Abstract
The role of the factor IXa heparin-binding exosite in coagulation was assessed with mutations that enhance (R170A) or reduce (R233A) stability of the protease-factor VIIIa A2 domain interaction. After tissue factor (TF) addition to reconstituted factor IX-deficient plasma, factor IX R170A supported a 2-fold increase in velocity index (slope) and peak thrombin concentration, whereas factor IX R233A had a 4- to 10-fold reduction relative to factor IX wild-type. In the absence of TF, 5 to 100 pM of factor IXa increased thrombin generation to approach TF-stimulated thrombin generation at 100% factor IX. Factor IXa R170A demonstrated a 2- to 3-fold increase in peak thrombin concentration and 5-fold increase in velocity index, whereas the response for factor IXa R233A was blunted and delayed relative to wild-type protease. In hemophilia B mice, factor IX replacement reduced the average time to hemostasis after saphenous vein incision, and the time to occlusion after FeCl3-induced saphenous vein injury. At 5% factor IX, the times to occlusion for factor IX wild-type, R170A, and R233A were 15.7 minutes, 9.1 minutes (P ≤ .003), and more than 45 minutes. These data support the role of the factor IXa heparin-binding exosite as a critical regulator of coagulation and novel antithrombotic target.
Introduction
Thrombin is the penultimate product of the coagulation cascade, generated in an explosive burst on stimulation of plasma with limiting concentrations of tissue factor.1,2 The measurement of plasma thrombin generation has merits as a global test of coagulation, and enhanced levels of thrombin generation have been associated with increased risk of recurrent venous thrombosis.3 Thus, the rate and magnitude of thrombin generation may be predictive of the coagulation phenotype of patients.4,5 In vitro and ex vivo modeling of the coagulation cascade indicates that factor X activation by the intrinsic tenase complex (factor IXa–factor VIIIa) is the rate-limiting step for thrombin generation.1,2,6 Intrinsic tenase activity is unstable because of the diffusional loss of the noncovalently bound factor VIIIa A2 domain.7,8 The instability of this enzyme complex is presumed to be an important regulator of the coagulation response
The mechanism(s) for activation of factor IXa within the intrinsic tenase complex are poorly understood. Factor VIII circulates as a heterodimer of A1-A2-B and A3-C1-C2 peptides with domain structure and metal-binding functions similar to ceruloplasmin.9 Factor VIII undergoes proteolytic activation by thrombin, resulting in an unstable, metal-dependent A1/A2/A3-C1-C2 heterotrimer with cofactor activity.10,11 Factor VIII or factor VIIIa light chain (A3-C1-C2 domains) bind to factor IXa on the phospholipid surface with an affinity that approaches intact factor VIIIa but lack cofactor activity.12,13 The isolated factor VIIIa A1 domain also lacks cofactor activity. In contrast, the factor VIIIa A2 domain directly modulates the catalytic activity of factor IXa, which is further enhanced by the A1 domain, markedly increasing the kcat for factor X activation.14,15 Although the isolated A2 domain binds with low affinity to factor IXa, it contributes significantly to protease-cofactor affinity in the membrane-bound enzyme complex.16 Thus, the factor IXa-A2 domain interaction appears to be the critical protein-protein interface for cofactor enhancement of factor X activation.
The heparin-binding exosite on the factor IXa protease domain is a cofactor interactive site that contributes significantly to stabilization of the factor VIIIa A2 domain, and allosteric activation of the protease within the enzyme complex.17,18 This exosite is the molecular target for antithrombin-independent inhibition of the intrinsic tenase complex by heparin and other glycosaminoglycans and may contribute to the antithrombotic properties of heparin.17,19 Mutations in the factor IXa heparin-binding exosite that modulate intrinsic tenase stability can be used to assess the importance of the factor IXa-A2 domain interaction in complex systems. Factor IXa R170A (chymotrypsinogen numbering system) demonstrates increased apparent cofactor affinity and enhanced stability of the enzyme complex with purified components, and increased coagulant activity in an activated partial thromboplastin time (APTT)-based assay. In contrast, factor IXa R233A demonstrates decreased apparent cofactor affinity and reduced stability of the enzyme complex with purified components, and moderately reduced coagulant activity.17 Coagulant activity measured in APTT-based assays is useful for the detection of factor deficiencies but represents a highly unphysiologic assessment of blood coagulation. The complexity of ex vivo plasma and in vivo injury models make predictions regarding the importance of specific molecular interactions difficult. However, the opposing phenotypes of these recombinant proteases provide valuable tools to investigate the role of the factor IXa-A2 domain interaction in more physiologic systems.
Because evidence suggests that interaction with the factor VIIIa A2 domain is critical to cofactor activation of factor IXa, factor X activation by the intrinsic tenase complex is rate-limiting for thrombin generation, and thrombin generation phenotype is associated with the risk of venous thrombosis, we hypothesized that the factor IXa heparin-binding exosite is a critical regulator of coagulation response. To test this hypothesis, we evaluated the effect of mutations in the heparin-binding exosite of factor IX (R170A and R233A) on tissue factor and factor IXa–initiated thrombin generation in factor IX–deficient plasma, bleeding from a saphenous vein incision, and FeCl3-induced saphenous vein thrombosis in the hemophilia B mouse. The results suggest that the heparin-binding exosite of factor IXa is a critical physiologic regulator of plasma thrombin generation and venous thrombus formation.
Methods
Materials
Human normal pooled plasma and factor IX–deficient patient plasma were purchased from George King (Overland Park, KS). Corn trypsin inhibitor (CTI) was obtained from Haematologic Technologies (Essex Junction, VT). Human plasma-derived factor IX, IXa, and thrombin were purchased from Enzyme Research Laboratories (South Bend, IN). Phosphatidylserine (PS) and phosphatidylcholine (PC) were purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol was purchased from Calbiochem (San Diego, CA). PC:PS:cholesterol (molar ratio 75:25:1) phospholipid vesicles (PC:PS vesicles) were prepared by extrusion through a 100-nm polycarbonate filter.20 Dimethyl sulfoxide (DMSO) was purchased from Mallinckrodt (St Louis, MO), bovine serum albumin (BSA) (A-9647) from Sigma-Aldrich (St Louis, MO), and urea from EM Sciences (Cherry Hill, NJ). Lyophilized bovine thrombin-α2-macroglobulin complex was purchased from Thrombinoscope BV (Maastricht, The Netherlands). Thromborel S, a human thromboplastin from Dade Behring (Deerfield, IL), was used as the source of relipidated human tissue factor. The tissue factor concentration was 200 ng/mL based on the mean of enzyme-linked immunosorbent assay results for multiple lots of Thromborel S (Stephanie Smith, University of Illinois, e-mail communication, March 2, 2006). The fluorogenic substrate Z-Gly-Gly-Arg-AMC HCl was obtained from Bachem Biosciences (King of Prussia, PA).
