For patients with discrepancies between the 2 sources of data, the corresponding data were discussed with the referring physician to resolve the discrepancies. In addition, 245 patients who were not in the previous report were documented through the questionnaire. Thus, 465 patients were followed from diagnosis to last follow-up.
Diagnosis
The diagnosis of PNH was defined by an unequivocal positive Ham test6 and/or by FC (patients with more than 5% GPI-AP–deficient polymorphonuclear cells were diagnosed with PNH).7 The date of diagnosis was based on the first positive Ham test or on FC analysis if there was no prior positive Ham test. The proportion of GPI-AP–deficient granulocytes by FC (clone size) was also collected at diagnosis.
Subcategories of PNH patients at diagnosis
Because of the recent proposed PNH working clinical classification,1 patients were a priori subdivided into disease subcategories.
The classic PNH subcategory includes patients with clinical evidence of intravascular hemolysis but no evidence of bone marrow failure. Patients with a thrombosis at diagnosis were included since this subcategory is associated with propensity to thrombosis.12 Patients with anemia only (less than 120 g/L [12 g/dL]) and/or thrombosis at diagnosis were thus defined as classic PNH. Neutrophil count and platelet count were per criteria greater than 1.5 × 109 cells/L and 120 × 109 cells/L, respectively.
Patients with aplastic anemia-PNH syndrome (AA-PNH syndrome) were diagnosed with, at least, 2 or 3 peripheral blood cytopenias (hemoglobin level: ≤ 100 g/L [10 g/dL], platelet count: ≤ 80 × 109 cells/L, and neutrophil count: ≤ 1 × 109 cells/L).
Patients who did not fulfill the last 2 subcategories' criteria were assigned to a subcategory referred to as intermediate PNH.
Patients whose data were insufficient to be assigned to 1 of the 3 subcategories were not classified.
Investigations
Analyses were performed for the overall population as well as for each of the 3 individual subcategories of patients. In addition to demographic data, the date of diagnosis and the presence or absence of aplastic anemia preceding PNH were documented. Details of presenting symptoms, as well as thrombosis, infections (more than one documented infection requiring specific treatment), and abdominal pains at diagnosis were also documented. Initial cytopenias were recorded at diagnosis. Patients were classified as anemic if the hemoglobin concentration was below 120 g/L (12 g/dL), neutropenic if the absolute granulocyte count was less than 1.5 × 109 cells/L, and thrombocytopenic if the platelet count was below 150 × 109 cells/L.13 Pancytopenia was defined at diagnosis according to international criteria.14 First, second, or subsequent treatments (with date) were documented. Data on the development of the following complications was recorded: first thrombosis (date and location), progression to bicytopenia or pancytopenia (date), development of myelodysplastic syndrome (MDS; French-American-British [FAB] classification and date), acute leukemia (AL; FAB classification and date), recurrent abdominal pain crises or infections, other complications, and last known follow-up status (alive, or date and cause of death). Progression from bicytopenia to pancytopenia or a new episode of bicytopenia or pancytopenia after recovery was not considered as a first progression to bicytopenia or pancytopenia in the AA-PNH subcategory.