Expression and purification of recombinant factor IX
HEK 293 cell lines stably transfected with human factor IX wild-type and R170A were provided by Darrel Stafford (University of North Carolina at Chapel Hill).21 A stable HEK 293 cell line expressing human factor IX R233A was constructed as described.17 Recombinant factor IX proteins were purified to homogeneity from conditioned media and quantitated by absorbance at 280 nm. A portion of the factor IX was activated to factor IXa with human factor XIa, and factor IXa catalytic sites quantitated by titration with antithrombin III, as previously described.18
Fluorogenic method for detection of plasma thrombin generation
Thrombin generation in human plasma was detected by modification of the methods of Hemker et al and Technothrombin TGA.3,22 The assay was performed in 96-well polystyrene flat-bottom clear plates from Greiner Bio-One (Longwood, FL). Thrombin activity was determined by 7-amino-4-methylcoumarin (AMC) release from the fluorogenic substrate Z-Gly-Gly-Arg-AMC, detected by a 360/40-nm-excitation and 460/40-nm-emission filter set in a Biotek Synergy HT fluorescent plate reader equipped with Gen 5 software (Biotek Instruments, Winooski, VT). The substrate Z-Gly-Gly-Arg-AMC HCl was reconstituted at 100 mM in DMSO and stored at −20°C. Before each assay, a fresh fluorogenic substrate and calcium solution (FluCa substrate) was prepared by adding 100 μL of 1.0 M of CaCl2 to 875 μL of 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.35, 60 g/L of BSA at 37°C, followed by 25 μL of 100 mM of Z-Gly-Gly-Arg-AMC in DMSO with vigorous mixing. FluCa substrate had a final concentration of 2.5 mM of Z-Gly-Gly-Arg-AMC HCl and 100 mM of CaCl2. The bovine thrombin-α2-macroglobulin complex calibrator was reconstituted in distilled water, followed by dilution in TGA buffer (100 mM of NaCl, 20 mM of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.35, and 1% BSA) to achieve final plasma concentrations of 500, 250, 50, and 5 nM, respectively. Factor IX–deficient plasma (60 μL) and TGA buffer containing calibrator (20 μL) were added to each well and preheated at 37°C for 10 minutes. FluCa substrate (20 μL) at 37°C was then added, mixed at medium intensity for 5 seconds, and readings were obtained at 30 seconds intervals for 60 minutes. The raw data for the first 10 minutes were imported into Technothrombin TGA evaluation software from Technoclone (Vienna, Austria) to construct the calibration curve.
Plasma thrombin generation was triggered by addition of a limiting tissue factor concentration (0.2 pM) or factor IXa to recalcified plasma. Plasma was thawed sequentially on ice for 5 minutes, at room temperature for 5 minutes, and in a 37°C water bath for 5 minutes. For tissue factor triggered assays, the initiator solution was composed of 0.12 mg/mL of CTI, 25 μM of PC:PS vesicles, 0.6 pM of tissue factor, and 0 to 270 nM of plasma-derived or recombinant FIX in TGA buffer. For factor IXa–triggered assays, tissue factor was omitted, and plasma-derived or recombinant factor IXa replaced factor IX. Plasma (60 μL) and initiator solution (20 μL) were added to each well and preheated at 37°C for 10 minutes. FluCa substrate (20 μL) at 37°C was then added, mixed at medium intensity for 5 seconds, and readings were obtained at 30-second intervals for 60 minutes. Final concentrations (extrapolated to the 60 μL plasma volume) were 0.2 pM of tissue factor, 8.3 μM of PC:PS vesicles, and 40 μg/mL of CTI. Fluorescent signal data were exported to Technothrombin TGA evaluation software, and thrombin generation over time was determined using the calibration curve. Numerical parameters generated by the software to describe each thrombin generation curve, included lag time (start until first burst in thrombin formation), peak thrombin concentration, time to thrombin peak, and velocity index (slope between the end of lag time and peak thrombin).
The time course of plasma thrombin generation by Western blotting
Tissue factor-dependent thrombin generation assays in factor IX-deficient plasma supplemented with 4.5 nM of recombinant factor IX (5% plasma levels) were prepared as described for the fluorogenic method in 1.5-mL Eppendorf tubes at 37°C. Individual reactions were quenched by addition of 100 μL 2× sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer containing 5 M of urea. Quenched samples were incubated at 37°C for 5 minutes, followed by boiling for 5 minutes to ensure complete clot dissolution. Samples were diluted 1:20 in either nonreducing or reducing SDS-PAGE loading buffer and subjected to 10% SDS-PAGE, followed by overnight transfer to Immobilon-P (Millipore, Billerica, MA). Membranes were blocked in 5% milk and 1% BSA for 60 minutes, incubated in a 1:2500 dilution of polyclonal sheep anti–human thrombin antibody (Haematologic Technologies) for 90 minutes, followed by incubation in a 1:10 000 dilution of peroxidase-conjugated affinity purified donkey anti–sheep IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 60 minutes. The washed Immobilon-P membrane was immersed in Supersignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL) for 5 minutes and exposed to Kodak BioMax Xar film (Eastman Kodak, Rochester, NY).
Wild-type and hemophilia B mice
All procedures were reviewed and approved by the University of North Carolina at Chapel Hill School of Medicine Institutional Animal Care and Use Committee. Wild-type C57BL/6 (wtC57B6) mice were purchased from Charles River Breeding Laboratories (Portage, MI) and/or bred in house. Hemophilia B knockout mice were the kind gift of Dr Dougald M. Monroe.23
Saphenous vein hemostasis and thrombosis models
Adult mice were anesthetized with 0.1 mL/g body weight of a 2.5% 2,2,2-tribromoethanol (Sigma-Aldrich) solution. Ventral hind limb hair was removed bilaterally and the animal placed supine on a THM 100 Temperature & ECG Monitoring Board (Indus Instruments, Houston, TX). The paws were gently restrained by Velcro attached the ECG pads and/or by a loop of polyethylene tubing attached to the board. The skin overlying both the left and right ventral hind limb was incised, a length of the saphenous neurovascular bundle exposed and covered with normal saline to prevent drying of the tissues. To allow for injection of therapeutic agents, the left saphenous vein was cannulated under a SZX12 dissecting microscope (Olympus, Tokyo, Japan) using a catheter constructed of pulled PE-10 tubing (Braintree Scientific, Braintree, MA) with a 3.0-mil cleaning wire (Hamilton, Reno, NV) placed into the lumen as a stylet. For the factor IX dose-response curves, mice were given weight-dependent doses of plasma-derived factor IX (Mononine, CSL Behring, King of Prussia, PA) in 0.9% sodium chloride to achieve 0% to 100% normal plasma concentrations (0-1 U/mL). For recombinant human factor IX wild-type, R170A, or R233A experiments, mice were given weight-dependent doses of a 200 nM of stock solution recombinant human factor IX to achieve 10% normal plasma concentration (0.1 U/mL) for the hemostasis model, or 5% normal plasma concentration (0.05 U/mL) for the thrombosis model. All factor IX injections were given 5 minutes before the initiation of saphenous vein incision or FeCl3 induced injury. All animals were killed at the end of the procedure.
Hemostasis was assessed by determination of serial bleeding times from a saphenous vein incision. The right saphenous vein was transected by piercing through and through with a 23-G needle (BD Biosciences, San Jose, CA). Blood was wicked away with a Kimwipe (Kimberly-Clark, Roswell, GA) until hemostasis occurred (typically 1-2 minutes for all mice). A longitudinal incision was made in the distal portion of the vessel using Student Vannas Spring Scissors (Fine Science Tools, Foster City, CA) by inserting one blade into the vessel as far as possible and then cutting. Blood was gently wicked away with a Kimwipe until hemostasis occurred. The clot was then disrupted using a 30-G needle, and blood wicked away until hemostasis occurred again. Clot disruption was repeated after every incidence of hemostasis until 30 minutes after the initial 23-G needle injury. All injury, clot disruption, and hemostasis time points were recorded on the Chart software.
Thrombosis in the saphenous vein was produced using FeCl3 (Sigma-Aldrich)-induced injury. Flow in the right saphenous vein was monitored using a 20-MHz Doppler flow probe (Indus Instruments). Data from the Doppler probe were collected on an Apple iMac using a PowerLab 4/20 and Chart software (ADInstruments, Colorado Springs, CO). Five minutes after recombinant factor IX injection, vessel injury was initiated by placing a 0.5 × 2 mm strip of filter paper soaked in 10% FeCl3 onto the vein. After 2 minutes, the filter paper was removed, the neurovascular bundle washed twice with normal saline and then covered again with normal saline and the flow signal reacquired. Injury and thrombosis were allowed to proceed until vessel occlusion. The time to occlusion (TTO) was determined from application of the filter paper until loss of the Doppler flow signal for one minute. The experiment was stopped after vessel occlusion, or 30 or 45 minutes (factor IX dose-response curve or recombinant human factor IX experiments, respectively) after filter paper was applied if no occlusion occurred.
Results
Effect of mutations in the factor IX heparin binding exosite on tissue factor–stimulated plasma thrombin generation
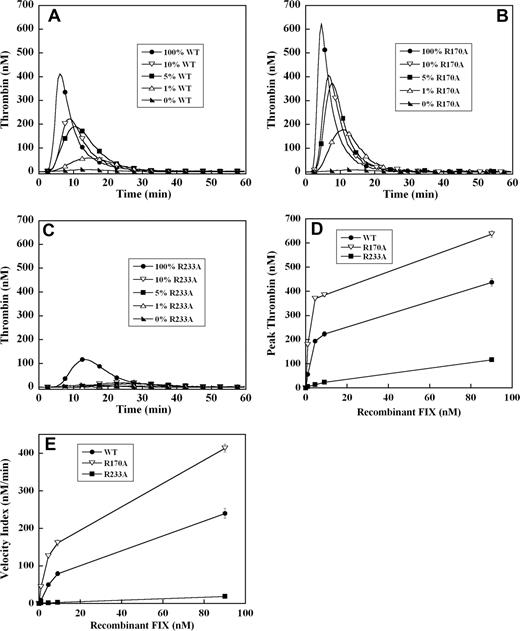
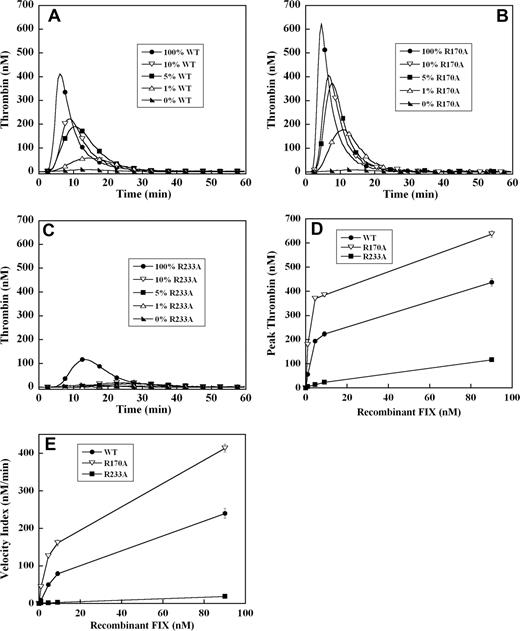
To assess the effect of these mutations on the ability of recombinant factor IX to support plasma thrombin generation triggered by tissue factor, factor IX–deficient plasma was supplemented with 0%, 1%, 5%, 10%, and 100% (90 nM) levels of each recombinant factor IX. A dose-dependent increase in velocity index (slope) and peak thrombin concentration, modest reduction in lag time, and shortening of the time to peak thrombin generation were observed between 0% and 100% factor IX levels in all mutants (Figure 1A-C). Thrombin generation curves of factor IX R170A were shifted modestly to the left compared with factor IX wild-type (Figure 1A,B). This mutant was able to generate significant amounts of thrombin, even at 1% factor IX levels (Figure 1B), and demonstrated 2-fold enhancement in velocity index and peak thrombin concentration between 1% and 5% levels compared with factor IX wild-type (Figure 1D,E). In contrast, factor IX R233A thrombin generation curves were shifted substantially to the right (Figure 1C) with significantly reduced velocity index and peak thrombin concentration (4- to 10-fold) compared with factor IX wild-type (Figure 1D,E). At factor IX R233A levels of 10% or less, thrombin generation was markedly blunted (Figure 1C), and even at 100% plasma levels still generated less thrombin than 5% levels of factor IX wild-type (Figure 1A,C).