Statistical analysis
Description of patients at presentation in the global population and in each of the 3 subcategories is presented as n/N (%) for qualitative variables and median (interquartile range, IQR) for continuous variables. The distributions of the presentation characteristics were compared among the 3 subcategories and between classic PNH and AA-PNH by chi-square test, or Fisher exact test when necessary, for qualitative characteristics and by Kruskal-Wallis (3 subcategories) or Mann-Whitney (2 groups) test for continuous characteristics. All but 6 patients were analyzed in terms of follow-up (5 patients have no follow-up data, and status at last follow-up was the only available data point for 1 patient). Survival rates (SE) were estimated by the Kaplan-Meier method in the overall population and in each subcategory.15 Patients who underwent transplantation were censored at that time. Follow-up time and survival time are presented as median (SE). Survival curves were compared between subcategories through log-rank tests.16 In each subcategory, cumulative incidences of progression to bicytopenia or pancytopenia (except in AA-PNH subcategory), thrombosis, myelodysplastic syndrome, or acute leukemia were estimated in a competing-risks setting, with death and transplantation treated as competing events.17 Cumulative incidence curves were compared using the Gray test.18 The influence of factors on overall survival was estimated in the whole population and within each subcategory by Cox proportional hazard models,19 including time-dependent covariates when necessary.20 The following prognostic factors were tested at diagnosis: age, previous history of aplastic anemia, thrombosis, infections, anemia, neutropenia, thrombocytopenia, and pancytopenia. Progressions to first bicytopenia or pancytopenia (except in AA-PNH subcategory), MDS, or AL and development of first thrombosis were tested as time-dependent covariates. Type of treatment during the first year after diagnosis was also tested. To take into account the availability of immunosuppressive treatments from the early 1990s, immunosuppressive treatment was studied as a time-dependent covariate. For the study of risk factors associated with the occurrence of the first episode of thrombosis after diagnosis, the same method was used including the same factors at diagnosis as in the survival study and considering each treatment as a time-dependent covariate (androgens, steroids, immunosuppressive therapy, transfusions, and warfarin). Patients who died without history of thrombosis were censored at that time. For survival and first thrombosis episode studies, the factors were analyzed separately in a first step. Then, to identify independent prognostic factors, a multivariate stepwise analysis was carried out including all factors with P value less than .20 in the previous analysis. In the multivariate procedure, P value less than .05 was used to define statistical significance. Hazard ratios (HRs) of death or first thrombosis occurrence are presented as estimates with 95% confidence interval (CI). SPSS statistical software was used for all statistical analyses (Chicago, IL).
Results
Demographic characteristics
This study includes 465 patients diagnosed with PNH between 1950 and 2005. The reference date was July 1, 2005. Five patients were excluded due to diagnosis after this date, leading to 460 patients for analysis. One hundred sixty-three patients (35%) were diagnosed before 1986, 144 patients (31%) were diagnosed between 1986 and 1995, and 153 patients (33%) were diagnosed after 1995 in 58 participating French centers. Diagnosis was made using the Ham test until 1990 (193/206, 94%), whereas 202 (80%) of 253 patients were diagnosed by FC after this date (Figure S1). Patient and disease characteristics and initial treatments are summarized in Table 1.
Natural history and complications in the overall population
The median (SE) follow-up time was 6.8 (0.5) years. The median (SE) survival time was 22 (2.5) years. Survival rate estimate (SE) was 76.3% (2.6) at 10 years after diagnosis (Figure 1A). Complications encountered in the study population are detailed in Table 2. Among the 454 patients with documented follow-up, 52 received bone marrow transplant. Twenty-two died after transplantation. Among the 402 patients who did not undergo transplantation, 96 died. The causes of these 96 deaths are detailed in Table 2.
Survival in the overall population and in the 3 subcategories. Kaplan-Meier survival curve estimates in the global population (A), in the 3 subcategories (B), and in the AA-PNH syndrome according to time period (C).
Survival in the overall population and in the 3 subcategories. Kaplan-Meier survival curve estimates in the global population (A), in the 3 subcategories (B), and in the AA-PNH syndrome according to time period (C).
From multivariate analysis, independent factors that increased the risk of first thrombosis were as follows: age older than 55 years (HR, 1.8 [95% CI: 1.2 to 2.9]; P = .01), use of transfusions (HR, 1.7 [95% CI: 1.1 to 2.5]; P = .009), thrombosis at diagnosis (HR, 3.7 [95% CI: 2.1 to 6.6]; P < .001), and warfarin as primary prophylaxis (HR, 5.2 [95% CI: 2.7 to 10.2]; P < .001). In the absence of thrombosis at diagnosis, 10 of 18 patients who received warfarin as primary prophylaxis developed at least one thromboembolic events, compared with 90 of 400 patients who did not. Among patients with thrombosis at diagnosis, 8 of 19 patients who received warfarin as secondary prophylaxis or treatment developed at least one thromboembolic event, compared with 7 of 14 patients who did not (HR, 1.3 [95% CI: 0.5 to 3.7]; P = .60). In addition, the use of an immunosuppressive treatment was independently associated with a decreased risk of thrombosis (HR, 0.51 [95% CI: 0.28 to 0.94]; P = .02).