Tissue factor–stimulated thrombin generation in factor IX–deficient plasma supplemented with 1%, 5%, 10%, and 100% levels of recombinant factor IX wild-type, R170A, and R233A. Thrombin generation was initiated with 0.2 pM of human tissue factor, 8.3 μM of PC:PS vesicles, and 40 μg/mL of CTI (plasma concentrations) in factor IX–deficient plasma supplemented with 0%, 1% (0.9 nM), 5% (4.5 nM), 10% (9 nM), and 100% (90 nM) levels of recombinant factor IX wild-type (A), R170A (B), or R233A (C). The time course of thrombin generation was measured as described in “Fluorogenic method for detection of plasma thrombin generation.” Thrombin generation curves representing the mean fluorescent data from replicate determinations (n = 10) are shown. Curves are identified by representative points. Peak thrombin concentration (D) and velocity index of thrombin generation (E) for each recombinant protein are plotted for comparison. Error bars represent SE.
Tissue factor–stimulated thrombin generation in factor IX–deficient plasma supplemented with 1%, 5%, 10%, and 100% levels of recombinant factor IX wild-type, R170A, and R233A. Thrombin generation was initiated with 0.2 pM of human tissue factor, 8.3 μM of PC:PS vesicles, and 40 μg/mL of CTI (plasma concentrations) in factor IX–deficient plasma supplemented with 0%, 1% (0.9 nM), 5% (4.5 nM), 10% (9 nM), and 100% (90 nM) levels of recombinant factor IX wild-type (A), R170A (B), or R233A (C). The time course of thrombin generation was measured as described in “Fluorogenic method for detection of plasma thrombin generation.” Thrombin generation curves representing the mean fluorescent data from replicate determinations (n = 10) are shown. Curves are identified by representative points. Peak thrombin concentration (D) and velocity index of thrombin generation (E) for each recombinant protein are plotted for comparison. Error bars represent SE.
Western Blot of plasma thrombin generation in the presence of 5% recombinant factor IX
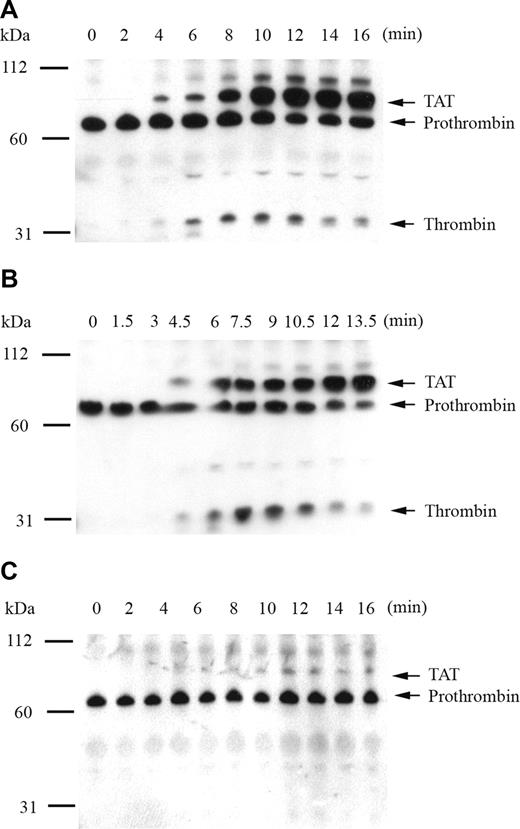
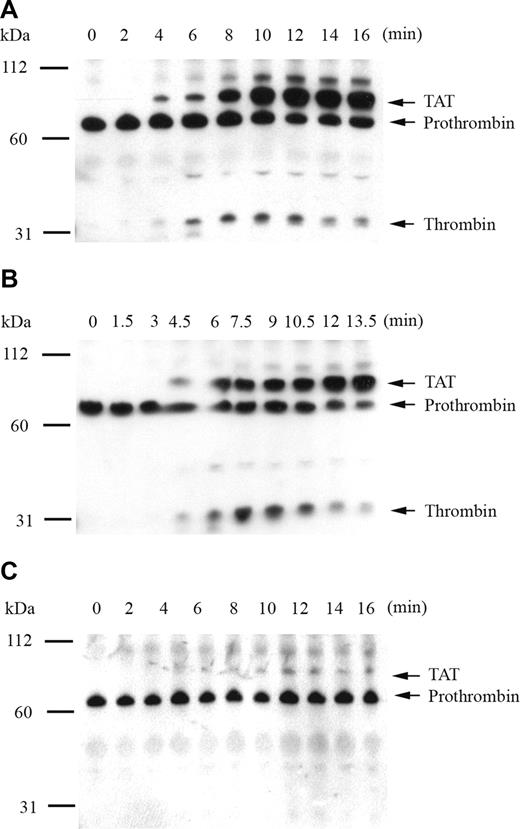
To confirm that fluorogenic substrate cleavage accurately represented plasma thrombin generation by recombinant factor IX mutants, the time course of thrombin activation and inhibition products was monitored by Western blot. The primary antithrombin antibody simultaneously detected prothrombin, thrombin-related cleavage products, and the thrombin-antithrombin complex (TAT). Thrombin generation was initiated by 0.2 pM of human tissue factor in factor IX–deficient plasma supplemented with 5% recombinant factor IX, and reaction products were analyzed by SDS-PAGE under nonreducing (Figure 2) and reducing (data not shown) conditions. Thrombin generation in the presence of 5% factor IX wild-type demonstrated appearance of the TAT complex at 4 minutes with progressive accumulation over time and peak free thrombin concentration at 10 minutes (Figure 2A). An unidentified higher molecular weight band also accumulated with time, which disappeared under reducing conditions (not shown). Modest reduction in the prothrombin/meizothrombin band was noted over time, without evidence of prothrombin depletion. In the presence of 5% factor IX R170A, the TAT complex was detected at 4.5 minutes, peak free thrombin accumulation occurred at 7.5 minutes, and the amount of free thrombin generated was enhanced relative to factor IX wild-type (Figure 2B). In the presence of 5% factor IX R233A, thrombin generation was markedly delayed and reduced in intensity, with small amounts of TAT complex appearing at 10 minutes, and no discernible amount of free thrombin detected up to 16 minutes (Figure 2C).