Prognostic factors affecting survival in the overall population (n = 415)
The following independent factors were related to poor survival: diagnosis before 1996 (HR, 2.6 [95% CI: 1.2-5.9]; P = .01); initial clinical features at time of diagnosis including age equal to 40 years or older (HR, 2.2 [95% CI: 1.4-3.4]; P = .001), hemoglobin level less than or equal to 100 g/L (10 g/dL) (HR, 3.0 [95% CI: 1.3-7.0]; P = .004), and neutropenia (HR, 1.7 [95% CI: 1.0-2.8]; P = .050); absence of specific treatment (no use of immunosuppressive therapy [antithymocyte globulin {ATG} and/or cyclosporin A {CsA}], no corticosteroids, no androgens or danazol, and no warfarin therapy) during the first year (HR, 2.2 [95% CI: 1.3-3.5]; P = .001) and severe complications occurring during follow-up such as progression to bicytopenia or pancytopenia (HR, 3.5 [95% CI: 1.8-6.6]; P = .001), development of thrombosis (HR, 15.4 [95% CI: 9.3-25.4]; P < .001); MDS or AL development (HR, 15.2 [95% CI: 6.6-35.0]; P < .001).
Patients' characteristics and outcome according to disease subcategory
One hundred thirteen patients were classified in the classic PNH subcategory, whereas 224 presented with an AA-PNH syndrome. Ninety-three patients (22%) did not fulfill the criteria for these 2 subcategories and were therefore assigned to the subcategory of intermediate PNH (detailed in footnote to Table 3). The remaining 30 patients could not be classified because of missing data at time of diagnosis. These proportions varied with time period. Before 1985, 42% of the patients presented with classic PNH compared with 17% after 1995 (P < .001), whereas for the same period, more AA-PNH syndromes were diagnosed (44% to 55%; P = .05) as well as more intermediate PNHs (14% to 28%; P = .004).
Patients, disease characteristics at diagnosis, and initial treatment of each subcategory are summarized in Table 3. Per criteria, these patients also differed according to biologic parameters (anemia and pancytopenia). In classic PNH, nearly half of the patients had more than 50% GPI-AP–deficient granulocytes, and abdominal pain was the initial symptomatic manifestation in approximately one-third of the patients (vs 12% to 16% in the other subcategories).
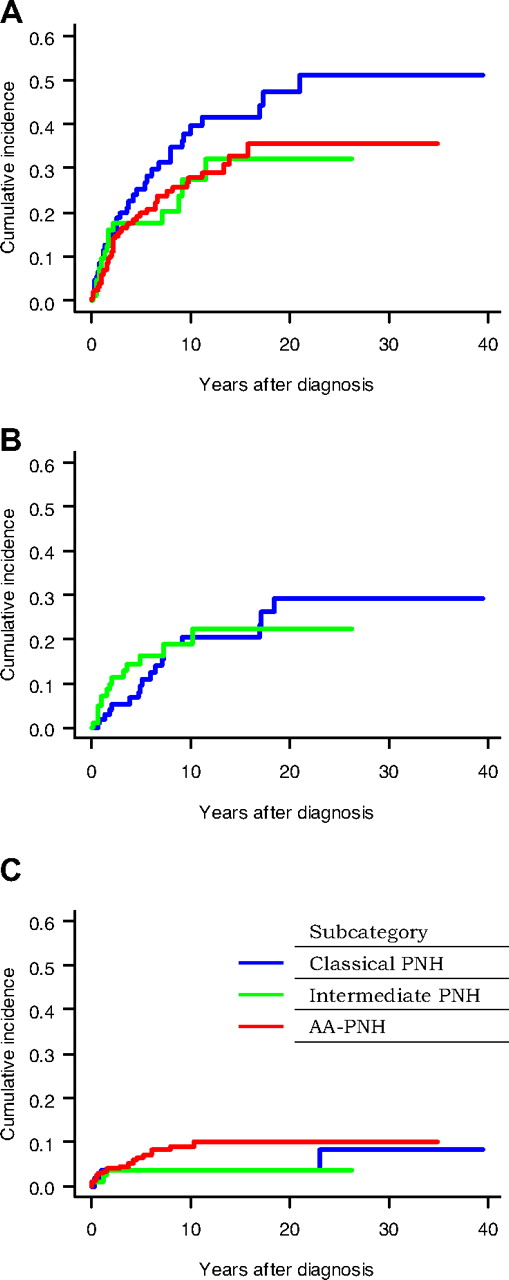
Survival curves for each subcategory are depicted in Figure 1B. Survival times in disease subcategories were analyzed according to period of time and showed an improvement in AA-PNH syndrome (Figure 1C). Cumulative incidence of first thrombosis, bicytopenia or pancytopenia (except for AA-PNH subcategory), and malignant disease are displayed per subcategory in Figure 2. There was a trend of a higher occurrence of thrombosis (Figure 2A) in classic PNH (10-year cumulative incidence: 37.9% [95% CI: 26.8 