Analysis of tissue factor–stimulated thrombin generation by Western blot in factor IX–deficient plasma supplemented with 5% recombinant factor IX wild-type, R170A, and R233A. Thrombin generation was initiated with 0.2 pM of human tissue factor, 8.3 μM of PC:PS vesicles, and 40 μg/mL of CTI (plasma concentrations) in factor IX-deficient plasma supplemented with 5% recombinant factor IX wild-type (A), R170A (B), and R233A (C). Individual reactions were quenched over time with loading buffer containing 5 M of urea and analyzed by 10% SDS-PAGE under nonreducing conditions as described in “The time course of plasma thrombin generation by Western blotting.” Proteins transferred to Immobilon-P were detected with a polyclonal sheep anti–human thrombin primary antibody, followed by a peroxidase-conjugated affinity-purified donkey anti–sheep IgG, and subsequent development of signal with chemiluminescent substrate. Prothrombin indicates prothrombin/meizothrombin band; TAT, thrombin-antithrombin complex.
Analysis of tissue factor–stimulated thrombin generation by Western blot in factor IX–deficient plasma supplemented with 5% recombinant factor IX wild-type, R170A, and R233A. Thrombin generation was initiated with 0.2 pM of human tissue factor, 8.3 μM of PC:PS vesicles, and 40 μg/mL of CTI (plasma concentrations) in factor IX-deficient plasma supplemented with 5% recombinant factor IX wild-type (A), R170A (B), and R233A (C). Individual reactions were quenched over time with loading buffer containing 5 M of urea and analyzed by 10% SDS-PAGE under nonreducing conditions as described in “The time course of plasma thrombin generation by Western blotting.” Proteins transferred to Immobilon-P were detected with a polyclonal sheep anti–human thrombin primary antibody, followed by a peroxidase-conjugated affinity-purified donkey anti–sheep IgG, and subsequent development of signal with chemiluminescent substrate. Prothrombin indicates prothrombin/meizothrombin band; TAT, thrombin-antithrombin complex.
Tissue factor–independent thrombin generation by recombinant factor IXa in factor IX–deficient plasma
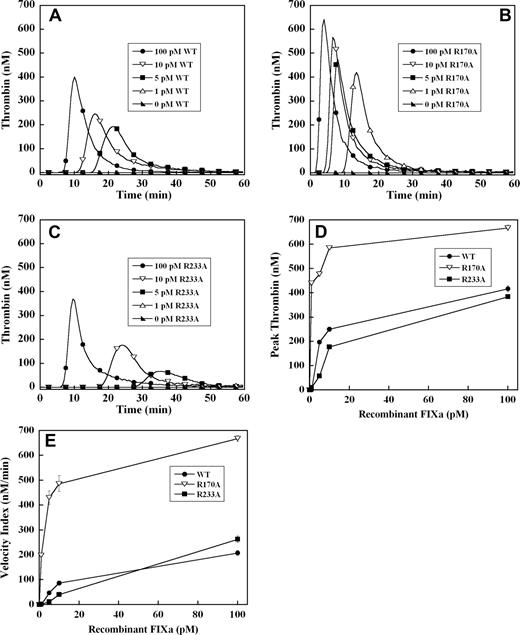
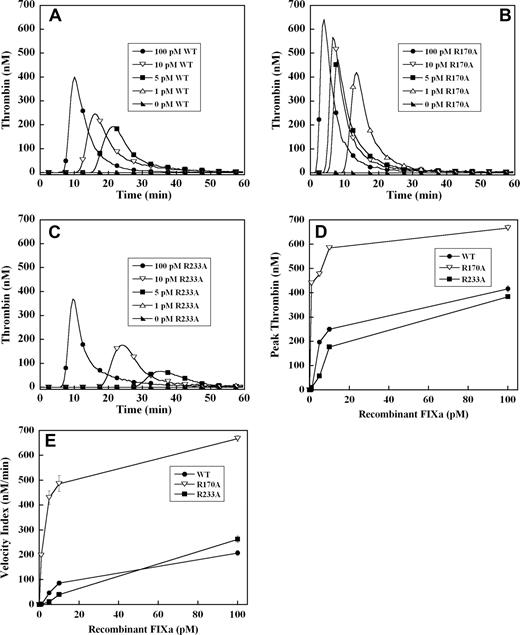
To exclude the potential effects of these mutations in the heparin binding exosite on factor IX activation by tissue factor–factor VIIa complex, thrombin generation was initiated with recombinant factor IXa in the absence of tissue factor. Significant thrombin generation was observed with as little as 5 pM of wild-type recombinant factor IXa, representing activation of less than 0.001% of the normal zymogen concentration (Figure 3A). A dose-dependent increase in the velocity index and peak thrombin concentration was observed between 5 and 100 pM factor IXa wild-type (Figure 3A). Increased concentrations of factor IXa R170A demonstrated a progressive increase in velocity index and peak thrombin concentration and shortened the time to peak thrombin (Figure 3B). The dose-response for factor IXa R170A was shifted significantly to the left relative to factor IX wild-type, with enhanced thrombin generation between 1 and 100 pM (Figure 3A,B). The peak thrombin concentration was increased 2- to 3-fold, and the velocity index up to 5-fold for factor IXa R170A relative to wild-type protease (Figure 3D,E). Addition of 1 pM of factor IXa R170A resulted in similar velocity index and peak thrombin to 100 pM of factor IXa wild-type (Figure 3D,E), whereas 1 pM of wild-type protease failed to trigger a significant thrombin response. Factor IXa R233A demonstrated reduced thrombin generation relative to wild-type protease between 5 and 100 pM (Figure 3C). Addition of 1 pM of factor IXa R233A to plasma resulted in no significant thrombin response, whereas the response to 5 to 10 pM of protease was blunted and delayed compared with wild-type factor IXa (Figure 3A,C). However, at 100 pM of factor IXa, peak thrombin generation by factor IXa R233A approached that of the wild-type protease (Figure 3A,C).