to 49.0]) than in AA-PNH syndrome (27.8% [95% CI: 20.5 to 35.1]; P = .095). The cumulative incidence of bicytopenia or pancytopenia (Figure 2B) was similar in patients with classic and intermediate disease (P = .63). The cumulative incidence of malignant disease (Figure 2C) was similar among the 3 subcategories (P = .30) even if the 10-year incidence rate appeared to be higher in the AA-PNH subcategory (9.0 [95% CI: 4.5 to 13.6]) than in the classic PNH (3.8 [95% CI: 0.1 to 7.5]) or in the intermediate PNH subcategory (3.7 [95% CI: 0.1 to 7.5]).
Cumulative incidence of complications in the 3 subcategories of patients. Cumulative incidence of thrombosis (A), bicytopenia or pancytopenia (B), and malignant disease (C) in the 3 subcategories of patients; * indicates except for AA-PNH subcategory.
Cumulative incidence of complications in the 3 subcategories of patients. Cumulative incidence of thrombosis (A), bicytopenia or pancytopenia (B), and malignant disease (C) in the 3 subcategories of patients; * indicates except for AA-PNH subcategory.
Prognostic factors affecting survival in the 3 disease subcategories
Independent prognostic factors affecting overall survival in each subcategory are presented in Table 4. Thrombosis as a complication was found to be strongly associated with worse prognosis in each of the 3 subcategories (more strongly associated in AA-PNH patients than in classic PNH patients). Evolution to bicytopenia or pancytopenia was associated with worse prognosis in patients with classic PNH, whereas evolution to malignant diseases impacted survival in patients with AA-PNH syndrome or intermediate PNH. At diagnosis, greater age was associated with lower survival in patients with classic PNH and in those with intermediate disease. In this last group, thrombocytopenia at diagnosis was associated with better outcome. Period of diagnosis had significant impact on survival, with better survival since 1986 in these 2 subcategories. Within the first year after diagnosis, the need for transfusions (before 1995) was associated with worse outcomes in patients with the AA-PNH syndrome. In this latter group, use of immunosuppressive treatment as well as the use of androgens/danazol in patients with classic PNH were associated with positive outcomes.
Discussion
This large retrospective study of 460 PNH patients has allowed us to analyze, for the first time, the disease subcategories at diagnosis and their complications during follow-up. Although clinical presentation and prognosis factors are different between classic PNH and AA-PNH syndrome, outcomes do not appear to be clearly different and are affected mainly by postdiagnosis complications, notably thrombosis, which is the main prognosis factor in all disease subcategories.