Factor IXa–stimulated thrombin generation in factor IX–deficient plasma in the absence of tissue factor. Thrombin generation was initiated by 1 to 100 pM of recombinant factor IXa wild-type (A), R170A (B), or R233A (C), in the presence of 8.3 μM of PC:PS vesicles, and 40 μg/mL of CTI (plasma concentrations) in factor IX–deficient plasma. Factor IXa was added just before recalcification at a final plasma concentration indicated in the figure. The time course of thrombin generation was measured as described in “Fluorogenic method for detection of plasma thrombin generation.” Thrombin generation curves representing the mean fluorescent data from replicate determinations (n = 10) are shown. Curves are identified by representative points. Peak thrombin concentration (D) and velocity index of thrombin generation (E) for each recombinant protein are plotted for comparison. Error bars represent SE.
Factor IXa–stimulated thrombin generation in factor IX–deficient plasma in the absence of tissue factor. Thrombin generation was initiated by 1 to 100 pM of recombinant factor IXa wild-type (A), R170A (B), or R233A (C), in the presence of 8.3 μM of PC:PS vesicles, and 40 μg/mL of CTI (plasma concentrations) in factor IX–deficient plasma. Factor IXa was added just before recalcification at a final plasma concentration indicated in the figure. The time course of thrombin generation was measured as described in “Fluorogenic method for detection of plasma thrombin generation.” Thrombin generation curves representing the mean fluorescent data from replicate determinations (n = 10) are shown. Curves are identified by representative points. Peak thrombin concentration (D) and velocity index of thrombin generation (E) for each recombinant protein are plotted for comparison. Error bars represent SE.
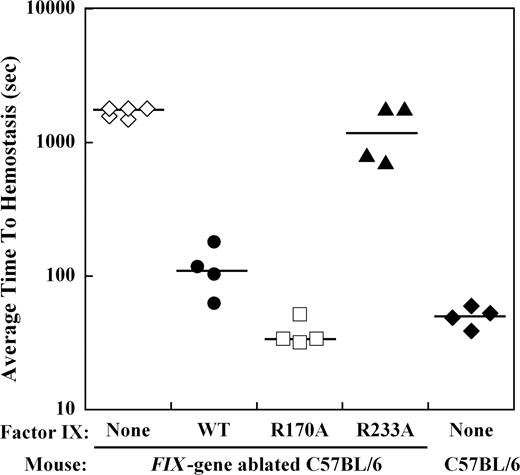
Effect of recombinant factor IX on the average time to hemostasis after saphenous vein incision in hemophilia B mice
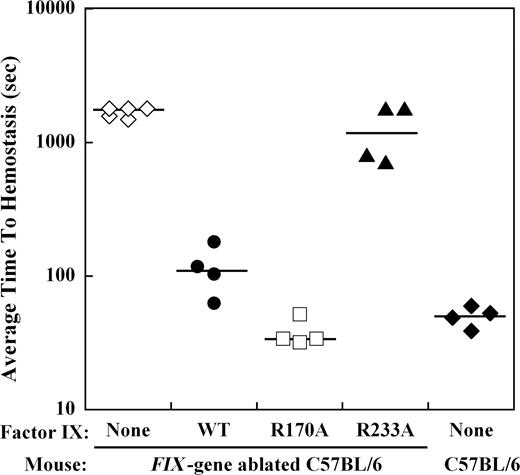
The effect of mutations in the heparin-binding exosite of factor IX(a) on hemostasis in the hemophilia B mouse was examined by bleeding times after a distal saphenous vein incision obtained with repeated clot disruption. The time to hemostasis for each bleeding episode was recorded for 30 minutes, and the average time to hemostasis (ATTH) for all episodes was calculated for each mouse. Wild-type C57BL/6 mice (n = 4) demonstrated a median ATTH of 51 seconds, whereas Factor IX gene–ablated C57BL/6 mice (n = 5) had a median ATTH of more than 1800 seconds (3 of 5 mice failed to achieve hemostasis within 30 minutes; Figure 4). Recombinant factor IX was infused at weight-based doses sufficient to achieve approximately 10% normal plasma levels before saphenous vein incision in hemophilia B mice. Median ATTH for factor IX wild-type (n = 4) was 111 seconds and factor IXa R170A (n = 4) was 34 seconds, both significantly shorter than untreated hemophilia B mice (P ≤ .008, Mann-Whitney test). In contrast, factor IX R233A (n = 4) demonstrated a median ATTH of 1304 seconds (2 of 4 mice failed to achieve hemostasis), not significantly different from untreated hemophilia B mice.
Effect of recombinant factor IX mutants at 10% plasma levels on the average time to hemostasis (ATTH) after saphenous vein incision in the hemophilia B mouse. Wild-type or Factor IX gene–ablated C57BL/6 mice were subjected to incision of the distal saphenous vein, and repeat bleeding times were determined with serial clot disruptions for 30 minutes, as described in “Saphenous vein hemostasis and thrombosis models.” The median ATTH (solid lines) for wild-type and Factor IX gene–ablated C57B6 mice was 51 seconds and more than 1800 seconds, respectively. Factor IX gene–ablated C57BL/6 mice were injected with recombinant factor IX wild-type, R170A, or R233A at doses calculated to result in 10% normal plasma levels before saphenous vein incision. Injection of factor IX into the gene-ablated mice shortened the median ATTH for factor IX wild-type and R170A to 111 seconds and 34 seconds, respectively (P ≤ .008, Mann-Whitney test). Median ATTH for factor IX R233A was 1304 seconds (not significant). The y-axis for ATTH represents a log scale.
Effect of recombinant factor IX mutants at 10% plasma levels on the average time to hemostasis (ATTH) after saphenous vein incision in the hemophilia B mouse. Wild-type or Factor IX gene–ablated C57BL/6 mice were subjected to incision of the distal saphenous vein, and repeat bleeding times were determined with serial clot disruptions for 30 minutes, as described in “Saphenous vein hemostasis and thrombosis models.” The median ATTH (solid lines) for wild-type and Factor IX gene–ablated C57B6 mice was 51 seconds and more than 1800 seconds, respectively. Factor IX gene–ablated C57BL/6 mice were injected with recombinant factor IX wild-type, R170A, or R233A at doses calculated to result in 10% normal plasma levels before saphenous vein incision. Injection of factor IX into the gene-ablated mice shortened the median ATTH for factor IX wild-type and R170A to 111 seconds and 34 seconds, respectively (P ≤ .008, Mann-Whitney test). Median ATTH for factor IX R233A was 1304 seconds (not significant). The y-axis for ATTH represents a log scale.
Effect of factor IX on the time to vessel occlusion after FeCl3 injury of the saphenous vein in wild-type and hemophilia B mice
To determine the factor IX dependence of venous thrombosis formation in response to FeCl3-induced saphenous vein injury, the time to vessel occlusion as monitored by a Doppler flow probe was determined in both wild-type and hemophilia B (Factor IX gene– ablated) C57BL/6 mice.23 After FeCl3-induced saphenous vein injury, wild-type C57BL/6 mice (n = 4) demonstrated a median TTO of 11.5 minutes, whereas hemophilia B mice (n = 4) failed to occlude the vessel within 45 minutes (data not shown). Infusion of plasma-derived factor IX before saphenous vein injury shortened the TTO in hemophilia B mice back to values observed in wild-type mice. The dose-response for factor IX demonstrated marked shortening of the TTO between 3% and 5% normal plasma factor IX levels and limited further effect between 5% and 100% levels (data not shown).
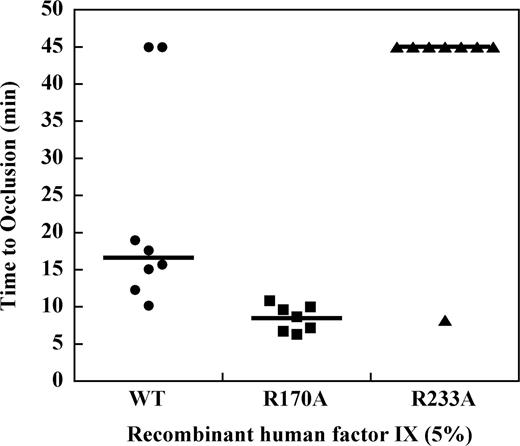
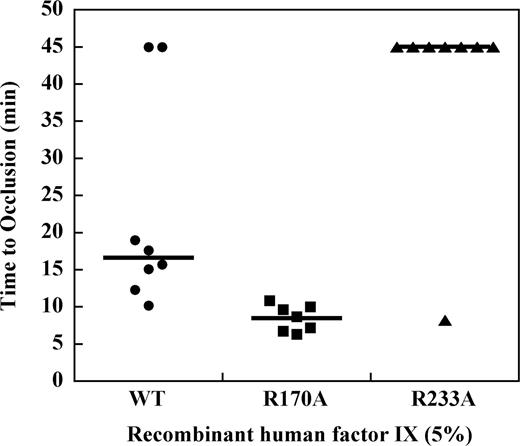
Effect of factor IX mutations on TTO in the hemophilia B mouse at 5% plasma levels
To determine the effect of mutations in the heparin-binding exosite of factor IX(a) on saphenous vein thrombosis in the hemophilia B mouse, recombinant factor IX was infused at doses sufficient to achieve approximately 5% normal plasma levels before vessel injury. Recombinant factor IX wild-type demonstrated a median TTO of 15.7 minutes, which was modestly prolonged relative to the plasma-derived factor IX preparation (Figure 5). Infusion of factor IX R170A at 5% normal plasma levels resulted in a TTO of 9.1 minutes, which was significantly shortened relative to the wild-type protease (P ≤ .003, Mann-Whitney test). In contrast, infusion of factor IX R233A at 5% plasma levels resulted in a median TTO greater than 45 minutes. To confirm that factor IX R233A has in vivo activity, the dose was increased to achieve approximately 50% normal plasma levels of factor IX, which resulted in a TTO similar to the wild-type protease (data not shown).
Effect of recombinant factor IX mutants at 5% plasma levels on the time to occlusion (TTO) after FeCl3-induced saphenous vein injury in the hemophilia B mouse.Factor IX gene–ablated C57B6 mice were infused with recombinant factor IX wild-type, R170A, or R233A at doses calculated to result in 5% normal plasma levels before undergoing FeCl3-induced saphenous vein injury as described in “Saphenous vein hemostasis and thrombosis models.” Saphenous vein flow was monitored by Doppler probe up to 45 minutes or until loss of flow was observed for 60 seconds to determine the time to occlusion (TTO). Median TTO (solid lines) was 15.7 minutes for factor IX wild-type, 9.1 minutes for factor IX R170A, and more than 45 minutes for factor IX R233A (P ≤ .003, Mann-Whitney).
Effect of recombinant factor IX mutants at 5% plasma levels on the time to occlusion (TTO) after FeCl3-induced saphenous vein injury in the hemophilia B mouse.Factor IX gene–ablated C57B6 mice were infused with recombinant factor IX wild-type, R170A, or R233A at doses calculated to result in 5% normal plasma levels before undergoing FeCl3-induced saphenous vein injury as described in “Saphenous vein hemostasis and thrombosis models.” Saphenous vein flow was monitored by Doppler probe up to 45 minutes or until loss of flow was observed for 60 seconds to determine the time to occlusion (TTO). Median TTO (solid lines) was 15.7 minutes for factor IX wild-type, 9.1 minutes for factor IX R170A, and more than 45 minutes for factor IX R233A (P ≤ .003, Mann-Whitney).