This data set represents the vast majority of patients diagnosed in, or referred to, hematologic services in France during the time period. Data on this analysis were carefully checked for discrepancies, and any that were identified were successfully resolved on a case-by-case basis, reaching back to the patients' physician if necessary. Demographic data, presenting features, and peripheral blood findings of our patients are similar to those of other studies.8,10,13,21-23 PNH is a disease mainly of the third decade, but was also observed in childhood and adolescence in our cohort, as previously reported by other authors.22
The introduction of FC as a diagnostic tool allows the detection of small clones that would otherwise not be identified. Of note, not only have diagnostic tools changed since the 1990s, but also clinical presentations (R.P.d.L., unpublished data, March 2007). The use of FC has thus made it possible to more frequently diagnose patients with PNH, compared with the classic Ham test, especially for patients with AA-PNH in the recent period. We have also found a time-dependent improved survival. Indeed, the median survival time in the present population is 22 years. Based on historical studies in 1995 and 1996, the median survival in PNH was 10 to 15 years from the time of diagnosis.8,9 This improvement is likely related to modern supportive measures, better management of thrombosis, as well as immune suppressive therapy for patients with AA-PNH syndrome. These improvements, as with all of the treatment results, have to be taken cautiously due to the retrospective nature of the survey and to the uncontrolled assignment of treatment to patients, which may have varied in relation to the patient's status, and may have been inconsistent over the long time periods involved.
Notwithstanding the interest of studying disease subcategory, it should be remembered that the main complications that impact survival include thromboses, infection, and evolution to MDS/leukemias. Myelodysplastic syndrome and acute myeloid leukemia are classic but rare complications of PNH. They occurred in similar proportions as in a previously published paper,10 with a more than 100-fold increased risk compared with age-matched controls. The prevalence of recurrent documented infections requiring specific treatment largely exceeded our first estimates9 (40% of the patients; second cause of death in our population) and confirms the joint American-Japanese study results, in which 20% of Duke patients developed severe infections during follow-up.10
Thromboses remain a major life-threatening complication affecting outcomes in the overall population, as well as in each of the disease subcategories. One-third of the patients suffered thrombotic events in hepatic, cerebral, or deep limb veins.8-10 The cumulative incidence of this complication was nearly 37%, in classic PNH 10 years after diagnosis, but also nearly 30% in AA-PNH syndrome.10,12,24 Risk factors for thrombosis included old age, thrombosis at diagnosis, transfusions, and lack of immunosuppressive therapy. Warfarin, given as prophylaxis, was also associated with an increased thrombotic risk. This provocative result does not ensure that warfarin increases the risk of thrombosis. It may illustrate the fact that higher risk patients were more likely to be placed on warfarin. However, our study is retrospective and does not allow us to ascertain the reason why these patients were placed on warfarin.
The cause of the thrombotic tendency in PNH is not clear and may be multifactorial. Other groups also reported new thrombosis events (TE) as well as progression of existing TE in patients with PNH, despite the use of anticoagulants and/or antiaggregative agents.24,25 One retrospective study showed a potential role of warfarin in thrombosis prophylaxis, with 2 serious hemorrhages in 100 patient-years of warfarin therapy.12 The risk for fatal hemorrhage in PNH patients with chronic anticoagulant therapy is related mainly to the frequent occurrence of thrombocytopenia.12,24 The complement inhibitor eculizumab,4,5 able to treat hemolysis-related anemia, also seemed to reduce the risk of clinical thromboembolism; but thrombosis prophylaxis was not the primary objective of this retrospective study.26 Prophylaxis against thromboembolism, which is still an issue of debate,1 could not have been scientifically assessed outside the setting of a prospective randomized trial that could assess whether eculizumab with or without warfarin initiation and/or continuation truly lowered the incidence of thrombosis or relapse thereof. Although immunosuppressive therapy is known to control immune attack, it also seems to decrease the risk of thromboembolism for reasons that are not clear. Finally, concerning the size of the PNH clone, we found a correlation between thromboembolism complications and larger size of the PNH clone (> 50%) (HR, 3.2 [95% CI: 1.4-7.5]; P = .004). However, our analysis is limited by the fact that the size of the clone was known for only 74% of the patients who were diagnosed by flow cytometry, corresponding to 35% of the overall population. For that reason, clone size was not introduced in the proportional hazard model to study independent factors of thrombosis occurrence.