Discussion
We hypothesized that mutagenesis of the factor IXa heparin-binding exosite would dramatically affect the rate of plasma thrombin generation and formation of venous thrombi. Plasma thrombin generation was detected by fluorogenic substrate cleavage and Western blotting for thrombin-related products. Factor IX–deficient plasma supplemented with 100% plasma levels of factor IX demonstrated enhanced thrombin generation compared with normal, pooled human plasma (data not shown). This result may reflect individual characteristics of the patient plasma or preanalytic variables related to collection and processing of the pooled human plasma.24,25 A single lot of hemophilia B patient plasma was used for all additional experiments, eliminating preanalytical variables as the source of variation in thrombin generation. Increasing amounts of wild-type factor IX (1%-100%) accelerated the time course and amplitude of thrombin generation. Minimal differences in thrombin generation were observed between 0% and 1% wild-type factor IX levels, whereas 5% levels resulted in significantly enhanced thrombin responses, achieving greater than half of the peak thrombin concentration observed with 100% factor IX levels (Figure 1A). These data suggest a threshold effect for enhancement of thrombin generation by factor IX that correlates with the observed clinical severity of mild (> 5%), moderate (1%-5%), and severe (< 1%) hemophilia phenotypes.26 Likewise, thrombin generation was exquisitely sensitive to factor IXa concentrations in the 5- to 100-pM range (Figure 3). Notably, 100 pM of factor IXa (0.11% normal zymogen concentration) resulted in thrombin generation similar in magnitude to factor IX–deficient plasma supplemented with 100% factor IX (Figures 1A, 2A). The sensitivity of plasma thrombin generation to picomolar factor IXa is consistent with results obtained from numerical simulations, and in synthetic plasma in the presence of platelets.27 These findings predict that excess factor IX activation will have dramatic effects on thrombin generation and the risk of throm-bosis, consistent with the role of factor XI in mouse models of thrombosis.28,29
The stability of intrinsic tenase activity at subnanomolar concentrations of factor IXa depends largely on diffusional loss of the factor VIIIa A2 domain.7 Recombinant factor IX(a) with mutations in the heparin-binding exosite that selectively enhance (R170A) or reduce (R233A) stability of the protease-A2 domain interaction had dramatic effects on plasma thrombin generation relative to factor IX wild-type (Figure 1D,E).17 Factor IX R170A demonstrated increased velocity-index and peak thrombin concentration relative to wild-type protein at all concentrations (Figure 1D), with the relative differences most pronounced at the lowest zymogen concentration (1% plasma levels). In contrast, factor IX R233A demonstrated markedly reduced peak thrombin concentration and velocity index relative to wild-type protein (Figure 1D,E), again most pronounced at the lower zymogen concentrations where no significant thrombin response was observed. This thrombin generation phenotype was more dramatic than suggested by the moderately reduced coagulant activity (59%) of factor IX R233A in an APTT-based assay.17 Evaluation of plasma thrombin generation by Western blot analysis in factor IX–deficient plasma supplemented with 5% factor IX wild-type, R170A, or R233A confirmed the validity of the fluorogenic substrate measurements (Figure 2A-C). When thrombin generation was initiated with factor IXa, the dose-response for factor IXa R170A was again shifted to the left relative to wild-type, with differences most pronounced at the lower protease concentrations. The dose-response for factor IXa R233A was likewise shifted to the right but approached factor IXa wild-type at the highest protease concentration (Figure 3A-C). Thus, the thrombin generation phenotype of these recombinant proteins largely reflects the effect of mutations on protease function. However, sufficiently high factor IXa concentration may compensate for destabilization of the protease-cofactor interaction. The dramatic effect of these mutations on plasma thrombin generation emphasizes the critical role of the interaction between the factor IXa heparin-binding exosite and the factor VIIIa A2 domain in regulation of the coagulation response.
The physiologic relevance of these findings was underscored by the effect of recombinant factor IX on saphenous vein hemostasis and thrombus formation in the hemophilia B mouse. Comparison of wild-type and Factor IX gene–ablated C57BL/6 mice demonstrated that both bleeding and thrombus formation were factor IX–dependent and restored in hemophilia B mice by infusion of factor IX before the injury. Infusion of 10% plasma levels of recombinant factor IX wild-type or R170A into hemophilia B mice dramatically shortened the ATTH after time saphenous incision, whereas 10% factor IX R233A was not significantly different from untreated mice (Figure 4). Similar to thrombin generation results, the factor IX dose dependence of thrombus formation after FeCl3-induced injury demonstrated a threshold effect less than 5% plasma factor IX levels (data not shown). Thus, the magnitude of bleeding risk in hemophilia, plasma thrombin generation, and thrombus formation appear closely linked with respect to factor IX levels. Infusion of a recombinant factor IX dose calculated to achieve 5% plasma concentrations before the injury resulted in a significantly shorter TTO for factor IX R170A compared with wild-type protease, whereas factor IX R233A failed to trigger thrombus formation at this concentration (Figure 5). These results demonstrate that mutations in the heparin-binding exosite of factor IX have significant effects on in vivo hemostasis and thrombus formation, consistent with their effects on plasma thrombin generation. Thus, stability of the intrinsic tenase complex is critical to both the rate of plasma thrombin generation and formation of venous thrombi in response to injury.
The dramatic effects on intrinsic tenase stability, plasma thrombin generation, hemostasis, and in vivo thrombus formation observed for factor IX R170A and R233A confirm that this heparin-binding exosite is a major physiologic regulator of coagulation. The relative stability of the factor IXa–A2 domain interaction appears to act as a “rheostat” that modulates the magnitude of the coagulation response. These results suggest that manipulation of this protein-protein interaction may have substantial therapeutic value in both hemophilia and thrombosis. Indeed, this protease exosite may contribute to the antithrombotic effects of heparin oligosaccharides and depolymerized holothurian glycosaminoglycan.17,19 As opposed to active site-directed inhibitors, targeting this protease exosite may allow down-regulation rather than complete disruption of the coagulation response, resulting in a broader therapeutic window. The ability of these mutations to modulate the factor IXa–A2 domain interaction suggests that this protease exosite is a robust target for novel antithrombotic agents and amendable to approaches designed to decrease the stability of this critical protein-protein interaction.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Darrell Stafford for providing factor IX wild-type and R170A cell lines and access to the factor IX knockout mouse, Dougald M. Monroe for breeding the factor IX knockout mice used in these experiments, Andreas Mueller-Beckhaus of Bayer HealthCare LLC for providing recombinant factor VIII (Kogenate FS), and Technoclone Ltd for providing the Technothrombin TGA software for analysis of plasma thrombin generation.
This work was supported by the National Institutes of Health (grant HL080452; J.P.S).
National Institutes of Health
Authorship
Contribution: Y.B. performed the thrombin generation assays and Western blotting, analyzed and interpreted data, and wrote the manuscript; H.C.W. designed and performed the murine hemostasis and thrombosis models and analyzed and interpreted data; and J.P.S. designed the research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John P. Sheehan, Department of Medicine/Hematology, University of Wisconsin, 1300 University Avenue, Medical Sciences Center, Room 4285, Madison, WI 53706; e-mail: jps@medicine.wisc.edu.
References
Author notes
*Y.B. and H.C.W. contributed equally to this study.