The protean manifestations of PNH with classic PNH27 and AA-PNH syndrome11 have long been recognized. The joint American-Japanese study confirmed 2 different presentations of the disease, and a panel of experts proposed this dichotomy as a working classification for the disease.1,10 Our cohort allows the analysis of these 2 different PNH subcategories. However, 61 patients presented with hemolytic anemia (but with at least one other cytopenia, and without bicytopenia or pancytopenia according to AA-PNH definition) and 32 patients presented without anemia at diagnosis (and no bicytopenia or pancytopenia according to AA-PNH definition); these patients were included in the intermediate subcategory (93 patients, 21.6%). Thus nearly 1 of 5 patients has either hemolytic anemia or one other cytopenia that could easily suggest a diagnosis of PNH, but 32 patients had isolated neutropenia and/or thrombocytopenia, a more unusual presentation. We nevertheless decided to follow the stringent criteria for classic and AA-PNH syndrome proposed by the international expert group10 for criteria consistency between reports. Hematologic characteristics at diagnosis were per definition different and the size of the PNH clone was larger in patients with classic PNH, as expected.10,12 The overall time-dependent improved survival was observed in all disease subcategories, but was not significant in classic and intermediate PNH. This is likely to be due to a lack of statistical power as a result of the limited number of deaths in those 2 subcategories (hazard ratios related to time trend within these subcategories were quite similar to those estimated in the overall population).
During the evolution, nearly 20% of the patients with classic PNH subsequently developed pancytopenia, which was associated with worse outcomes. Thrombosis occurred in classic PNH, but occurred as well in patients with the AA-PNH syndrome, although it was never previously reported. This latter finding (ie, 30% rate of thrombosis in patients with the AA-PNH syndrome) should be emphasized since it clearly has important consequences in patient counseling and major prognostic importance. The incidence of subsequent hematologic malignancies appeared to be similar in the different subcategories. Although the natural histories are very similar between subcategories, specific treatments are distinct. A time effect was observed, with improved survival in recent years, related mainly to the use of immunosuppressive therapy in the AA-PNH subcategory that has greatly improved survival of such patients. Immunosuppressive therapy remains an important treatment modality for patients with aplastic anemia, with or without PNH clone.28 Bone marrow transplantation, which is the reference treatment in young patients with a sibling donor, was not, however, studied in the present analysis, since patients were censored at the time of transplantation. A formal comparison of the outcome of PNH patients who received or did not receive a transplant in the different disease subcategories is under analysis in collaboration with the European Group for Blood and Marrow Transplantation (EBMT). Prognosis of patients with classic PNH has already improved since the mid 1980s, probably because of better symptomatic therapy, since none of the therapeutic measures seemed to improve prognosis in this disease in 50 years (data not shown). However, although no effective therapy in classic PNH was available, androgens seem to have been associated with better outcomes.
In conclusion, the 2 PNH disease subcategories (classic PNH and AA-PNH subcategory) present similar type of complications. Thrombosis emerged as the main factor influencing survival in all disease categories including for a patient with the AA-PNH syndrome. Future basic and clinical research ought to (1) focus on a better understanding of the thrombotic tendency in PNH and how to treat this effectively, (2) take into consideration the risk of thromboses in AA-PNH that warrants aggressive monitoring and consideration of prophylaxis, and (3) study how eculizumab impacts these factors and overall survival.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Leonard Bell is greatly thanked for his helpful comments and editing. Peter Weber is specially thanked for the grammatical and syntax editing of this paper. Raphael Porcher is thanked for help in the cumulative incidence analysis. Cendrine Chaffaud is thanked for technical assistance. This study was possible because of the collaboration between the French Society of Hematology and the French Association of Young Hematologists. See Document S1 for a list of study participants.
This work has been supported partially by unrestricted grants from France HPN (Paris, France) and by Alexion France (Paris, France).
Authorship
Contribution: R.P.d.L., J.Y.M., and G.S. designed the study, performed research, collected, analyzed, and interpreted data, and drafted the paper; J.Y.M. did the statistical analysis; J.Y.M. and R.P.d.L. validated the data; G.S., R.P.d.L., and J.Y.M. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; and C.S., L.T., G.E., M.M., S.R., S.G., S.M., and J.Y.C. performed research, collected data, and reviewed drafts of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: G. Socié, Service d'Hématologie-Greffe de Moelle, Hôpital Saint Louis, Paris, France; e-mail: gerard.socie@sls.aphp.fr.